Abstract
We investigated the mechanisms underlying the effects of sustained and transient covert attention on contrast sensitivity. The aim of this study was twofold: (1) Using a zero-noise display, we assessed whether sustained (endogenous) attention enhances contrast sensitivity via signal enhancement, and compared the magnitude of the effect with that of transient (exogenous) attention. (2) We compared the contrast psychometric functions for both sustained and transient attention and evaluated them in terms of contrast gain and response gain models. Observers performed a 2AFC orientation discrimination task on a tilted target Gabor, presented alone at 1 of 8 iso-eccentric locations. Either a neutral (baseline), peripheral (to manipulate transient attention), or a central cue (to manipulate sustained attention) preceded the target. Even in the absence of external noise, and using suprathreshold stimuli, observers showed an attentional effect, evidence in support of signal enhancement underlying both sustained and transient attention. Moreover, sustained attention caused a strictly leftward threshold shift in the psychometric function, supporting a contrast gain model. Interestingly, with transient attention we observed a change in asymptote in addition to a threshold shift. These findings suggest that whereas sustained attention operates strictly via contrast gain, transient attention may be better described by a mixture of response gain and contrast gain
Keywords: Covert attention, Contrast sensitivity, Contrast gain, Response gain, Signal enhancement
1. Introduction
Covert attention allows us to monitor our periphery in the absence of eye movements (Posner, 1980). A growing body of behavioral evidence demonstrates that there are two components of covert attention: 'sustained' and 'transient' (Cheal & Lyon, 1991; Corbetta & Shulman, 2002; Nakayama & Mackeben, 1989). Sustained, or endogenous, attention corresponds to what we usually think of as attention: at will, we monitor information at a given location. Transient, or exogenous, attention corresponds to a faster, involuntary capture of attention to a location where sudden, salient stimulation has occurred. Previous studies have shown that we can engage these systems differentially by using different cues: a central or symbolic cue is presented in the center of the visual field to direct sustained, or endogenous attention in a conceptually driven fashion in ~300 ms, whereas a peripheral cue flashed briefly in a location adjacent to the relevant location captures transient, or exogenous attention in a stimulus-driven, automatic manner in ~100 ms (Nakayama & Mackeben, 1989). Whereas the shifts of attention by sustained cues appear to be under conscious control, it is hard or impossible for observers to ignore transient cues, even when they are known to be irrelevant (Carrasco, Ling, & Read, 2004; Giordano, McElree, & Carrasco, 2003; Muller & Rabbit, 1989; Pestilli & Carrasco, 2005).
There is no consensus as to whether common neurophysiological substrates underlie sustained and transient attention. Some have suggested that whereas sustained attention is cortical in nature, transient attention also activates subcortical processing (Robinson & Kertzman, 1995; Zackon, Casson, Zafar, Stelmach, & Racette, 1999). However, whereas some suggest that the preparatory control signals of sustained and transient attention are mediated by partially segregated networks (Corbetta & Shulman, 2002; Kanwisher & Wojciulik, 2000; Kastner & Ungerleider, 2000), others have found no difference in the brain networks mediating these systems (Peelen, Heslenfeld, & Theeuwes, 2004).
The goal of this study is to compare sustained and transient covert attention psychophysically. Specifically, we tested whether a signal enhancement mechanism underlies both types of attention. Moreover, we investigated the neural model underlying signal enhancement by measuring the psychometric functions for both sustained and transient attention, to assess whether they have similar or different effects on the contrast response function.
1.1. Mechanisms of attention: signal enhancement and external noise reduction
How does covert attention exert its effects? Psychophysically, the impact of covert attention on visual performance is well documented across a range of perceptual tasks, such as visual search (Carrasco & McElree, 2001; Carrasco & Yeshurun, 1998; Nakayama & Mackeben, 1989) and letter identification (Prinzmetal, Presti, & Posner, 1986; Talgar, Pelli, & Carrasco, 2004), and improves performance in visual domains such as contrast sensitivity (Carrasco, Penpeci-Talgar, & Eckstein, 2000; Cameron, Tai, & Carrasco, 2002; Lu & Dosher, 1998, 2000; Dosher & Lu, 2000a, 2000b; Huang & Dobkins, 2005; Smith, Wolfgang, & Sinclair, 2004; Solomon, 2004) and spatial resolution (Carrasco, Williams, & Yeshurun, 2002; Golla, Ignashchenkova, Haarmeier, & Their, 2004; Yeshurun & Carrasco, 1998, 1999). It has also been established that transient attention alters the appearance of contrast (Carrasco et al., 2004) and spatial frequency (Gobell & Carrasco, 2005).
Although it is well established that covert attention improves performance in early visual tasks, the underlying mechanisms responsible for these effects are not well understood. Explanations of how attention improves performance range from claims that the deployment of attention affects processing at the decisional level (Kinchla, Chen, & Evert, 1995; Palmer, 1994; Shiu & Pashler, 1994; Sperling & Dosher, 1986) to claims that attention actually enhances perceptual sensitivity. At the perceptual level, two prominent models have been proposed: signal and external noise reduction. According to signal enhancement, attention strengthens and improves the representation of the signal within the locus of attention enhancement (Cameron et al., 2002; Carrasco et al., 2000, 2002; Lu & Dosher, 1998, 2000; Luck, Hillyard, Mouloua, & Hawkins, 1996; Smith et al., 2004). According to external noise reduction, attention affects performance in a given area by actively suppressing the strength of representation of areas outside the locus of attention (Baldassi & Burr, 2000; Dosher & Lu, 2000a, 2000b; Lu & Dosher, 1998, 2000; Lu, Lesmes, & Dosher, 2002; Morgan, Ward, & Castet, 1998; Shiu & Pashler, 1994).
