Abstract
The extracellular glutathione S-transferase from the filarial parasite Onchocerca volvulus (Ov-GST1) is a glutathione-dependent prostaglandin D synthase. Ov-GST1, located in the outer hypodermal lamellae and in parts of the cuticle, produces prostaglandin D2 directly at the parasite-host interface. Ov-GST1 therefore has the potential to participate in the modulation of the host immune response by contributing to the production of prostanoids; this supports the predominant hypothesis that parasite-derived eicosanoids influence host inflammatory and immune cells.
Onchocerciasis, caused by the filarial nematode Onchocerca volvulus, affects 18 million people in sub-Saharan Africa. This chronic disease is entirely caused by microfilarial stages. The resulting immunopathological changes, which can lead to severe dermatitis and blindness, reflect the close relationship between the parasite and the immunological mechanisms of the host. Eosinophils, elevated levels of serum immunoglobulin E (IgE) and IgG4, and mast cell proliferation have been shown to be associated with infections caused by filarial parasites, indicating a pronounced bias toward Th2 responses. Whether this Th2 response is protective or is present to amend the Th1 pathology is still a matter of debate (12; Special program for research and training in tropical diseases, final report series 27, http://www.who.int/tdr/research/finalreps/no27.htm, 2000). However, the long-term survival of the parasites in the host despite their close proximity to inflammatory and immune cells is remarkable and has initiated a search for parasite-derived molecules that are involved in the modulation of host immune responses.
Eicosanoids are lipid mediators that are produced from polyunsaturated fatty acids, most notably arachidonic acid. The conversion of arachidonic acid to prostanoids is catalyzed by cyclooxygenase (COX) and leads to the formation of the unstable hydroxyl endoperoxide prostaglandin (PGH2). By the action of specific PG synthases, PGH2 is converted to PGF2α, PGE2, and PGD2.
Glutathione S-transferases (GSTs) are multifunctional enzymes that are involved in the biotransformation of xenobiotics and endogenously derived cytotoxic compounds, displaying a broad specificity for hydrophobic electrophilic compounds. Molecular evolutionary studies revealed that the hematopoietic (spleen-type) PGD synthase belongs to the sigma GST class (6). This enzyme exclusively occurs in antigen-presenting, dendritic, Langerhans, Kupffer, megakaryoblastic, and mast cells, suggesting that it functions in the production of PGD2 as an allergic and inflammatory mediator (22). Our previous studies of the O. volvulus GST1 (Ov-GST1) demonstrated an overall sequence homology of 47% with the hematopoietic PGD synthase, clearly showing that the enzyme belongs to the sigma class. However, Ov-GST1 displays unique structural features: it has a signal peptide and a 25-amino-acid N-terminal extension before sequence identity to the sigma class GSTs begins. Ov-GST1 is located in the outer zone of the hypodermis, in the basal layer of the cuticle, and in the epicuticle, directly at the parasite-host interface; furthermore, it carries highly antigenic truncated mannose residues (20). Although sigma class GSTs are also found in the free-living nematode Caenorhabditis elegans, they are cytosolic enzymes, suggesting that the unique features of the Ov-GST1 are perhaps evolutionary designs of parasitism.
Investigations of specific activities with model substrates has proven useful for classifying newly described GSTs and identifying their potential physiological substrates. For substrate profiling, the Ov-GST1a was recombinantly expressed in Escherichia coli and affinity purified (9). The Ov-GST1 catalyzed glutathione (GSH) conjugation with a wide range of substrates such as 1-chloro-2,4-dinitrobenzol (10), ethacrynic acid (0.649 ± 0.015 μmol min−1 mg of protein−1), 7-chloro-4-nitrobenzofurazan (27.83 ± 4.1 μmol min−1 mg of protein−1), benzylisothiocyanate (34.0 ± 3.1 μmol min−1 mg of protein−1), and n-butylnitrite (0.87 ± 0.21 μmol min−1 mg of protein−1) under standard assay conditions (3, 13); however, no activity with α,β-unsaturated carbonyls or GSH peroxidase activity toward cumene hydroperoxide was detected, ruling out a possible involvement of the Ov-GST1 in antioxidant defense mechanisms.
