Abstract
The leading cause of food poisoning in both Taiwan and Japan is Vibrio parahaemolyticus infection, whose mechanism of enteropathogenesis is still unclear. To evaluate whether surface components are responsible for the intestinal adhesion of V. parahaemolyticus, we have developed a novel method for isolating the capsular polysaccharide (CPS) from V. parahaemolyticus (serotype O4:K8). We found that culturing of V. parahaemolyticus in broth for 1 week or more changed the colony form of the bacteria on an agar plate from opaque to translucent. The translucent colonies of V. parahaemolyticus contained little CPS and exhibited a much lower level of adherence to epithelial cells (Int-407) than the opaque colonies of the bacteria. Incubation of V. parahaemolyticus in medium supplemented with bile increased the levels of CPS and adherence. Treatment of V. parahaemolyticus with anti-CPS but not anti-LPS serum decreased the level of bacterial adherence. In addition, purified CPS bound to epithelial cells in a dose-dependent manner. Intranasal administration of CPS to mice in the presence of adjuvants such as immunostimulatory sequence oligodeoxynucleotides or cholera toxin elicited CPS-specific mucosal and systemic immune responses. These results indicate that CPS plays an important role in the adherence of V. parahaemolyticus to its target cells and may be considered a potential target for the development of a vaccine against this pathogen.
Vibrio parahaemolyticus, a widely distributed gram-negative facultative anaerobic bacillus that causes acute gastroenteritis, was first isolated from its victims in 1950 by Fujino et al. (14). Under favorable conditions, the doubling time of V. parahaemolyticus can be as short as 9 min (26), enabling the organism to multiply rapidly. The onset of the illness usually takes place 3 to 24 h after consumption of V. parahaemolyticus-contaminated food. The symptoms include diarrhea, abdominal cramps, nausea, vomiting, headache, and low-grade fever (19). Exotoxins of the organism, such as thermostable direct hemolysin and its related toxin, have been intensively studied. It is generally believed that these exotoxins alone are not sufficient to generate the pathogenicity of the organism (19, 36).
Before enteric pathogens can cause disease, they usually adhere to, replicate in, and produce virulence factors on or in epithelial cells of the enteric tract. The adherence of V. parahaemolyticus to epithelial cell lines has been evaluated in several in vitro studies (6, 7, 16, 43). The association of pili of a V. parahaemolyticus strain with bacterial colonization of epithelial cells has been documented (30, 44). However, this result has not yet been generally verified for other strains. Whether there is a mechanism of bacterial colonization other than pili needs further elucidation.
V. parahaemolyticus synthesizes three major surface antigens: heat-stable somatic O antigens (lipopolysaccharide [LPS]), heat-labile capsular K antigens (capsular polysaccharide [CPS]), and H antigens (flagellar antigens). Although H antigens of all types are serologically identical, 13 different O antigens and 71 different K antigens have been identified (8, 22, 41). Recently, culturing of V. parahaemolyticus with bile or deoxycholate was shown to increase not only the bacterial capsule size but also the production of a uronic acid-containing capsule and to enhance the level of adherence to epithelial cells (32). For other enteric bacteria, it has been shown that capsule size can play a major role in virulence (8, 45) and that the production of a uronic acid-containing extracellular capsule enables the bacteria to aggregate and adhere to intestinal cells (32). Therefore, it is possible that the adherence ability and pathogenicity of V. parahaemolyticus are related to its capsule. However, which major surface components are responsible for adherence remains to be determined.
In this study, we have purified and characterized a CPS from V. parahaemolyticus and evaluated the inhibitory effect of anti-CPS antibodies on the adherence of the bacterium to an intestinal cell line (Int-407). In addition, we have used CPS as an antigen together with either cholera toxin (CT) or immunostimulatory sequence oligodeoxynucleotides (ODN) to immunize mice (18, 20). CPS-specific IgA in the feces and IgG in the plasma of these immunized mice have been analyzed to determine the ability of CPS to elicit mucosal and systemic immunity.
MATERIALS AND METHODS
Materials.
Sera against O4:K8 V. parahaemolyticus (anti-O4:K8 whole-bacterium sera) were obtained from the Institute of Biological Chemistry, Academia Sinica. Serotyping antibodies against O4 antigens (LPS) and K8 antigens (CPS) were purchased from Denka (Seiken, Japan).
Culturing of bacteria and intestinal cell line.
V. parahaemolyticus isolates (13,023 clinical isolates, serotype O4:K8) obtained from the Culture Collection and Research Center in Taiwan (CCRC) were grown at 37°C in brain heart infusion (BHI) broth (Difco, Detroit, Mich.) supplemented with 3% NaCl. Intestinal cell line Int-407, purchased from CCRC, was routinely cultured as monolayers in basal Eagle medium (GIBCO BRL, Gaithersburg, Md.) containing 15% calf serum at 37°C in a humidified atmosphere of 5% CO2 in air.
Isolation of CPS.
A single bacterial colony isolated from BHI agar (1.5% agar in BHI broth supplemented with 3% NaCl) was inoculated into a 2-liter glass flask containing 500 ml of BHI culture broth and 3% NaCl. The mixture was shaken at 200 rpm overnight at 37°C. Bacterial cell particles and their debris were removed by centrifugation at 16,000 × g and 4°C for 20 min, and the supernatant was concentrated 10-fold by ultrafiltration (Labscale TFF system; 300,000-molecular-weight-cutoff membrane [Millipore, Bedford, Mass.]). The concentrated supernatant was centrifuged at 48,000 × g and 4°C for 2 h and subjected to enzymatic digestion with RNase (100 μg/ml), DNase I (50 μg/ml plus 1 mM MgCl2), and pronase (250 μg/ml) at 37°C for 1 h. The enzyme-treated sample was dialyzed (100,000-molecular-weight-cutoff membrane [Millipore]), and the resultant solution was lyophilized.
The lyophilized material was subsequently mixed with sample buffer solution (0.05 M glycine- 0.001 M EDTA-10% sodium deoxycholate [pH 9.6]) and adjusted to a concentration of 10 mg of lyophilized powder per ml of buffer. The sample was passed through a 0.45-μm-pore-size nitrocellulose membrane (Amersham Biosciences, Uppsala, Sweden) to remove large particles. Thirty milliliters of the filtered sample was subsequently applied to a column of Sephacryl S-400 (5.0 by 90 cm; Amersham) that had been preequilibrated with running buffer solution (0.05 M glycine- 0.001 M EDTA- 3% sodium deoxycholate [pH 9.6]) (24). The effluent was monitored for the presence of CPS by a Western blot assay as described below. Fractions containing CPS were pooled, dialyzed with multiple changes of distilled water, and lyophilized. The lyophilized sample was mixed with 85% (vol/vol) ethanol and centrifuged at 16,000 × g and 4°C for 30 min. The pellet was collected, washed with 85% ethanol twice, dissolved in distilled water, and adjusted to a concentration of 10 mg of dry pellet per ml of distilled water.