Psychophysically, transient attention has been shown to increase contrast sensitivity for detection and discrimination tasks, even under low-or zero-noise conditions —results which can only be explained by signal enhancement (Cameron et al., 2002; Carrasco et al., 2000). This finding has been corroborated using the external noise plus attention paradigm; transient attention operates via signal enhancement under low-noise conditions, and via noise reduction under high-noise conditions (Lu & Dosher, 1998, 2000). With regard to sustained attention, these authors have stated that it works primarily via an external noise reduction mechanism. Indeed, effects of sustained attention only arise in high-noise conditions, and not under low-noise conditions (Dosher & Lu, 2000a, 2000b; Lu, Liu, & Dosher, 2000; Lu et al., 2002).
The first goal of the present study was to systematically assess whether sustained and transient attention can enhance contrast sensitivity in the absence of added external noise (i.e., masks, distracters), and compare their effects. An attentional benefit with sustained attention in the absence of noise would be direct empirical evidence for signal enhancement.
1.2. Contrast response functions: contrast gain and response gain
What neural mechanism underlies signal enhancement? Neuronal firing rate increases as a function of stimulus contrast, resulting in a contrast response function. There are two predictions as to how attentional modulation may affect the contrast response function: contrast gain and response gain (Fig. 1; Sclar, Lennie, & DePriest, 1989). Contrast gain: if the neurons responding to the contrast of a stimulus combined with attentional modulation when processing the signal, the effect on the contrast response function could lead to an increase in sensitivity, with no change in relative firing rate. This would render the response no different from an actual change in the physical contrast of the stimulus. The signature of contrast gain is a leftward shift in threshold (C50; see equations in Fig. 1) of the contrast response function. Response gain: if attention and the contrast response were modulated independently, attention would have a multiplicative effect over the entire contrast response function, reflecting a multiplicative increase in firing of a neuron as a function of contrast, with no change in threshold. This would amplify the response as a function of stimulus intensity, thereby changing the actual shape of the function. The signature of response gain is a higher asymptote (Rmax; see equations in Fig. 1).
Fig. 1.
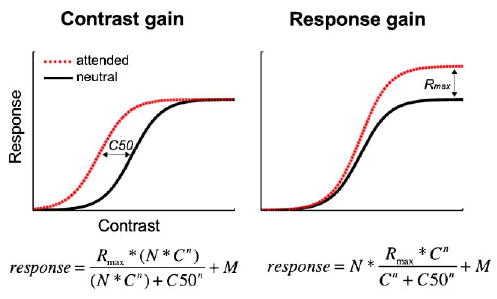
Possible effects of attention on the contrast response function. The left panel depicts a contrast gain model for attention. Contrast gain predicts an increase in sensitivity that is a function of stimulus intensity, and is characterized by a leftward threshold (C50) shift in the contrast response function. The dashed curve represents the signature curve shift brought about by attentional contrast gain; the shape of the function does not change, but rather shifts leftward—boosting the effective contrast of the stimulus. In the right panel, the dashed curve (attended) represents the effects of attention according to response gain models. Response gain predicts an increase in firing rate, which is characterized by a change in the shape of the curve—in slope and asymptote (Rmax). C50, threshold; Rmax, asymptote; n, slope; C, contrast level; N, attentional modulation; and M, response at lowest stimulus intensity.
An open question in the literature is how attentional changes are manifested at the neural level. Neurophysiologically, only sustained covert attention has been investigated, and most studies have found support for a contrast gain model (Di Russo, Spinelli, & Morrone, 2001; Martinez-Trujillo & Treue, 2002; McAdams & Maunsell, 1999; Reynolds, Pasternak, & Desimone, 2000 Reynolds, Pasternak, & Desimone, 2004), whereas others have reported findings consistent with a response gain model with feature-based attention (Treue & Martinez Trujillo, 1999).
Psychophysically, a number of studies have addressed contrast gain vs. response gain using transient attention. In an orientation discrimination task, a peripheral precue enhanced contrast sensitivity across the psychometric function, rendering response functions suggestive of a contrast gain model (Cameron et al., 2002). Likewise, in a task assessing the effect of transient attention on perceived contrast, Carrasco et al. (2004) measured appearance psychometric functions where transient attention shifted functions leftward, indicative of contrast gain. However, as the authors acknowledged in those studies, the high asymptote left little to no room to test for response gain. Neurophysiological studies of sustained attention that have evaluated these two models have highlighted the importance of avoiding levels at which neural saturation occurs (Reynolds et al., 2000). Similarly, to properly compare contrast gain and response gain psychophysically, the psychometric functions should arise from a demanding task that ensures that performance on the neutral baseline condition does not asymptote at 100%, thus leaving room to test for response gain. In this study, we obtained contrast psychometric functions for both sustained and transient attention, and assessed whether their effects are consistent with contrast and/or response gain.
In a sustained attention task, using a dual task paradigm in which observers performed tasks under conditions of full- or poor-attention, Morrone, Denti, and Spinelli (2004) found evidence for pure response gain. However, Huang and Dobkins (2005) subsequently found psychophysical evidence suggesting that dualtask, sustained attention may operate via a hybrid model, involving both contrast gain and response gain.
The second goal of the present study was to systematically compare the contrast psychometric functions of sustained and transient covert attention, further bridging the gap between neurophysiological and psychophysical findings. Using the same task, stimuli and observers, we measured psychometric functions under conditions of sustained and transient attention, and assessed whether their effects on the contrast psychometric function are consistent with contrast or response gain models, or with a combination of both.
2. Experiment
How similar are sustained and transient covert attention? The aim of this study was twofold: (1) To evaluate whether signal enhancement underlie both sustained and transient attention; (2) To evaluate how sustained and transient attention affect the contrast psychometric function.
3. Methods
3.1. Observers
Four observers participated in this study. Three were trained psychophysical observers, naïve as to the purpose of the experiment, and the fourth was an author (SL). All observers had normal or corrected-to-normal vision.