Based on the observations of Pinzar et al. (19), the conserved residues required for PGD synthase activity in the Ov-GST1 were identified (Y32, R38, W129, K138, and K222, without the signal peptide) (20) and PGD2 synthesis was examined by two different enzymatic assays. PGD synthase activity was measured in a direct assay using freshly diluted 40 μM PGH2, 1 or 10 mM GSH, and various amounts of Ov-GST1 (between 0.5 and 12 μg) in the 50-μl reaction mixture (50 mM NaCl, 0.5 mM tryptophan, 0.2 μM hematin in 2 mM sodium phosphate [pH 7.4]) (15). For additional verification, a coupled assay was performed as described by Meyer and Thomas (16) with COX-1 and arachidonic acid. This ensured continuous synthesis of fresh PGH2. Control experiments were performed without the addition of GSH or by using different concentrations of arachidonic acid in the reaction mixture (data not shown). Furthermore, GSTs from other parasites (Echinococcus granulosus and Plasmodium falciparum) not belonging to the sigma class were used as negative controls. Assays were performed for 2 min under physiological conditions of pH, temperature, and ionic strength. The PGs were extracted twice by the addition of 250 μl of 2 M citric acid and ice-cold chloroform-1 mg of triphenylphosphine (1.5:1,000 [vol/vol]) ml−1. Addition of triphenylphosphine prevented decomposition of PGH2 during extraction. Following chloroform extraction, the sample was dried under a stream of nitrogen and dissolved in 200 μl of acetonitrile-water-trifluoroacetic acid (31:69:0.02 [vol/vol/vol]). An aliquot of 30 μl was used for analysis by high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS). Liquid chromatography was performed with a Hewlett-Packard model 1100 series. The mixture was analyzed by straight-phase HPLC at an operating temperature of 20°C on an RP-18 LiChrosphere column (particle size, 5 μm; length, 20 cm; with Lichroprep precolumn) operated isocratically, with acetonitrile-water-trifluoroacetic acid (31:69:0.02 [vol/vol/vol]) at a flow rate of 1.1 ml min−1. The effluent passed a UV detector (214 nm) and was then subjected to ion electrospray ionization. For mass spectrometry, a G1946A mass-selective detector, operating in full or selected scan and atmospheric pressure electrospray-positive modes at a fragmentor voltage of 60 mV, was used.
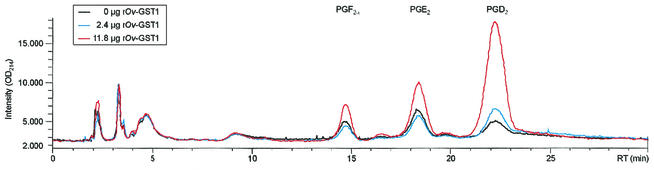
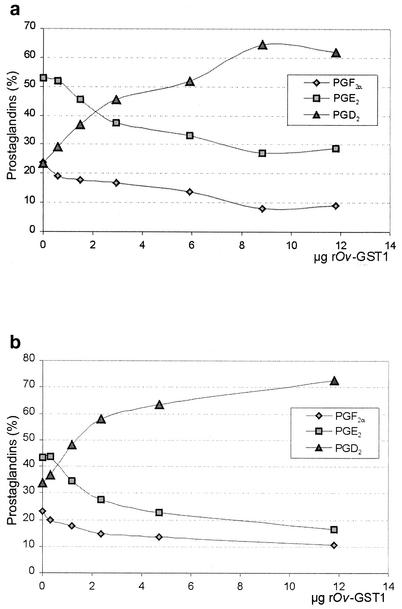
Figure 1 shows an overlay of UV traces (optical density at 214 nm) from two individual experiments, typical for the retention pattern observed in several similar experiments carried out in the absence or presence of recombinant Ov-GST1 (rOv-GST1). Due to the instability of PGH2 when added directly or synthesized by COX-1 in the assay, we were not able to quantify the specific PGD synthase activity; however, the results clearly show that the Ov-GST1 specifically promotes PGD2 formation. Analysis of the PGD synthase activity of native Ov-GST1 isolated from female adult worms revealed that the N-glycans did not influence the catalytic activity (data not shown). Figure 2a (direct assay) and b (coupled assay) show that rOv-GST1 is able to synthesize PGD2 in a concentration-dependent manner. In contrast to other sigma class GSTs, whose nonselective isomerization properties lead to a mixture of PGF2α, PGD2, and PGE2 (21), rOv-GST1 isomerizes PGH2 to PGD2 selectively, as depicted in Fig. 1 and 2. The small amounts of PGE2 and PGF2α observed can be explained only in terms of rapid degradation of the highly unstable PGH2; increasing the concentration of rOv-GST1 notably decreases the formation of these by-products because of the higher consumption of PGH2 in the catalytic formation of PGD2.
FIG. 1.
PGD synthase activity of rOv-GST1. The PGD synthase activity was measured in a coupled assay using ovine COX-1 and arachidonic acid as the substrate. PG synthesis was performed for 2 min in the presence of 12 (red) or 2.4 μg (blue) of rOv-GST1 and in the absence of rOv-GST1 (black). The HPLC-ESI-MS chromatogram shows the major PG products. RT, retention time; OD214, optical density at 214 nm.