Western blot analysis.
Samples were subjected to electrophoresis on a sodium dodecyl sulfate (SDS)-12% polyacrylamide gel and then transferred to a nitrocellulose membrane (Amersham). The membrane was blocked by treatment with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBST) and 5% nonfat dry milk for 1 h at room temperature. After the blocking procedure, the membrane was incubated for 1 h with anti-O4:K8 whole-bacterium, anti-K8 CPS, or anti-O4 LPS serum (diluted 1/10,000 in PBS) and then washed with PBST three times. The membrane was subsequently treated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) secondary antibodies (diluted 1/10,000 in PBS; Serotec, Oxford, United Kingdom) for 1 h. Bands were detected by chemiluminescence with an ECL kit (Amersham).
Monosaccharide and fatty acid composition analyses.
Purified CPS was hydrolyzed with 48% (wt/wt) hydrofluoric acid (2°C, 36 h) and dried with nitrogen gas at 2°C. The lipid portion and dephosphorylated chain fragments were separated twice by phase partitioning in CHCl3-methanol-0.05 M NH4HCO3 (1:1:0.9). Water-soluble and organic-soluble fractions were dried by using nitrogen gas. The water-soluble fraction was used for monosaccharide composition analysis, while the organic-soluble fraction was used for fatty acid composition analysis. In brief, the water-soluble carbohydrates were methanolyzed with 0.5 M methanolic HCl (Supelco, Bellefonte, Pa.) at 80°C for 16 h. They were subsequently N acetylated again with 500 μl of methanol, 10 μl of pyridine, and 50 μl of acetic anhydride; treated with Sylon HTP trimethylsilylating reagent (Supelco) for 20 min at room temperature; air dried with nitrogen gas; redissolved in hexane; and subjected to gas chromatography (GC)-mass spectrometry (MS) analysis. The organic-soluble fatty acids were methanolyzed with 0.5 M methanolic HCl at 80°C for 16 h. They were subsequently treated with Sylon HTP trimethylsilylating reagent for 20 min at room temperature,air dried with nitrogen gas, redissolved in hexane, and used for GC-MS analysis.
GC-MS analysis was carried out with a Hewlett-Packard model 5890 gas chromatograph connected to a Hewlett-Packard 5790 mass-selective detector. The trimethylsilylated derivatives were chromatographed by using a Hewlett-Packard HP-5MS fused-silica capillary column (30 m; 0.25-mm inner diameter) with a temperature gradient of 60 to 140°C at 25°C min−1, 200°C at 5°C min−1, and an increase to 300°C at 10°C min−1.
Adherence assay.
The assay for the adherence of V. parahaemolyticus to Int-407 human epithelial cells was carried out as described previously (32) with minor modifications. In brief, bacteria were cultured in BHI culture broth overnight, subcultured in BHI culture broth with or without bile (1% [wt/vol] preheated at 100°C for 30 min and then cooled to room temperature; Sigma, St. Louis, Mo.) for 3 h, and then cultured for another 30 min in freshly prepared, prewarmed BHI medium (without bile) at 37°C. The bacteria (about 106 bacteria per 10 μl) were added to the Int-407 cell monolayer (105 epithelial cells per well in a 24-well tissue culture plate) overlaid with antibiotic-free Hanks' balanced salt solution (HBSS) (GIBCO BRL). After incubation for 30 min, the plate was washed with HBSS five times to remove loose bacteria, and cultured cells were lysed in 0.5 ml of HBSS containing 0.1% sodium deoxycholate. The solution was diluted 100-fold, and 50 μl was plated on BHI culture agar. Bacterial colonies were counted after overnight incubation at 37°C. Under the same conditions, in the absence of the Int-407 cell monolayer, there were few (1,000-fold less) bacterial colonies. The numbers of colonies were thus indicative of bacterial adherence to Int-407 cells.
To evaluate the inhibitory effect of antibodies, sera were pretreated by heating at 56°C for 30 min, and bacteria were incubated with the sera or preimmune serum at a predesignated dilution (1/5 to 1/100) for 30 min at room temperature prior to the adherence assay.
Immunization of mice with CPS.
Female BALB/c mice (6 to 8 weeks old; National Laboratory Animal Breeding and Research Center, Taipei, Taiwan) were immunized intranasally while under light anesthesia with ketamine. CPS (3 or 10 μg), alone or in combination with 10 μg of CT (Sigma) or CpG ODN (TGACTGTGAACGTTCGAGATGA) (21) in 20 μl of water, was used for immunization. Each animal was inoculated at days 0, 14, and 28, and feces and serum samples were collected 2 weeks after each treatment. All animal handling processes and experiments complied with federal guidelines of the United States and institutional policies of Academia Sinica.
Collection and processing of fecal samples.
Freshly voided feces were collected and dried in a SpeedVac concentrator. The dried feces were mixed with PBS containing 5% skim milk (Difco) and protease inhibitor (Boehringer Mannheim, Mannheim, Germany) at a concentration of 1 mg of feces in 15 μl of solution. The mixture (ca. 600 to 900 μl) was resuspended completely in a 1.5-ml centrifuge tube by vortexing and then was centrifuged at 16,000 × g for 15 min to remove residual solids. The supernatant was collected and frozen at −20°C.
ELISA.
The CPS-specific IgA in feces (or IgG in serum samples) was determined by an enzyme-linked immunosorbent assay (ELISA) as described previously (31). In brief, V. parahaemolyticus CPS (20 μg/ml in 0.06 M carbonate buffer [pH 9.6]) was used to coat a flat-bottom 96-well microtiter plate (100 μl per well; Nunc, Roskilde, Denmark) overnight at room temperature. After three washes with PBST, the plate was blocked by treatment with PBS containing 5% skim milk for 1 h at room temperature. The plate was washed again, and 50-μl samples of fecal extracts (or sera) in twofold serial dilutions in PBS were added to designated wells. After 1 h of incubation at room temperature, followed by washes, the plate was incubated with 50 μl of biotin-conjugated goat anti-mouse IgA (or anti-mouse IgG) antibodies (diluted 1/3,000 in PBS; Serotec) for 1 h. The plate was washed again and incubated with 100 μl of Vectastain ABC reagent (Vector Laboratories, Burlingame, Calif.) for 1 h. After thorough washing, 100 μl of 3,3′,5,5′-tetramethylbenzidine (Sigma) was added as a substrate for color development. The reaction was stopped by the addition of 100 μl of 0.5 M H2SO4 15 min later, and the absorbance was measured at 450 nm. The end-point titer was defined as the reciprocal of the highest dilution that resulted in an absorbance value two times greater than that of the negative controls. A nonpaired t test was used to analyze the significance of the difference in the antibody titers.