3.2. Apparatus
The stimuli were created using Matlab and the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997). Observers viewed the stimuli on a γ-corrected monitor. A video attenuator was used to drive just the green gun of a 21"IBM P260 monitor (1024 × 768; 120 Hz; Pelli & Zhang, 1991)—thus providing a larger set of distinct luminance levels (12 bits). Mean luminance was set at 14.1 cd/m2. Eye movements were monitored via an iScan Infrared camera.
3.3. Stimuli and design
A black fixation cross was presented at the center of the screen throughout the experiment (0.1°× 0.1°; Fig. 2). Observers performed a 2AFC orientation discrimination task on a target Gabor (sinusoidal grating enveloped in a Gaussian window; 2° × 2°), tilted ±4° to the left or right. The Gabor appeared at one of 8 iso-eccentric locations (4° eccentricity, center-to-center). To capture full psychometric functions, we used the method of constant stimuli; in each trial the contrast of the Gabor was randomly sampled from a set of contrasts ranging from 9 to 62% in 14 log increments.
Fig. 2.
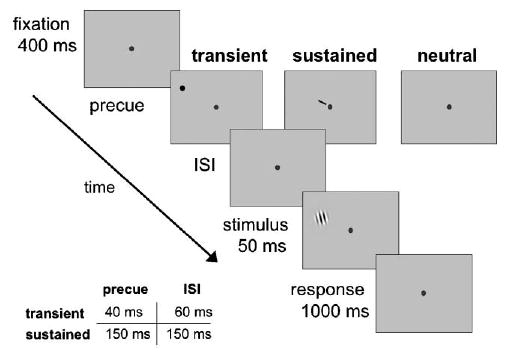
Sequence of events in a given trial. Observers performed a 2AFC orientation discrimination task on a tilted target Gabor patch, which appeared at one of eight iso-eccentric locations. The target was preceded by either a sustained cue (instructing observers to deploy their attention to the upcoming target location), a transient cue (reflexively capturing attention to the upcoming target location), or a neutral cue (baseline). The timings (precue and ISI) for sustained and transient conditions differed (along with their respective neutral conditions), to maximize the effectiveness of the cues. We used the method of constant stimuli to obtain psychometric functions, varying the contrast of the Gabor stimuli from trial-to-trial.
To manipulate attention, one of three types of cues preceded the target display: peripheral, central, or neutral. The transient peripheral cue was a black dot (0.3° × 0.3°), which appeared adjacent to the upcoming target Gabor location (1.5°, center-to-center from the Gabor), to elicit transient attention while avoiding masking. The central cue was a small line (0.1° × 0.8°) near fixation, pointing towards the upcoming target locations to direct sustained attention. The neutral cue was a dot (0.3° × 0.3°) appearing at fixation. Both the peripheral and central cue indicated target location, but did not contain information regarding the orientation of the stimulus. All three cues indicated the temporal stimulus onset.
3.4. Procedure
Each trial began with a screen containing only the fixation point (50 ms). This was followed by one of three types of cues. In the transient attention condition, fixation was followed by a peripheral cue, which briefly flashed adjacent to the upcoming target location (40 ms), followed by a blank ISI (60 ms), drawing attention reflexively to the target location. In the sustained attention condition, a central cue appeared near fixation pointing towards where the target was about to appear (150 ms), followed by a blank ISI (150 ms), allowing observers to voluntarily allocate attention to the target location. In the neutral, baseline condition, a dot flashed at the center of the screen, indicating when the target was about to appear, but not where it would appear. There were two timings for the neutral condition: One matched the timing for the transient cue condition (40 and 60 ms ISI), and one matched that for the sustained cue (150 and 150 ms ISI). After a brief ISI, a tilted Gabor appeared at one of eight locations (50 ms) and observers performed a 2AFC orientation discrimination task.
The cue timings were chosen to optimize the effects of transient and sustained attention (Cameron et al., 2002; Carrasco et al., 2000, 2002, 2004; Cheal & Lyon, 1991; Nakayama & Mackeben, 1989). Additionally, in the transient attention condition, the timing between cue onset and stimulus onset (100 ms) was brief enough to prevent observers from making any goal-directed saccades (Mayfrank, Kimmig, & Fischer, 1987). Given that the timing of the sustained attention condition (300 ms) could have allowed eye movements, observers' eye movements were monitored using an infrared camera. Breaks from fixation were very rare (<1%), and blocks in which breaks were observed were re-run.
Observers completed 25 sessions, which were comprised of 5 blocks per session, and 200 trials per block. Sessions were blocked by attentional manipulation, such that a particular session tested only transient or sustained attention and its respective neutral condition. Within each block the Gabor contrast, orientation, location and cue type (peripheral or central vs. neutral) were randomly selected from trial to trial. The order of the cue-condition blocks was randomized.
4. Results
4.1. Signal enhancement
To determine whether signal enhancement underlies sustained and transient attention, we measured whether an attentional effect arose when the display contained no added external noise. Data were fit (via maximum likelihood) to the Naka–Rushton contrast response model (Albrecht & Hamilton, 1982; Sclar, Maunsell, & Lennie, 1990)
| (1) |
where response represents performance, C is the contrast intensity level, C50 is the contrast at half the saturating response (threshold), n is the exponent which determines the steepness of the function (slope), Rmax is the level at which the response saturates (asymptote), and M is the response at the lowest contrast level. To fit the data to each condition (sustained, transient, and their respective neutral conditions), we allowed threshold (C50), slope (n) and asymptote (Rmax) to vary freely.
Fig. 3 depicts the psychometric functions (accuracy as a function of stimulus contrast) for each observer, under the sustained (Fig. 3A) and transient (Fig. 3B) conditions, along with their respective neutral conditions. Attention consistently improved performance, even though the display was completely void of any external noise—evidence in support of signal enhancement for both sustained and transient attention. A nested hypothesis test (separate fits for each condition vs. one fit for both conditions collapsed together; Mood, Graybill, & Boes, 1974) revealed significant effects of attention for all observers (p < .01) in both the sustained (BM: χ2(3, n = 5000) = 10.9, p < .01; FP: χ2 (3, n = 5000) = 25.5, p < .0001; JG: χ2 (3, n = 5000) = 13.2, p < .001; SL: χ2 (3, n = 5000) = 31.1, p < .0001), and transient conditions (BM: χ2 (3, n = 5000) = 18.8, p < .005; FP: χ2 (3, n = 5000) = 26.8, p < .0001; JG: χ2 (3, n = 5000) = 17.7, p < .0001; SL: χ2 (3, n = 5000) = 62.6, p < .0001).