FIG. 2.
Analysis of concentration-dependent PGD2 formation by rOv-GST1. Isomerase activity of rOv-GST1 was determined in a coupled assay with COX-1 and arachidonic acid (a) and in a direct assay using PGH2 at a constant concentration of 40 μM (b). While PGF2α and PGE2 are breakup products of PGH2, PGD2 is formed in a concentration-dependent manner by increasing concentrations of rOv-GST1, indicating the specific isomerization of PGH2 to PGD2.
The production of PGs by sigma class GSTs can be attributed to the unique architecture of the hydrophobic binding pocket. Like those of other sigma class GSTs, the catalytic pocket of the Ov-GST1 has a wide, open cleft, unlike the narrow, shallow cavities of other GST classes (20). Whereas the Ov-GST1 specifically forms PGD2, analysis of the Ascaridia galli sigma class GST revealed a specific PGH2-PGE2 isomerase activity (15).
The divergent functions of PGD2 and its metabolite 15-deoxy-delta-12,14-PGJ2 in the development and/or modulation of inflammatory and immunological responses have been studied intensively (for a recent review see reference 4). Recently it was shown that PGD2 exerts its biological effects on different cell types by means of two different receptors, explaining the often opposing actions observed. While well-known functions like vasodilation, relaxation of smooth muscles, inhibition of platelet aggregation, and dendritic cell migration are mediated by the DP-1 receptor (18), induction of chemokinesis and chemotaxis of eosinophils, basophils, and TH2 lymphocytes is due to the activation of the CRTH2 pathway (5, 17). Recently, Angeli et al. (1) demonstrated that skin infection of mice with Schistosoma mansoni activated but also retained Langerhans cells in the epidermis. This inhibitory effect appears to be due to parasite-produced PGD2 rather than host-derived anti-inflammatory cytokines, representing an additional strategy of the parasite to actively modulate the host immune system. It is conceivable that skin microfilariae of O. volvulus use the same trick to escape the cutaneous immune response.
Even though the biochemical pathway of the filarial eicosanoid metabolism is poorly characterized, several studies have demonstrated that filarial parasites and their hosts mutually influence various physiological aspects of their counterpart by means of lipid mediators (for a recent review, see reference 2). The first evidence of a possible involvement of filaria-derived eicosanoids in the modulation of the host's immune response came from studies analyzing the effects of diethylcarbamazine (DEC). This inhibitor of the eicosanoid synthesis is used for the treatment of lymphatic filariasis. Whereas in vitro studies demonstrate that DEC itself has no direct microfilaricidal activity, the drug rapidly reduces the number of circulating microfilariae in the bloodstream and skin (8, 14). Brugia malayi microfilariae were shown to inhibit platelet aggregation by secreting platelet-suppressive prostanoids (11). In canine heartworm disease, relaxation of the aorta is caused by whole worms or products of Dirofilaria immitis. Here, analysis of the D. immitis supernatant demonstrated the production of PGD2, indicating that this prostanoid is involved in the pathogenesis of dirofilariasis (7).
This study reports the brief observation that, beside being a classical detoxification enzyme, the extracellular Ov-GST1 is a GSH-dependent PGD synthase. The Ov-GST1 therefore has the potential to participate in the modulation of immune cell functions by contributing to the production of prostanoids. The physiological importance of PGD2 synthesis by Ov-GST1 for the clinical manifestation of onchocerciasis is an interesting subject that needs to be further elucidated.
Acknowledgments
This work was supported by the DFG (projekt Li 793/1-5).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Angeli, V., C. Faveeuw, O. Roye, J. Fontaine, E. Teissier, A. Capron, I. Wolowczuk, M. Capron, and F. Trottein. 2001. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 193:1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daugschies, A., and A. Joachim. 2000. Eicosanoids in parasites and parasitic infections. Adv. Parasitol. 46:181-240. [DOI] [PubMed] [Google Scholar]
- 3.Günzler, W. A., and L. Flohe. 1987. Glutathione peroxidase, p. 285-289. In R. A. Greenwald (ed.), Handbook of methods for oxygen radical research. CRC Press, Inc., Boca Raton, Fla.