RESULTS
Extraction and purification of CPS.
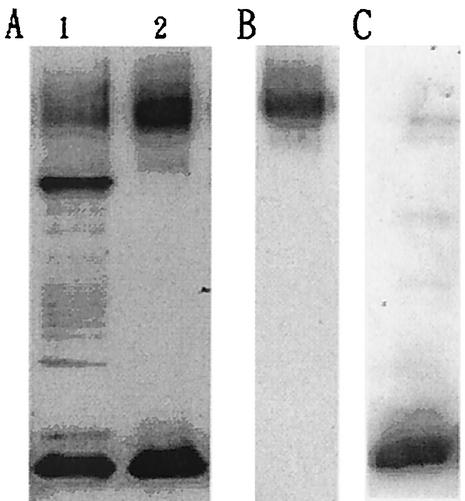
To isolate capsular materials, V. parahaemolyticus cell cultures were shaken overnight. To separate CPS from cellular DNA, RNA, and protein contaminants, culture media were digested with DNase, RNase, and pronase and then subjected to ultrafiltration. Western blot analysis of treated media revealed two major bands (Fig. 1A, lane 2) reactive with anti-O4:K8 whole-bacterium serum. One of them (the upper band) also reacted with anti-K8 CPS serum (Fig. 1B), while the lower band reacted with anti-O4 LPS serum (Fig. 1C).
FIG. 1.
Western blot analysis of V. parahaemolyticus capsular materials. The capsular materials obtained from bacterial cultures (lane 1) were treated with enzymes to digest DNA, RNA, and protein contaminants. Ultrafiltration separated capsular materials (A, lane 2, B, and C) from contaminants. Materials in lanes 1 and 2 of panel A were labeled with anti-O4:K8 whole-bacterium serum; materials in panels B and C were labeled with anti-K8 CPS and anti-O4 LPS sera, respectively. For details, see Materials and Methods.
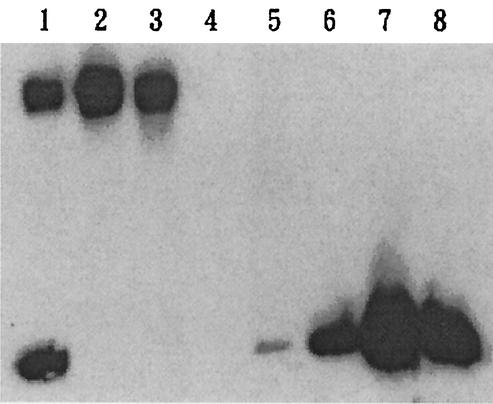
To separate the components contained in the upper and lower bands, Sephacryl S-400 gel filtration was used. After elution of the column with 3% sodium deoxycholate buffer, the fractions that contained K8 CPS (fractions 44 to 46, shown in Fig. 2, lanes 2 and 3) and O4 LPS (fractions 52 to 56, shown in Fig. 2, lanes 6, 7, and 8) were collected and lyophilized. The yield of purified CPS (pooled fractions 44, 45, and 46) was about 120 mg per liter of culture medium.
FIG. 2.
Western blot analysis of purified K8 CPS. Anti-O4:K8 whole-bacterium serum was used in the Western blot analysis to identify CPS purified by Sephacryl S-400 chromatography with a 3% sodium deoxycholate buffer solution as the eluent. Lane 1, isolated polysaccharides prior to Sephacryl S-400 purification. Lanes 2 and 3, fractions 44 and 46, respectively, of the Sephacryl S-400 eluate. Lanes 4, 5, 6, 7, and 8, fractions 48, 50, 52, 54, and 56, respectively.
Composition analysis of K8 CPS.
UV spectral analysis of the isolated K8 CPS revealed the absence of absorption at 260 and 280 nm (data not shown), indicating that it contained little, if any, nucleic acid or protein. The sugar content in K8 CPS, as measured by the phenol-sulfuric acid method, was about 70% (wt/wt). The monosaccharide composition analysis showed that this CPS contained glucose, galactose, fucose, and N-acetylgalatosamine in a ratio of 2:2:5:16. Fatty acid composition analysis showed that it also contained palmitic acid and stearic acid in a ratio of 1:4.
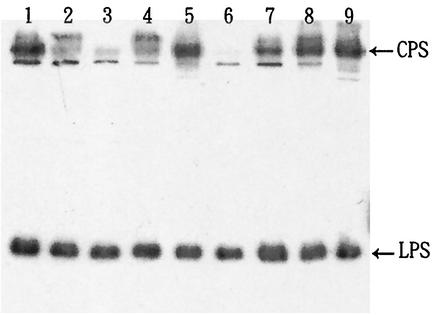
Expression of K8 CPS in medium containing bile or surfactants.
The size and composition of the capsule may play an important role in the pathogenicity of enteric bacteria (8, 17, 43). It has been reported that the addition of 1.5% bile or 0.1% deoxycholate to V. parahaemolyticus cultures enhances not only bacterial capsule size on agar plates but also adherence to epithelial cells (32). To determine whether the amount of K8 CPS or O4 LPS was increased by the addition of bile, V. parahaemolyticus was incubated overnight in tubes containing medium with serially increasing concentrations of bile. After incubation, all tubes were adjusted to contain the same concentration of bile and were then rocked at 4°C for 3 h to minimize any differences due to the potential detergent effect of bile. The culture media were subsequently collected and analyzed by Western blotting. Figure 3 shows that the amount of CPS but not LPS increased as the bacteria were cultured with increasing concentrations of bile (from 0.1% up to 1.0%). Deoxycholate, one of the major active components of bile (4, 32), had a similar enhancing effect on CPS of V. parahaemolyticus (Fig. 4). Since deoxycholate is a surfactant, we examined other surfactants to determine whether they have a similar effect (Fig. 4). Our results indicated that in terms of potency for increasing the expression of CPS, the order of these surfactants was as follows: deoxycholate ≥ SDS > Triton X-100 ≥ NP-40 > Tween 20.