Fig. 3.
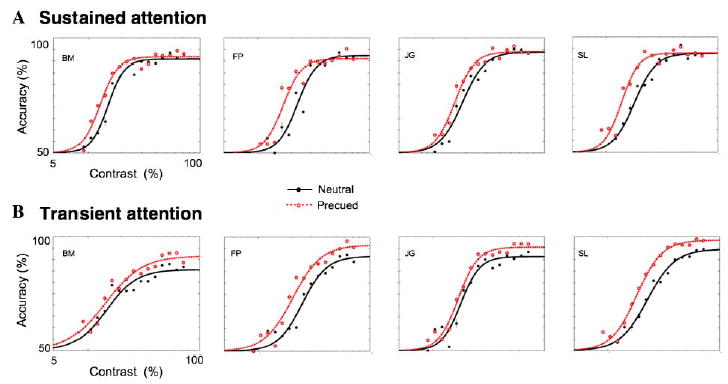
Psychometric functions for sustained and transient attention. The solid line represents the fits for the neutral condition, and the dashed line represents the fits for the precued. (A) Sustained attention consistently shifted the function to the left, having little impact on its shape, but increasing contrast sensitivity. (B) Transient attention consistently led to an elevation in asymptote, and the fits suggest a decrease in contrast threshold as well. Error bars correspond to mean ± 1 standard error.
4.2. Contrast gain vs. response gain models
We then assessed what model better predicted the data in each condition: contrast gain or response gain. What is particularly important is that although performance in the neutral condition asymptotes at a high contrast, it does not reach ceiling levels (100% accuracy). Rather, the task was rendered difficult enough such that observers’ performance asymptotes at ~90% accuracy, thus leaving room for response gain (if any) to manifest itself in the attended condition.
Sustained attention (Fig. 3A) led to a consistent decrease in threshold, and almost no change in asymptote. Transient attention (Fig. 3B) led to a consistent elevation in asymptote, along with a consistent decrease in threshold. Fig. 4 plots the C50 and Rmax parameter estimations taken from the aforementioned fits against each other under cued (sustained or transient) and neutral conditions. For every observer, in both the sustained and transient conditions, there was a reduction in contrast threshold (C50),—evidence in support of a contrast gain model (Fig. 4A). However, the magnitude of threshold shift was smaller for transient than sustained attention for all observers. The effects of attention on asymptote (Rmax; Fig. 4B) depended on the type of covert attention being deployed. For all four observers, transient attention led to a large increase in Rmax, consistent with a response gain model, whereas sustained attention had little-to-no effect on asymptote. Taken together, these results suggest a mixed model for transient attention.
Fig. 4.
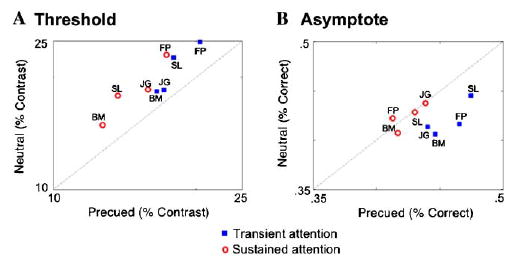
The effect of sustained and transient attention on threshold (C50) and asymptote (Rmax) for individual observers. The blue squares represent an observer’s parameter estimates in the transient condition vs. neutral, and the red circles represent estimates for sustained condition vs. neutral. Points falling on the dashed line represent unity, where there is no difference between precued and neutral conditions. (A) Threshold (C50) decreased for both sustained and transient covert attention. (B) Asymptote (Rmax) did not change with sustained attention, but increased for all observers with transient attention. (For interpretation of the references to colors in this figure legend, the reader is referred to the web version of this paper.)
To directly compare the two models, data from the sustained and transient conditions were fit to modified versions of the Naka–Rushton function where, for the precued conditions, an additional attentional parameter N was introduced (Martinez-Trujillo & Treue, 2002).
The response gain model tested was:
| (2) |
where the parameters were the same as in the original Naka–Rushton model Eq. (1), and the additional N parameter represents attentional modulation with a multiplicative effect on the overall response. The contrast gain model tested was
| (3) |
where the additional N parameter represents attention modulating the psychometric function through multiplying by contrast intensity level.
We fit data for the sustained and transient conditions to both models by first obtaining parameter estimates (C50, Rmax and n) under the original Naka–Rushton model Eq. (1) for the neutral condition. Next we fit the precued data to the response gain Eq. (2) and contrast gain Eq. (3) models by fixing those parameters obtained via Eq. (1), and only let the new ‘attention’ parameter N vary freely, obtaining a best estimate for attentional modulation under each model.
To test the mixed model, we then fit the data for sustained and transient attention to a mixed model, incorporating both contrast and response gain with attention:
| (4) |
where the additional N1 parameter represents the response gain component with attention (as in Eq. (1)), and the N2 parameter represents the contrast gain component with attention (as in Eq. (2)). For sustained attention, a likelihood ratio test revealed that for all observers the mixed model was not superior to the contrast gain model: (BM: χ2 (1, n = 5000) = 1.1, p > .1; FP: χ2 (1, n = 5000) = 2.0, p > .1; JG: χ2 (1, n = 5000) = 0.5, p > .1; SL: χ2 (1, n = 5000) = 0.5, p > .1). For transient attention, the mixed model was superior over the response gain model for three observers (BM: χ2 (1, n = 5000) = 6.11, p < .01; SL: χ2 (1, n = 5000) = 33.9, p > .0001; FP: χ2 (1, n = 5000) = 12.2, p < .0001), but the response gain model provided a marginally better fit for the fourth observer (JG: χ2 (1, n = 5000) = 2.3, p = 0.08).