- 4.Harris, S. G., J. Padilla, L. Koumas, D. Ray, and R. P. Phillips. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23:144-150. [DOI] [PubMed] [Google Scholar]
- 5.Hirai, H., K. Tanaka, O. Yoshie, K. Ogawa, K. Kenmotsu, Y. Takamori, M. Ichimasa, K. Sugamura, M. Nakamura, S. Takano, and K. Nagata. 2001. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jowsey, I. R., A. M. Thomson, J. U. Flanagan, P. R. Murdock, G. B. T. Moorre, D. J. Meyer, G. J. Murphy, S. A. Smith, and J. D. Hayes. 2001. Mammalian class sigma glutathione S-transferases: catalytic properties and tissue-specific expression of human and rat GSH-dependent prostaglandin D2 synthases. Biochem. J. 359:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser, L., V. L. Lamb, P. K. Tithof, D. A. Gage, B. A. Chamberlin, J. T. Watson, and J. F. Williams. 1992. Dirofilaria immitis: do filarial cyclooxygenase products depress endothelium-dependent relaxation in the in vitro rat aorta? Exp. Parasitol. 75:159-167. [DOI] [PubMed] [Google Scholar]
- 8.Kanesa-Thasan, N., J. G. Douglas, and J. W. Kazura. 1991. Diethylcarbamazine inhibits endothelial and microfilarial prostanoid metabolism in vitro. Mol. Biochem. Parasitol. 49:11-19. [DOI] [PubMed] [Google Scholar]
- 9.Liebau, E., G. Wildenburg, R. D. Walter, and K. Henkle-Dührsen. 1994. A novel type of glutathione S-transferase in Onchocerca volvulus. Infect. Immun. 62:4762-4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebau, E., R. D. Walter, and K. Henkle-Dührsen. 1994. Isolation, sequence and expression of an Onchocerca volvulus glutathione S-transferase cDNA. Mol. Biochem. Parasitol. 63:305-309. [DOI] [PubMed] [Google Scholar]
- 11.Liu, L. X., and P. F. Weller. 1992. Intravascular filarial parasites inhibit platelet aggregation: a role of parasite-derived prostanoids. J. Clin. Investig. 89:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maizels, R. M., D. A. P. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 13.Mannervik, B., and C. Guthenberg. 1981. Glutathione transferase (human placenta). Methods Enzymol. 77:231-235. [DOI] [PubMed] [Google Scholar]
- 14.Medina-de la Garca, C. E., N. W. Brattig, F. W. Tischendorf, and J. M. Jarrett. 1990. Serum-dependent interaction of granulocytes with Onchocerca volvulus microfilariae in generalised and chronic hyperreactive onchocerciasis and its modulation by diethylcarbamazine. Trans. R. Soc. Trop. Med. Hyg. 84:701-706. [DOI] [PubMed] [Google Scholar]
- 15.Meyer, D. J., R. Muimo, M. Thomas, D. Coates, and R. E. Isaac. 1996. Purification and characterization of prostaglandin-H E-isomerase, a sigma-class glutathione S-transferase, from Ascaridia galli. Biochem. J. 313:223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer, D. J., and M. Thomas. 1995. Characterization of rat spleen prostaglandin H D-isomerase as a sigma-class GSH transferase. Biochem. J. 311:739-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monneret, G., S. Gravel, M. Diamond, J. Rokach, and W. S. Powell. 2001. Prostaglandin D2 is a potent chemoattractant for human eosinophils that act via a novel DP receptor. Blood. 98:1942-1948. [DOI] [PubMed] [Google Scholar]
- 18.Narumiya, S., Y. Sugimoto, and F. Ushikubi. 1999. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79:1193-1226. [DOI] [PubMed] [Google Scholar]
- 19.Pinzar, E., M. Miyano, Y. Kanaoka, Y. Urade, and O. Hayaishi. 2000. Structural basis of hematopoietic prostaglandin D synthase activity elucidated by site-directed mutagenesis. J. Biol. Chem. 359:31293-31344. [DOI] [PubMed] [Google Scholar]
- 20.Sommer, A., M. Nimtz, H. S. Conradt, N. Brattig, K. Boettcher, P. Fischer, R. D. Walter, and E. Liebau. 2001. Structural analysis and antibody response to the extracellular glutathione S-transferases from Onchocerca volvulus. Infect. Immun. 69:7718-7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ujihara, M., S. Tsuchida, K. Satoh, K. Sato, and Y. Urade. 1988. Biochemical and immunological demonstration of prostaglandin D2, E2 and F2α formation from prostaglandin H2 by various rat glutathione S-transferase isoenzymes. Arch. Biochem. Biophys. 264:428-437. [DOI] [PubMed] [Google Scholar]
- 22.Urade, Y., M. Ujihara, Y. Horiguchi, M. Igarashi, A. Nagata, K. Ikai, and O. Hayaishi. 1990. Mast cells contain spleen-type prostaglandin D synthetase. J. Biol. Chem. 265:371-375. [PubMed] [Google Scholar]