FIG. 3.
Effect of bile on the expression of CPS. Anti-O4:K8 whole-bacterium (A) and anti-K8 CPS (B) sera were used in the Western blot analysis to show the effect of bile on V. parahaemolyticus. Lanes 1, crude extracts obtained from bacteria cultured in BHI culture medium. Lanes 2 to 5, crude extracts from bacteria cultured overnight in BHI culture medium supplemented with 0.1, 0.3, 1.0, and 1.5% bile, respectively. Lane 6, purified CPS. The number of bacteria used in each lane was 5 × 106. Densitometric analysis of the data in panel A indicated that the relative intensity of the CPS band increased from 1.31 (without bile treatment) to 1.93, 2.68, 3.03, and 2.84 with 0.1, 0.3, 1.0, and 1.5% bile treatments, respectively.
FIG. 4.
Effect of surfactants on the expression of CPS. Anti-O4:K8 whole-bacterium serum was used in the Western blot analysis. Lanes 1 to 5, crude extracts from bacteria cultured overnight in BHI culture medium supplemented with 0.1% SDS, NP-40, Tween 20, Triton X-100, and sodium deoxycholate, respectively. Lanes 6 to 9, crude extracts from bacteria cultured overnight in BHI culture medium supplemented with 0, 0.1, 0.3, and 1.0% bile, respectively.
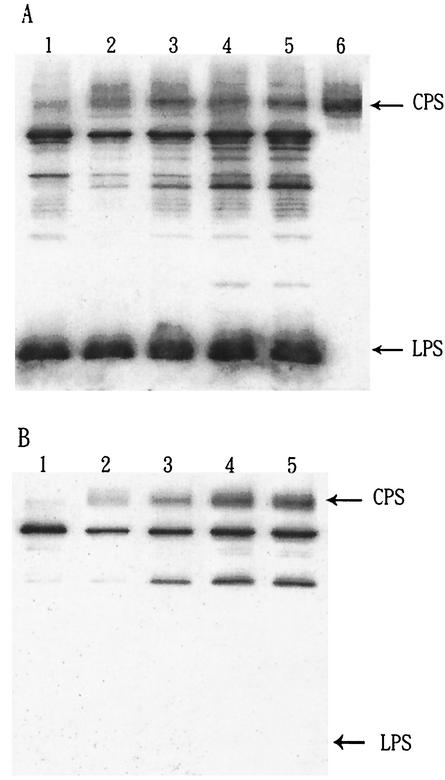
Relationship between K8 CPS content and adherence of opaque and translucent V. parahaemolyticus colonies.
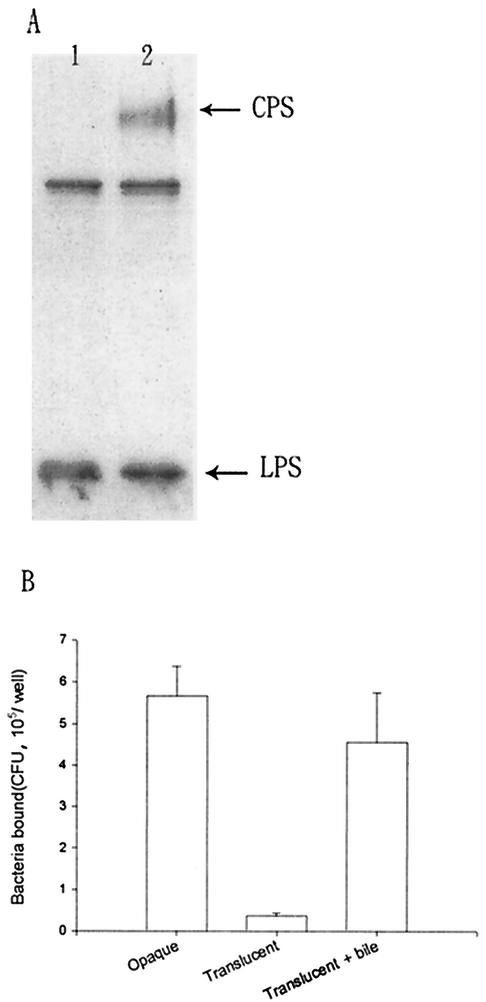
Colonies of V. parahaemolyticus CCRC strain 13023 on BHI agar plates were usually in the opaque form. However, after 1 week or more of culturing in BHI culture broth, translucent colonies appeared upon plating on BHI agar plates again. Western blot analysis of the capsular antigen compositions in the translucent and opaque colonies showed that the translucent-colony bacteria had a much smaller amount of K8 CPS (Fig. 5A). Although the rates of growth of translucent and opaque colonies were equivalent (both exhibited similar doubling times of 15 min under our experiment conditions), we found that the adherence ability of the translucent colonies was at least 10-fold lower than that of the opaque colonies (Fig. 5B). Culturing of the translucent-colony bacteria in broth containing bile converted them to opaque-colony bacteria and increased their CPS content as well as their adherence to Int-407 cells (Fig. 5B).
FIG. 5.
Correlation between CPS and adherence ability of V. parahaemolyticus. (A) Anti-O4:K8 whole-bacterium serum was used in the Western blot analysis to detect extracellular antigens of opaque and translucent forms of V. parahaemolyticus. Lane 1, crude extract obtained from the translucent colony. Lane 2, crude extract obtained from the opaque colony. Bands other than CPS and LPS are due to contaminants in the crude extracts that are recognized by anti-O4:K8 whole-bacterium serum, as shown in Fig. 1A, lane 1. These contaminants can be removed by further purification, as shown in Fig. 1A, lane 2. (B) V. parahaemolyticus was cultured in BHI culture broth overnight before addition to Int-407 cells for the adherence assay. Values for opaque and translucent forms of V. parahaemolyticus as well as values for translucent bacteria after bile treatment (cultured in BHI medium with 1% bile for 1 week) are reported as means and standard deviations (n = 3). In the negative controls (i.e., wells without Int-407 cells), we found very few colonies (less than 5 × 102 CFU/well) for both translucent- and opaque-colony bacteria.
Relationship among CPS, anti-CPS antibodies, and adherence.