Fig. 5 depicts the contrast gain and response gain model fits for sustained and transient attention. For the sustained attention data (Fig. 5A), a contrast gain model fit the data much better than a response gain model. Transient attention (Fig. 5B) was less consistent. The mixed model provided a better fit for the data in the transient condition for all observers but one (JG), for which the response gain model provided a marginally better fit.
Fig. 5.
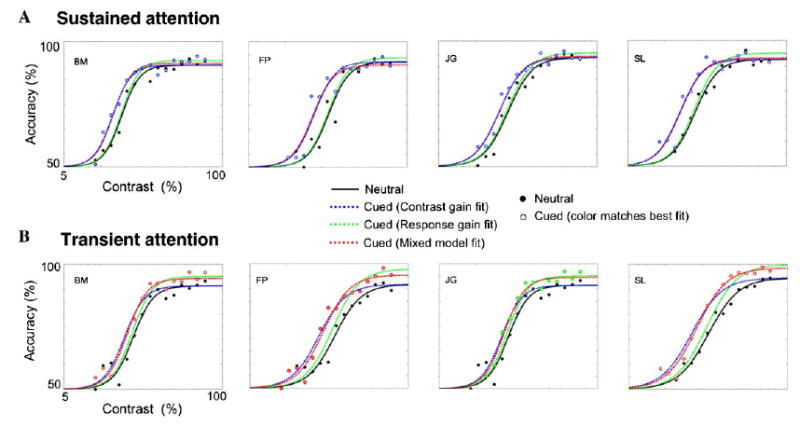
Contrast gain, response gain and mixed model fits to the data for sustained (A) and transient (B) covert attention. Filled circles correspond to the neutral data, and the hollow circles represent the precued data. The color of the hollow circles corresponds to the fit that best describes the attentional data. The solid black line is the fit to the neutral condition, the dashed green line corresponds to the attentional response gain model fit Eq. (2), the dashed blue line is the fit to the attentional contrast gain model Eq. (3), and the dashed red line corresponds to the mixed model Eq. (4).
5. Discussion
The goals of this study were: (1) to investigate whether sustained attention could operate via a signal enhancement mechanism, and (2) to characterize the contrast response functions for sustained and transient attention.
Our results indicate that both sustained and transient covert attention lead to a rise in contrast sensitivity for a target stimulus, even in the absence of any added external noise, such as distracters or masks. Given that the zero-noise display left nothing to be suppressed, our results can only be explained by a signal enhancement mechanism.
Moreover, we find differences in the psychometric functions for contrast sensitivity between sustained and transient attention. Sustained attention consistently led to a leftward shift in the psychometric function, which is characteristic of a contrast gain model of attention. Transient attention also led to a consistent, yet smaller, reduction in contrast threshold, consistent with contrast gain. However, with transient attention we also found consistently pronounced elevation of asymptote, which supports a response gain model. Taken together, these results suggest that whereas sustained attention operates via a strict contrast gain model, transient attention operates via a mix of both contrast and response gain.
5.1. Uncertainty
Signal enhancement and external noise reduction explanations propose that attention improves discriminability via changes to the actual perceptual signal. However, an alternative school of thought proposes that attention simply reflects a reduction of spatial uncertainty. The statistical uncertainty model asserts that each location we have to monitor adds decisional noise, thereby increasing the overall probability of erroneously confusing the signal with the noise (Pelli, 1985). Uncertainty reduction assumes that valid spatial cueing of an upcoming target location improves performance simply because it reduces the number of locations to be monitored from all possible target locations to just that one target location (Eckstein, 1998; Foley & Schwartz, 1998; Palmer, 1994; Solomon, Lavie, & Morgan, 1997).
As the detectability of a stimulus decreases, the more likely it is to be confused with the background, thereby increasing uncertainty. In the current study, some of the stimulus contrasts we presented were of a relatively low contrast. A strict uncertainty reduction model of attention would predict that the attentional effect should be most prominent with low contrast stimuli (where uncertainty is greatest, and performance would benefit most from uncertainty reduction) and decrease with increasing stimulus contrast (where uncertainty diminishes, and performance would not benefit from uncertainty reduction). However, this was not the case in our experiment.
Because the orientation discrimination task was fairly difficult, most of the stimulus contrasts had to be fairly high (9–62%) to capture the entire psychometric function. This rendered contrast thresholds much higher than detection threshold. Due to the high target-distracter discriminability, any performance benefit from uncertainty reduction would be insignificant—thus making the task sub-optimal for uncertainty reduction explanations. In addition, we did not observe a decrease in attentional benefit as stimulus contrast increased. In fact, in the case of transient attention there was often a larger attentional effect at the highest contrast values presented (Rmax elevation), which is a result that uncertainty reduction cannot account for. Thus, our current results cannot be explained via an uncertainty reduction model, but are more consistent with an actual change in the stimulus signal representation: a signal enhancement mechanism.
Other studies have supported the finding that attentional benefits go beyond that predicted by uncertainty reduction. Precues have been shown to improve performance better than that predicted by signal detection models of uncertainty (Morgan et al., 1998). Moreover, transient attention has been shown to increase contrast sensitivity across the psychometric function to the same extent for stimuli that differed in their spatial uncertainty (Cameron et al., 2002), or even when localization performance indicates observers have no target location uncertainty (Carrasco et al., 2000). Attentional benefits have been observed with spatial resolution using full contrast stimuli where uncertainty should have negligible effects (Carrasco et al., 2002). Taken together, these studies suggest that, while uncertainty reduction may play a role in performance benefits, it is not the sole source of attentional benefits.