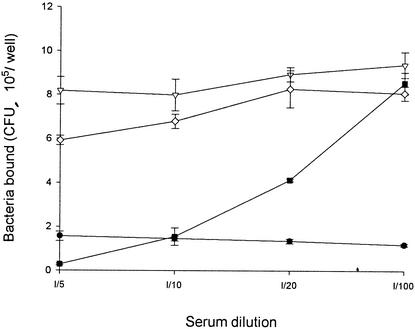
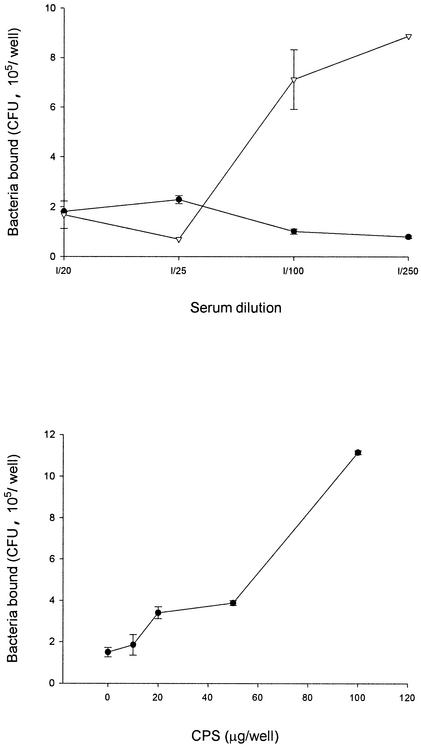
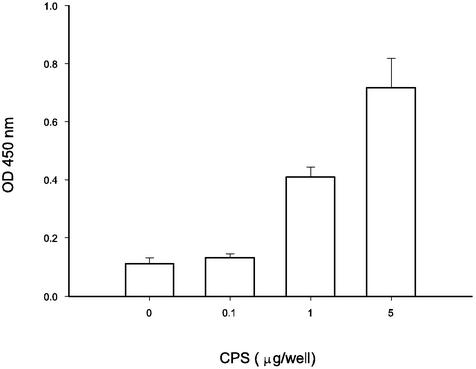
To evaluate whether the adherence of V. parahaemolyticus to Int-407 cells can be inhibited by any specific antibodies, the interactions of anti-O4:K8 whole-bacterium, anti-O4 LPS, and anti-K8 CPS sera with the bacteria and their effects were investigated. By mixing serum with V. parahaemolyticus for 1 h at room temperature, filtering the mixture through a 0.22-μm-pore-size membrane to isolate antibody-bacterium complexes, and using enzyme-labeled secondary antibodies to identify the complexes, we found that anti-O4 LPS serum, like anti-K8 CPS and anti-O4:K8 whole-bacterium sera, contained antibodies that bound to the bacteria, while preimmune and normal sera did not (data not shown). Although antibodies in all three kinds of sera bound to the bacteria, the adherence of bacteria to Int-407 cells was inhibited by anti-O4:K8 whole-bacterium and anti-K8 CPS sera but not by anti-O4 LPS serum (Fig. 6). Moreover, the inhibitory effect of the anti-O4:K8 whole-bacterium serum was affected by preincubation of the serum with CPS in a dose-dependent manner (Fig. 7). Preincubation of the anti-O4:K8 whole-bacterium serum with LPS (at a concentration even as high as 400 μg/well), on the other hand, had no effect (data not shown). In addition, we found that purified CPS bound to Int-407 cells in a dose-dependent manner (Fig. 8). These results suggest that K8 CPS may play an important role in the adherence of V. parahaemolyticus to enteric cells.
FIG. 6.
Inhibitory effect of antibodies on the adherence ability of V. parahaemolyticus. Bacteria were incubated with anti-O4:K8 whole-bacterium serum (•), anti-O4 LPS serum (▿), anti-K8 CPS serum (▪), or preimmune serum (⋄) before addition to Int-407 cells for the adherence assay. Values are reported as means and standard deviations (n = 3). Some variances, however, were too small to be shown. Normal serum seems to contain some factors that increase colony formation by bacteria.
FIG. 7.
Influence of CPS on the inhibitory effect of antibodies. (Top) Bacteria were incubated with anti-O4:K8 whole-bacterium serum (•) or CPS (20 μg/well) plus anti-O4:K8 whole-bacterium serum (▿) before addition to Int-407 cells for the adherence assay. (Bottom) Bacteria were incubated with anti-O4:K8 whole-bacterium serum (1/100 dilution) and CPS at predesignated amounts as indicated. Values are reported as means and standard deviations (n = 3). CPS (100 μg/well) prevented the inhibitory effect of anti-O4:K8 whole-bacterium serum (1/250 to 1/100 dilutions) on the adherence of V. parahaemolyticus (P < 0.05).
FIG. 8.
Evaluation of adherence of CPS to Int-407 cells. Purified CPS (0.1, 1, or 5 μg/well) was added to the Int-407 cell monolayer (104 epithelial cells per well) in a 96-well tissue culture plate. After 30 min of incubation, the plate was washed to remove unbound CPS. The amount of CPS bound to Int-407 cells was determined by using anti-CPS antibodies and an ELISA. OD, optical density. Values are reported as means and standard deviations (n = 3). For details, see Materials and Methods.
Elicitation of immune responses by K8 CPS.
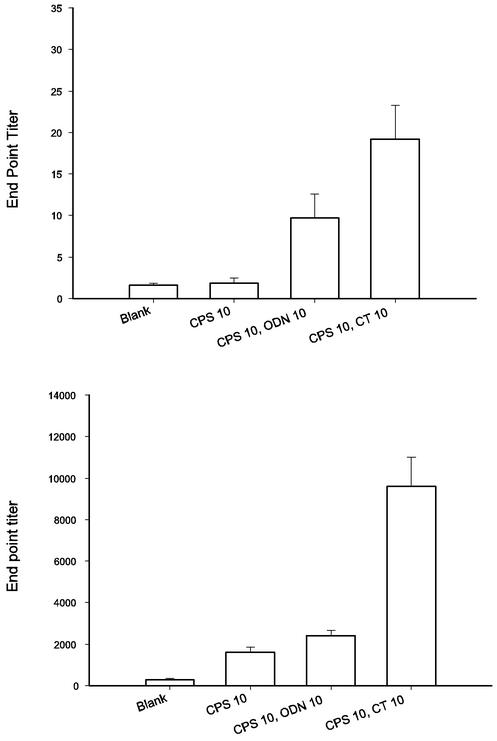
To evaluate whether CPS is a potent immunogen suitable for vaccination purposes, mice were immunized intranasally with CPS of V. parahaemolyticus alone or in combination with adjuvants. To monitor the induction of mucosal and systemic immune responses, an ELISA was used to detect CPS-specific IgA in feces and IgG in plasma. The top panel of Fig. 9 shows IgA titers in animal feces after CPS vaccination. The administration of CPS together with either ODN or CT as an adjuvant resulted in much higher responses (P < 0.05). In the ODN group, fecal IgA could be detected in almost every mouse (8 out of 9), compared to 0 out of 10 in the negative control group and 4 out of 10 in the group receiving CPS alone. The average IgA titer in the ODN group was three times higher than that in the group given CPS alone. When CT was used as an adjuvant, 10 out of 10 mice had CPS-specific IgA in their feces, and the average titer was eight times higher than that obtained with CPS alone. The trend for CPS-specific IgG in plasma was similar to that for IgA in feces. Higher CPS-specific IgG titers were detected in the plasma of mice immunized with adjuvants and CPS (Fig. 9, bottom panel) (P < 0.05). These results demonstrated clearly that in the presence of adjuvants, such as ODN and CT, V. parahaemolyticus CPS can elicit both mucosal and systemic immune responses.
FIG. 9.
Analysis of antibody titers elicited by CPS inoculation. BALB/c mice were primed intranasally with CPS, CPS plus ODN, or CPS plus CT (10 μg each) as indicated. The mice were boosted intranasally twice every other week. Feces and plasma samples were collected 2 weeks after immunization and assayed for CPS-specific antibodies by an ELISA. As the titers of IgA in the animal feces after the first boost were quite low (mean value, 2.5), only the results obtained after the second boost are shown. (Top) Feces CPS-specific IgA. (Bottom) Plasma CPS-specific IgG. Values are reported as means and standard errors of the means (n = 10).
DISCUSSION
As the major bacterium causing food-borne diseases in Southeast Asia, V. parahaemolyticus has been studied for more than 50 years. The surface antigens CPS (K antigens) and LPS (O antigens) have been used to serotype V. parahaemolyticus. For a total of 3,729 pathogenic isolates that were recovered from 1995 to 1999 in Taiwan, 40 serotypes were identified. Among these, O3:K6 was the most prevalent. A total of 2,234 isolates of O3:K6 were identified in 351 of the 542 V. parahaemolyticus outbreaks, while O4:K8 and O4:K10 isolates caused 107 outbreaks over the same period (8). In this study, a V. parahaemolyticus strain from Taiwan, CCRC strain 13023 (serotype O4:K8) (39, 42), was chosen mainly because sera against CPS, LPS, and whole bacteria of this strain were conveniently available. Once a mechanism of action is identified, other strains will be analyzed to examine its generality.
Enteric pathogens usually adhere to, replicate in, and produce virulence factors on or in epithelial cells of the enteric tract before they cause the syndrome. To evaluate whether major surface components of V. parahaemolyticus are responsible for bacterial colonization of enteric epithelium, we have successfully designed a method that easily separates CPS and LPS of V. parahaemolyticus CCRC strain 13023 (Fig. 1 and 2). This novel and simple method has helped us to characterize and evaluate CPS in this O4:K8 strain. Since other bacteria, such as Staphylococcus aureus and Salmonella enterica serovar Typhi, also contain CPS and LPS (3, 9, 25, 38), the purification scheme (with or without minor modifications) may be useful for the isolation of CPS from not only V. parahaemolyticus but also such CPS-encapsulated bacteria.
Composition analysis of the fraction containing purified CPS showed that it contained 70% sugar moieties and 30% fatty acids, i.e., palmitic acid and stearic acid. Since, during the purification process, we used sodium deoxycholate, a detergent, to separate CPS from LPS, it is unlikely that the fatty acid components are due to the formation of mixed CPS-LPS micelles. However, our results do not conclusively show that CPS is a glycolipid. Using 32P to intrinsically label polysaccharide, Gotschlich and coworkers (17) showed that there was a lipid material covalently linked to the reducing end of CPS of meningococcal groups A, B, and C. A similar approach is needed to demonstrate that V. parahaemolyticus CPS is indeed a glycolipid.
The adherence of V. parahaemolyticus to epithelial cell lines has been evaluated in several studies (5, 7, 43). Culturing of bacteria with bile or deoxycholate has been found to increase the size of the extracellular capsule and to enhance bacterial adherence to epithelial cells (32). Similar results were observed in our experiments. In addition, we found that the capsule content of CPS but not LPS increased upon supplementation of the bacterial culture with bile or deoxycholate (Fig. 3 and 4). Furthermore, we discovered incidentally that after 1 week or more of culturing of V. parahaemolyticus in BHI culture broth, the colonies of bacteria on agar plates had been transformed from opaque to translucent. The ability of the translucent-colony bacteria to adhere to epithelial cells was much lower than that of the opaque-colony bacteria. A comparison of the capsule composition of the translucent-colony bacteria with that of the opaque-colony bacteria showed that they apparently contained small quantities of CPS (Fig. 5). Culturing of translucent colonies of V. parahaemolyticus in broth containing bile converted them to opaque colonies and increased their CPS content (Fig. 5B) as well as their ability to adhere to Int-407 cells. We also showed that both anti-O4:K8 whole-bacterium and anti-K8 CPS sera inhibited the adherence of the bacterium to intestinal cells in vitro (Fig. 6). This inhibitory effect of the sera could be blocked by pretreatment of the sera with purified CPS but not LPS (Fig. 7). Moreover, we found that purified CPS bound to Int-407 cells in a dose-dependent manner (Fig. 8). These results clearly demonstrate that CPS rather than LPS is associated with the ability of V. parahaemolyticus to adhere to epithelial cells. It must be noted, however, that in other bacteria, such as Haemophilus influenzae, unencapsulated bacteria actually adhere better because surface adhesins (13, 35) that promote intimate interactions with epithelial cells and an extracellular matrix are unmasked from CPS. Whether factors other than CPS are also responsible for the adherence of V. parahaemolyticus to epithelial cells remains to be elucidated.
The human liver secretes about 1 liter of bile daily, and approximately 50 to 75% of the hepatic bile secreted during fasting enters the gallbladder, which concentrates, stores, and delivers concentrated bile into the duodenum when food leaves the stomach (33, 40). The concentration of bile in the human gut thus may vary not only from time to time but also from one location to another. However, the in vitro effective concentrations of bile (0.3%) and deoxycholate (0.1%) are within the physiological range in the human gut (≥0.1% deoxycholate) (4, 33, 37, 40); therefore, bile also could have an effect on V. parahaemolyticus in vivo.