5.2. Signal enhancement
Although it is very likely that signal enhancement and external noise reduction mechanisms co-exist (Cameron, Tai, Eckstein, & Carrasco, 2004; Carrasco et al., 2002; Lu & Dosher, 2000; Pestilli & Carrasco, 2005), in this study we tested the signal enhancement mechanism under low noise conditions. Regarding transient attention, the present findings are consistent with what our lab has previously shown: signal enhancement can underlie transient attention.
Transient attention enhances contrast sensitivity in zero-noise conditions across a wide range of spatial frequencies (Carrasco et al., 2000). Moreover, this signal enhancement occurs across the psychometric function (Cameron et al., 2002). Using a similar zero-noise display paradigm, in conjunction with a Landolt acuity task, it was found that transient attention enhances spatial resolution via signal enhancement (Carrasco et al., 2002). Similarly, a recent study that implemented the zero-noise paradigm to measure acuity for both humans and rhesus monkeys revealed that sustained attention improved acuity via signal enhancement as well (Golla et al., 2004).
Using the external noise paradigm, Lu and Dosher (1998, 2000) reported that transient covert attention seems to operate via both signal enhancement and external noise reduction. They showed that transient attention increases contrast sensitivity in conditions of low noise, indicative of signal enhancement, and also improves performance in high noise conditions, indicative of external noise reduction. However, they have attributed sustained attention effects only to an external noise reduction mechanism (Dosher & Lu, 2000a, 2000b; Lu et al., 2000, 2002).
With regard to transient attention, the current and previous findings are in agreement; under low external noise conditions, it operates via signal enhancement. However, the current results regarding sustained attention are inconsistent with those reported previously (Dosher & Lu, 2000a, 2000b; Lu et al., 2000, 2002). Why do Lu and Dosher find no evidence for signal enhancement with sustained attention? The most relevant difference in experimental parameters that could help reconcile this discrepancy lies in the amount of time observers were given to deploy their sustained attention. In the present study observers were given a 300 ms SOA to deploy their attention to the target location, whereas in their study the SOA was only 150 ms. Because of its voluntary nature, the optimal amount of time necessary to deploy sustained attention has been reported to be ~300 ms (Nakayama & Mackeben, 1989). The reasoning behind their shorter SOA was based on findings by Cheal and Lyon (1991), reporting that experienced observers were capable of deploying their attention in less time, in ~150 ms. Perhaps this short timing precluded the emergence of the signal enhancement mechanism. Indeed, consistent with this mechanism, in a minority of observers and conditions sustained attention increased contrast sensitivity even in low noise conditions (Dosher & Lu, 2000b; Lu et al., 2002). It is possible that the observers that failed to show any signal enhancement were not trained optimally to deploy sustained attention within the allotted time.
5.3. Contrast gain vs. response gain
Single-cell recordings from visual area MT have found that sustained attention shifts the contrast response function leftwards for neurons tuned to the target stimulus (C50), an effect equivalent to increasing the effective contrast of the actual stimulus (Martinez- Trujillo & Treue, 2002; Reynolds et al., 2000 Reynolds et al., 2004). The present results for sustained attention corroborate these neurophysiological studies; sustained attention operates via a contrast gain model. However, transient attention has not been tested with single unit recordings, possibly because it would be hard to tease apart the sensory component of the cue from the target. Our current psychophysical results suggest that transient attention manifests itself differently from sustained attention; transient attention appears to operate via a combination of both contrast gain and response gain.
Clearly, generalizations made from psychophysical data to neurophysiological findings should be made with caution. For instance, whereas psychometric functions presumably represent the output response from the entire visual system network, neurometric response functions are taken from measurements of only a modest subset of neurons responding to visual stimuli, confined to particular regions of the visual field. In addition, most neurophysiological studies of attention deal with sustained attention, whereas in this study we investigate both sustained and transient attention. Nevertheless, the link between psychometric and neurometric findings is tenable; for simple visual tasks such as motion discrimination, responses from single-unit recordings in MT are capable of accounting for behavioral psychometric functions (Britten, Shadlen, Newsome, & Movshon, 1992).
5.3.1. Transient attention—mixed model
Psychophysically, only a few other studies have investigated the issue of contrast vs. response gain. Research from our lab has suggested that transient attention shifts the psychometric function leftwards in an orientation discrimination task (Cameron et al., 2002), as well as when measuring perceived contrast (Carrasco et al., 2004). However, as the authors acknowledged, these findings were limited by the constraints imposed at the upper bounds of the psychometric function; by having the neutral condition’s psychometric function asymptote close to 100%, they left no room for a possible response gain mechanism to manifest itself. The present study overcame this hurdle by crippling performance in the neutral condition with a difficult discrimination task, thereby forcing asymptote to around 90% accuracy. Under these conditions, transient attention does not simply follow a contrast gain or response gain model, but rather a mixture of both (small shift in C50 and elevated Rmax).
Our results for transient attention may reflect the outcome of different stages of processing, in which the signal first undergoes a contrast gain modulation (as has been shown in visual areas V4 and MT), followed by response gain modulation at a later processing stage—an idea proposed by Huang and Dobkins (2005) with respect to sustained attention.
5.3.2. Sustained attention—contrast gain
With regard to sustained attention, even when asymptote was set to ~90% accuracy our results indicate that it operates strictly via contrast gain. There was a consistent shift in C50, and the contrast gain model was superior to the response gain model at accounting for the data for all four observers. In other words, voluntarily attending to a stimulus in one’s periphery changes the effective contrast of the stimulus.
Previous studies have found inconsistent results. In a study measuring threshold vs. contrast (TvC) functions, Morrone et al. (2004) showed that sustained attention in a dual task paradigm led to a change in the response function suggestive of a response gain model. Using a similar dual-task paradigm, a subsequent study by Huang and Dobkins (2005) tested whether attention operates via contrast or response gain. They found evidence for both contrast gain and response gain, and proposed a hybrid model in which attention first undergoes contrast gain, followed by a later-stage response gain modulation. Huang and Dobkins (2005) attributed the differing findings to experimental parameters; the contrasts they tested did a better job of capturing the entire response functions, and the dual task used by Morrone et al., was not demanding enough.