Polysaccharide vaccines based on CPSs of various bacteria linked to a carrier protein have been developed and tested in animals and humans (1-3, 12, 23, 24, 28). However, there is limited information on the ability of such CPS vaccines to induce antibodies on mucosal surfaces (10, 11, 15, 27, 34). In this study, the CPS of V. parahaemolyticus was administrated intranasally to mice either alone or with an adjuvant (ODN or CT) (20, 29) to evaluate the ability of CPS to induce mucosal and systemic immune response. Since V. parahaemolyticus commonly colonizes the intestinal mucosa, CPS-specific IgA levels in fecal samples from immunized animals were analyzed. We found that fecal IgA titers were threefold higher in sera from both the CPS-ODN and the CPS-CT experimental groups than in sera from the CPS-only group (Fig. 9). Higher CPS-specific IgG titers also were detected in sera from both the CPS-ODN and the CPS-CT experimental groups. These results indicate that with the help of an adjuvant, such as ODN or CT, CPS given intranasally can affect the levels of CPS-specific IgA in the intestine and IgG in plasma. Further studies are needed to determine whether the increased levels of CPS-specific IgA are sufficient to prevent enteric colonization by V. parahaemolyticus in vivo. Once the efficacy of CPS vaccination has been demonstrated, multivalent vaccines based on a cocktail of selected CPS serotypes could be developed for use against V. parahaemolyticus.
In summary, we have successfully isolated the CPS from V. parahaemolyticus and shown that it is positively correlated with the ability of the bacterium to adhere to intestinal cells. In addition, we have shown that the CPS of V. parahaemolyticus can induce mucosal and systemic immune responses. Our results suggest that the CPS of V. parahaemolyticus is a potential target for the development of vaccines against this pathogen.
Acknowledgments
We thank Kay-Hooi Khoo for instruction in GC-MS composition analysis and Jei-Ming Peng for skillful technical assistance.
We gratefully acknowledge the financial support of the Biotechnology Research Program of Academia Sinica.
Editor: J. D. Clements
REFERENCES
- 1.Baker, C. J., L. C. Paoletti, M. R. Wessels, H. K. Guttormsen, M. A. Rench, M. E. Hickman, and D. L. Kasper. 1999. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J. Infect. Dis. 179:142-150. [DOI] [PubMed]
- 2.Baxendale, H. E., Z. Davis, H. N. White, M. B. Spellerberg, F. K. Stevenson, and D. Goldblatt. 2000. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur. J. Immunol. 30:1214-1223. [DOI] [PubMed] [Google Scholar]
- 3.Burgeot, C., F. B. Gilbert, and B. Poutrel. 2001. Immunopotentiation of Staphylococcus aureus type 5 capsular polysaccharide co-entrapped in liposomes with alpha-toxin. Vaccine 19:2092-2099. [DOI] [PubMed] [Google Scholar]
- 4.Cabral, D. J., and D. M. Small. 1989. Physical chemistry of bile, p. 621-662. In S. G. Schultz, J. G. Forte, and B. R. Bauner (ed.), Handbook of physiology, sect. 6, vol. 3. American Physiology Society, Bethesda, Md.
- 5.Carruthers, M. M. 1977. In vitro adherence of Kanagawa positive Vibrio parahaemolyticus to epithelial cells. J. Infect. Dis. 136:588-592. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers, M. M., and B. Anderson. 1979. Inhibition by polyanions of adherence by Kanagawa-positive Vibrio parahaemolyticus: a physicochemical effect. J. Infect. Dis. 140:119-122. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti, M. K., A. K. Sinha, and T. Biswas. 1991. Adherence of Vibrio parahaemolyticus to rabbit intestinal epithelial cells in vitro. FEMS Microbiol. Lett. 84:113-118. [DOI] [PubMed] [Google Scholar]
- 8.Chiou, C. S., S. Y. Hsu, S. I. Chiu, T. K. Wang, and C. S. Cho. 2000. Vibrio parahaemolyticus serovar O3:K6 as cause of unusually high incidence of food-borne disease outbreaks in Taiwan from 1996 to 1999. J. Clin. Microbiol. 38:4621-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costerton, J. W., R. T. Irvin, and K. J. Cheng. 1981. The role of bacterial surface structures in pathogenesis. Crit. Rev. Microbiol. 3:303-338. [DOI] [PubMed] [Google Scholar]
- 10.Czerkinsky, C., and J. Holmgren. 1995. The mucosal immune system and prospects for anti-infectious and anti-inflammatory vaccines. Immunologist 3:97-103. [Google Scholar]
- 11.Czerkinsky, C., M. Quiding, K. Eriksson, I. Nordstrom, M. Lakew, C. Weneras, A. Kilander, S. Bjork, A. M. Svennerholm, E. Butcher, and J. Holmgren. 1995. Induction of specific immunity at mucosal surfaces: prospects for vaccine development. Adv. Exp. Med. Biol. 371B:1409-1416. [PubMed] [Google Scholar]
- 12.Fattom, A. I., J. Sarwar, L. Basham, S. Ennifar, and R. Naso. 1998. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66:4588-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink, D. L., B. A. Green, and J. W. St. Geme III. 2002. The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect. Immun. 70:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujino, T., Y. Okuno, D. Nakada, A. Aoyama, K. Fukai, T. Mukai, and T. Uebo. 1953. On the bacteriological examination of Shirasu food poisoning. Med. J. Osaka Univ. 4:299-304. [Google Scholar]
- 15.Gallichan, W. S., D. C. Johnson, F. L. Graham, and K. L. Rosenthal. 1993. Mucosal immunity and protection after intranasal immunization with recombinant adenovirus expressing herpes simplex virus glycoprotein. Br. J. Infect. Dis. 168:622-629. [DOI] [PubMed] [Google Scholar]
- 16.Gingras, S. P., and L. V. Howard. 1980. Adherence of Vibrio parahaemolyticus. Appl. Environ. Microbiol. 39:369-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotschlich, E. C., B. A. Fraser, O. Nishimura, J. B. Robbins, and T.-Y. Liu. 1981. Lipid on capsular polysaccharides of gram-negative bacteria. J. Biol. Chem. 256:8915-8921. [PubMed] [Google Scholar]
- 18.Gupta, R. K., and G. R. Siber. 1995. Adjuvants for human vaccines: current status, problems and future prospects. Vaccine 13:1263-1276. [DOI] [PubMed] [Google Scholar]
- 19.Honda, T., and T. Iida. 1993. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev. Med. Microbiol. 4:106-113. [Google Scholar]
- 20.Horner, A. A., and E. Raz. 2000. Immunostimulatory sequence oligodeoxynucleotide: a novel mucosal adjuvant. Clin. Immunol. 95:S19-S29. [DOI] [PubMed] [Google Scholar]
- 21.Hunolstein, C., S. Mariotti, R. Teloni, G. Alfarone, G. Romagnoli, G. Orefici, and R. Nisini. 2001. The adjuvant effect of synthetic oligodeoxynucleotide containing CpG motif converts the anti-Haemophilus influenzae type b glycoconjugates into efficient anti-polysaccharide and anti-carrier polyvalent vaccines. Vaccine 19:3058-3066. [DOI] [PubMed] [Google Scholar]
- 22.Iguchi, T., S. Kondo, and K. Hisatsune. 1995. Vibrio parahaemolyticus O serotypes from O1 to O13 all produce R-type lipopolysaccharide: SDS-PAGE and compositional sugar analysis. FEMS Microbiol. Lett. 130:287-292. [DOI] [PubMed] [Google Scholar]
- 23.Jann, K., and B. Jann. 1992. Capsules of Escherichia coli, expression and biological significance. Can. J. Microbiol. 38:705-710. [DOI] [PubMed] [Google Scholar]
- 24.Kasper, D. L., L. C. Paoletti, M. R. Wessels, H. K. Guttormsen, V. J. Carey, H. J. Jennings, and C. J. Baker. 1996. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugated vaccine. J. Clin. Investig. 98:2308-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasper, D. L., A. Weintraub, A. A. Lindberg, and J. Lonngren. 1983. Capsular polysaccharides and lipopolysaccharides from two Bacteroides fragilis reference strains: chemical and immunochemical characterization. J. Bacteriol. 153:991-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh, H. 1965. Studies on the growth rate of various food bacteria. 3. The growth of V. parahaemolyticus in raw fish meat. Nippon Saikingaku Zasshi 20:541-544. [DOI] [PubMed] [Google Scholar]
- 27.Kauppi, M., L. Saarinen, and H. Kayhty. 1993. Anti-capsular polysaccharide antibodies reduce nasopharyngeal colonization by Haemophilus influenzae type b in infant rats. J. Infect. Dis. 167:365-371. [DOI] [PubMed] [Google Scholar]
- 28.Kotloff, K. L., A. Fattom, L. Basham, A. Hawwari, S. Karkonen, and R. Edelman. 1996. Safety and immunogenicity of a tetravalent group B streptococcal polysaccharide vaccine in healthy adults. Vaccine 14:446-450. [DOI] [PubMed] [Google Scholar]
- 29.McCluskie, M. J., R. D. Weeratna, J. D. Clements, and H. L. Davis. 2001. Mucosal immunization of mice using CpG DNA and/or mutants of the heat-labile enterotoxin of Escherichia coli as adjuvants. Vaccine 19:3759-3768. [DOI] [PubMed] [Google Scholar]
- 30.Nakasone, N., and M. Iwanaga. 1990. Pili of a Vibrio parahaemolyticus strain as a possible colonization factor. Infect. Immun. 58:61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen, K. H., L. Kelly, D. Gall, P. Nicoletti, and W. Kelly. 1995. Improved competitive enzyme immunoassay for the diagnosis of bovine brucellosis. Vet. Immunol. Immunopathol. 46:285-291. [DOI] [PubMed] [Google Scholar]
- 32.Pace, J. L., T. J. Chai, H. A. Rossi, and X. Jiang. 1997. Effect of bile on Vibrio parahaemolyticus. Appl. Environ. Microbiol. 63:2372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaffer, E. A., P. McOrmond, and H. Duggan. 1980. Quantitative cholescintigraphy: assessment of gallbladder filling and emptying and duodenogastric reflux. Gastroenterology 79:899-906. [PubMed] [Google Scholar]
- 34.Shen, X., T. Lagergard, Y. Yang, M. Lindblad, M. Fredriksson, G. Wallerstrom, and J. Holmgren. 2001. Effect of pre-existing immunity for systemic and mucosal immune responses to intranasal immunization with group B Streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate. Vaccine 19:3360-3368. [DOI] [PubMed] [Google Scholar]
- 35.St. Geme, J. W., III, M. L. de la Morena, and S. Falkow. 1994. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol. Microbiol. 14:217-233. [DOI] [PubMed] [Google Scholar]
- 36.Takeda, Y. 1982. Thermostable direct hemolysin of Vibrio parahaemolyticus. Pharmacol. Ther. 19:123-146. [DOI] [PubMed] [Google Scholar]
- 37.van Faassen, A., M. J. Hazen, P. A. van den Brandt, A. E. van den Bogaaard, R. J. J. Hermus, and R. A. Janknegt. 1993. Bile acids and pH values in total feces and in fecal water from habitually omnivorous and vegetarian subjects. Am. J. Clin. Nutr. 58:917-922. [DOI] [PubMed] [Google Scholar]
- 38.Waldor, M. K., R. R. Colwell, and J. J. Mekalanos. 1994. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc. Natl. Acad. Sci. USA 91:11388-11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, T. K., S. I. Ho, J. L. Tsai, and T. M. Pan. 1996. K-serotype analyses of Vibrio parahaemolyticus isolated in northern Taiwan, 1983 through 1993. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 29:210-224. [PubMed] [Google Scholar]
- 40.Winkelstein, A., and P. W. Aschner. 1926. The mechanism of the flow of bile from the liver into the intestines. Am. J. Med. Sci. 171:104-111. [Google Scholar]
- 41.Wong, H. C., K. T. Lu, T. M. Pan, C. L. Lee, and D. Y. C. Shih. 1996. Subspecies typing of Vibrio parahaemolyticus by pulsed-field gel electro-phoresis. J. Clin. Microbiol. 34:1535-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong, H. C., S. H. Liu, L. W. Ku, I. Y. Lee, T. K. Wang, Y. S. Lee, C. L. Lee, L. P. Kuo, and D. Y. Shih. 2000. Characterization of Vibrio parahaemolyticus isolates obtained from food born illness outbreaks during 1992 through 1995 in Taiwan. J. Food Prot. 63:900-906. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto, T., and T. Yokota. 1989. Adherence targets of Vibrio parahaemolyticus in human small intestines. Infect. Immun. 57:2410-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto, T., K. Fujita, and T. Yokota. 1990. Piliated Vibrio parahaemolyticus adherent to human ureteral mucosa. J. Infect. Dis. 161:361-362. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto, T., M. J. Albert, and R. B. Sack. 1994. Adherence to human small intestines of capsulated Vibrio cholerae O139. FEMS Microbiol. Lett. 119:229-235. [DOI] [PubMed] [Google Scholar]