These results differ from our findings; for sustained attention we only found a consistent change in contrast gain. A major difference that may account for this discrepancy is how attention is manipulated. Both sets of studies used a concurrent task paradigm in which observers either performed a demanding rapid serial visual presentation (RSVP) task at fixation along with a peripheral task, drawing attentional resources away from the peripheral task, or they viewed the RSVP passively, allowing more attention to be allocated to the peripheral task. While the dual task paradigm has advantages, such as eliminating uncertainty reduction as an alternative explanation, it has disadvantages that may have hampered their conclusion. Dual task paradigms do not control the deployment of attention very well and make it difficult to isolate the source of possible processing differences (Pashler, 1998; Sperling & Dosher, 1986). In dual task paradigms, attention is not directed to a specific spatial location, but rather the amount of resources spread to all locations is manipulated. Considering that our task directed focused attention to only one target location, and theirs required that observers spread their resources equally to both stimuli (pedestal and test patch), the nature of their manipulation is quite different from ours.
Another difference arising from their use of a dual task is that the ‘full attention’ condition, where observers did not perform the RSVP task, is more analogous to the neutral condition in our study. Our neutral condition did not direct observers’ attention to any specific target location, much like their ‘full attention’ condition. To manipulate attention, in their ‘poor attention’ condition they drew resources away from the stimuli with an RSVP task, while we directed attention towards a specific target stimulus with a spatial cue. Therefore, it is possible that the discrepancy between our results and theirs is due to a fundamental difference in how attention is being deployed and the task demands; whereas our task is more consistent with the idea of directed covert spatial attention towards a given location, theirs is a manipulation of the drawing of resources away from stimuli.
6. Conclusion
The present study systematically compared sustained and transient covert attention using the same task, stimuli and observers. We demonstrated that both types of attention can operate via a signal enhancement mechanism under zero-noise conditions. This pattern of responses was consistent for all observers tested. Because this effect occurred even with very high-contrast stimuli, it cannot be explained by uncertainty reduction.
Although they both enhance the signal, sustained and transient attention have different effects on the contrast response function. When spatial covert attention is directed to the target location, sustained attention enhances sensitivity strictly via contrast gain, whereas transient attention seems to involve a mixture of both contrast gain and response gain.
Acknowledgments
The authors thank the observers who participated in this study. In addition, we thank members of the Carrasco lab and the anonymous reviewers for their valuable comments and suggestions. This work was funded by NRSA to S.L. and NSF to M.C.
References
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: Contrast response function. Journal of Neurophysiology. 1982;48(1):217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Baldassi S, Burr DC. Feature-based integration of orientation signals in visual search. Vision Research. 2000;40:1293–1300. doi: 10.1016/s0042-6989(00)00029-8. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Britten K, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: A comparison of neuronal and psychophysical performance. Journal of Neuroscience. 1992;12:4745–4767. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EL, Tai JC, Carrasco M. Covert attention affects the psychometric function of contrast sensitivity. Vision Research. 2002;42:949–967. doi: 10.1016/s0042-6989(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Cameron EL, Tai JC, Eckstein MP, Carrasco M. Signal detection theory applied to three visual search tasks: Identification, yes/no detection and localization. Spatial Vision. 2004;17:295–325. doi: 10.1163/1568568041920212. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, McElree B. Covert attention accelerates the rate of visual information processing. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5363–5367. doi: 10.1073/pnas.081074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Eckstein MP. Spatial covert attention increases contrast sensitivity across the CSF: Support for signal enhancement. Vision Research. 2000;40:1203–1215. doi: 10.1016/s0042-6989(00)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Williams PE, Yeshurun Y. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision. 2002;2:467–479. doi: 10.1167/2.6.4. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Yeshurun Y. The contribution of covert attention to the set-size and eccentricity effects in visual search. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:673–692. doi: 10.1037//0096-1523.24.2.673. [DOI] [PubMed] [Google Scholar]
- Cheal M, Lyon D. Central and peripheral precuing of forced-choice discrimination. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 1991;43A:859–880. doi: 10.1080/14640749108400960. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Spinelli D, Morrone MC. Automatic gain control contrast mechanisms are modulated by attention in humans: Evidence from visual evoked potentials. Vision Research. 2001;41:2435–2447. doi: 10.1016/s0042-6989(01)00134-1. [DOI] [PubMed] [Google Scholar]
- Dosher B, Lu ZL. Mechanisms of perceptual attention in precuing of location. Vision Research. 2000a;40:1269–1292. doi: 10.1016/s0042-6989(00)00019-5. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Noise exclusion in spatial attention. Psychological Science. 2000b;11:139–146. doi: 10.1111/1467-9280.00229. [DOI] [PubMed] [Google Scholar]
- Eckstein MP. The lower efficiency for conjunctions is due to noise and not serial attentional processing. Psychological Science. 1998;9:111–118. [Google Scholar]
- Foley JM, Schwartz W. Spatial attention: Effect of position uncertainty and number of distracter patterns on the threshold—vs.-contrast function for contrast discrimination. Journal of the Optical Society of America. 1998;15:1036–1047. [Google Scholar]
- Giordano, A. M., McElree, B., & Carrasco, M. (2003). On the automaticity of transient attention. Psychonomics Society
- Gobell J, Carrasco M. Attention alters the appearance of spatial frequency and gap size. Psychological Science. 2005;16(8):644–651. doi: 10.1111/j.1467-9280.2005.01588.x. [DOI] [PubMed] [Google Scholar]
- Golla H, Ignashchenkova A, Haarmeier T, Their P. Improvement of visual acuity by spatial cueing:Acomparative study in human and non-human primates. Vision Research. 2004;44(13):1589–1600. doi: 10.1016/j.visres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Huang, L., & Dobkins, K.R. (2005). Attentional effects on contrast discrimination in humans: Evidence for both contrast gain and response gain, Vision Research (in press). [DOI] [PubMed]
- Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nature Reviews Neuroscience. 2000:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kinchla RA, Chen Z, Evert D. Precue effects in visual search: Data or resource limited. Perception & Psychophysics. 1995;57:441–450. doi: 10.3758/bf03213070. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. External noise distinguishes attention mechanisms. Vision Research. 1998;38:1183–1198. doi: 10.1016/s0042-6989(97)00273-3. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. Spatial attention: Different mechanisms for central and peripheral temporal precues? Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1534–1548. doi: 10.1037//0096-1523.26.5.1534. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Liu CQ, Dosher BA. Attention mechanisms for multi-location first and second-order motion perception. Vision Research. 2000;40:173–186. doi: 10.1016/s0042-6989(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Lesmes LA, Dosher BA. Spatial attention excludes external noise at the target location. Journal of Vision. 2002;2(4):312–323. doi: 10.1167/2.4.4. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Hawkins HL. Mechanisms of visual–spatial attention: Resource allocation or uncertainty reduction. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:725–737. doi: 10.1037//0096-1523.22.3.725. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo J, Treue S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron. 2002;35:365–370. doi: 10.1016/s0896-6273(02)00778-x. [DOI] [PubMed] [Google Scholar]
- Mayfrank, L., Kimmig, H., & Fischer, B. (1987). In J. K. O’Regan & A. Levy-Schoen (Eds.), Eye movements: From physiology to cognition (pp. 37–45). New York: North-Holland.
- McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. The Journal of Neuroscience. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mood, A. M., Graybill, F. A., & Boes, D. C. (1974). Introduction to the theory of statistics 3rd edition (pp. 440–442) Boston: McGraw Hill.
- Morgan MJ, Ward RM, Castet E. Visual search for a tilted target: Tests of spatial uncertainty models. The Quarterly Journal of Experimental Psychology. 1998;51A:347–370. doi: 10.1080/713755766. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Denti V, Spinelli D. Different attentional resources modulate the gain mechanisms for color and luminance contrast. Vision Research. 2004;44(12):1389–1401. doi: 10.1016/j.visres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Muller HJ, Rabbit PM. Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Research. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Palmer J. Set-size effects in visual search: The effect of attention is independent of the stimulus for simple tasks. Vision Research. 1994;34:1703–1721. doi: 10.1016/0042-6989(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Pashler, H. (1998). The psychology of attention Cambridge MA: MIT Press.
- Peelen MV, Heslenfeld DJ, Theeuwes J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage. 2004;22:822–830. doi: 10.1016/j.neuroimage.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Pelli DG. Uncertainty explains many aspects of visual contrast detection and discrimination. Journal of the Optical Society of America A. 1985;2:1508–1532. doi: 10.1364/josaa.2.001508. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Pelli DG, Zhang L. Accurate control of contrast on microcomputer displays. Vision Research. 1991;31:1337–1360. doi: 10.1016/0042-6989(91)90055-a. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M. Contrast sensitivity is enhanced at cued and impaired at uncued locations. Vision Research. 2005;45:1867–1875. doi: 10.1016/j.visres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Posner M. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W, Presti DE, Posner MI. Does attention affect visual feature integration? Journal of Experimental Psychology: Human Perception and Performance. 1986;12:361–369. doi: 10.1037//0096-1523.12.3.361. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Kertzman C. Covert orienting of attention in macaques. III. Contributions of the superior colliculus. Journal of Neurophysiology. 1995;74:713–721. doi: 10.1152/jn.1995.74.2.713. [DOI] [PubMed] [Google Scholar]
- Sclar G, Lennie P, DePriest DD. Contrast adaptation in striate cortex of macaque. Vision Research. 1989;29:747–755. doi: 10.1016/0042-6989(89)90087-4. [DOI] [PubMed] [Google Scholar]
- Sclar G, Maunsell JHR, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Research. 1990;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- Shiu LP, Pashler H. Neglible effect of spatial precuing on identification of single digits. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:1037–1054. [Google Scholar]
- Smith P, Wolfgang B, Sinclair A. Mask-dependent attentional cuing effects in visual signal detection: The psychometric function for contrast. Perception & Psychophysics. 2004;66(6):1056–1075. doi: 10.3758/bf03194995. [DOI] [PubMed] [Google Scholar]
- Solomon JA, Lavie N, Morgan MJ. The contrast discrimination function: spatial cuing effects. Journal of the Optical Society of America A. 1997;14:2443–2448. doi: 10.1364/josaa.14.002443. [DOI] [PubMed] [Google Scholar]
- Solomon J. The effects of spatial cues on visual sensitivity. Vision Research. 2004;44(12):1209–1216. doi: 10.1016/j.visres.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Sperling, G., & Dosher, B. A. (1986). Strategy and optimization in human information processing. In K. R. Bo., L. Kaufman, & J. P. Thomas (Eds.). Handbook of perception and human performance (Vol. 1, pp. 1–65). New York: Wiley.
- Talgar CP, Pelli DG, Carrasco M. Covert attention enhances letter identification without affecting channel tuning. Journal of Vision. 2004;4:23–32. doi: 10.1167/4.1.3. [DOI] [PubMed] [Google Scholar]
- Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;39:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature. 1998;396:72–75. doi: 10.1038/23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. Spatial attention improves performance in spatial resolution tasks. Vision Research. 1999;39:293–306. doi: 10.1016/s0042-6989(98)00114-x. [DOI] [PubMed] [Google Scholar]
- Zackon DH, Casson EJ, Zafar A, Stelmach L, Racette L. The temporal order judgment paradigm: subcortical attentional contribution under exogenous and endogenous cueing conditions. Neuropsychologia. 1999;37:511–520. doi: 10.1016/s0028-3932(98)00134-1. [DOI] [PubMed] [Google Scholar]


