Abstract
Proteus mirabilis, a common cause of nosocomial and catheter-associated urinary tract infection, colonizes the bladder and ascends the ureters to the proximal tubules of the kidneys, leading to the development of acute pyelonephritis. P. mirabilis is capable of swarming, a form of multicellular behavior in which bacteria differentiate from the short rod typical of members of the family Enterobacteriaceae, termed the swimmer cell, into hyperflagellated elongated bacteria capable of rapid and coordinated population migration across surfaces, called the swarmer cell. There has been considerable debate as to which morphotype predominates during urinary tract infection. P. mirabilis(pBAC001), which expresses green fluorescent protein in both swimming and swarming morphotypes, was constructed to quantify the prevalence of each morphotype in ascending urinary tract infection. Transurethral inoculation of P. mirabilis(pBAC001) resulted in ascending urinary tract infection and kidney pathology in mice examined at both 2 and 4 days postinoculation. Using confocal microscopy, we were able to investigate the morphotypes of the bacteria in the urinary tract. Of 5,087 bacteria measured in bladders, ureters, and kidneys, only 7 (0.14%) were identified as swarmers. MR/P fimbria expression, which correlates with the swimmer phenotype, is prevalent on bacteria in the ureters and bladder. We conclude that, by far, the predominant morphotype present in the urinary tract during ascending infection is the short rod-the swimmer cell.
Proteus mirabilis, a common uropathogen, causes urinary tract infections (UTIs) in individuals with structural abnormalities of the urinary tract and is frequently isolated from the urine of elderly patients undergoing long-term catheterization (38, 40). Complications from infection with this organism are frequently serious and include bladder and kidney stone formation, encrustation and obstruction of the urinary catheter, acute pyelonephritis, and bacteremia (9, 33, 35, 38, 39). Indeed, this bacterium has a propensity for colonization of the kidney; in bladder washout studies, P. mirabilis was found in the kidneys more often than Escherichia coli (15).
Several virulence factors have been identified and characterized for P. mirabilis. These factors include a potent urease that catalyzes formation of ammonia from urea and leads to urinary stone formation (22), a pore-forming hemolysin (16), ZapA metalloprotease which cleaves both immunoglobulin G (IgG) and IgA (37), a capsular polysaccharide (19), four distinct fimbrial types (6, 7, 24-29), and peritrichous flagella for swimming (8, 11, 18) and swarming motility (1, 2, 4, 5, 10, 12, 13, 31, 41).
In liquid medium, P. mirabilis exists as a short (1.5 to 2 μm) motile rod with peritrichous flagella typical of members of the family Enterobacteriaceae. When transferred from liquid to an agar surface, the cells undergo striking changes in morphology and physiology, which result in a differentiated morphotype, called a swarmer cell, that is capable of multicellular rapid movement. Soon after encountering the solid surface of the agar, bacteria elongate dramatically due to an inhibition of septation, reaching 10 to 40 μm with minimal increase in cell width. During elongation, DNA replication is not affected, while the synthesis rates of certain proteins, most notably, urease, hemolysin, ZapA, and flagellin, are markedly increased (3, 5, 32, 37, 43).
The importance of swarming motility of P. mirabilis in UTIs has been debated yet remains unclear. Alison and colleagues (5) showed that hemolysin, urease, and metalloprotease are induced in swarmer cells to levels 30- to 80-fold higher than the levels in the swimmer cells. They also showed that swarming-specific induction of the virulence factors is controlled by coordinated differential gene expression (2). In vitro studies by Alison and colleagues showed that the differentiation from swimmer to swarmer cells is coupled to the ability of P. mirabilis to invade uroepithelial cells (1).
However, other reports suggest that perhaps swarming is not critical for infection. A nonflagellated isolate of P. mirabilis, unable to swarm, was recovered from a human with UTI and with UTI symptoms (44). When this isolate and a flagellum-negative mutant generated in the laboratory were separately compared with a flagellated isolate in an ascending mouse model of UTI, colonization rates were similar for all three strains (23). Therefore, there is no clear consensus in the literature as to whether P. mirabilis swarming is important or whether it even exists in ascending UTIs.
In this study, we used confocal microscopy, P. mirabilis expressing green fluorescent protein (GFP), and the mouse model of ascending UTI to quantify the morphotypes present during infection. Using these techniques, we demonstrate that the swarmer cell is only rarely observed during ascending UTIs.
Bacterial strains and media.
P. mirabilis HI4320 (produces urease, hemolysin, and MR/P, Pmf, and ATF fimbriae) was isolated from an elderly woman with urinary catheter-associated bacteriuria (22). E. coli DH5α [supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 gyrA96 thi-1 relA1] was used as a recipient for transformations and as a storage strain for plasmids. Luria broth (per liter, 10 g of peptone, 5 g of yeast extract, 10 g of NaCl) and Luria agar (Luria broth containing 1.5% [wt/vol] agar) were used as culture media. Nonswarming agar (per liter, 10 g of peptone, 5 g of yeast extract, 0.5 g of NaCl, 5 ml of glycerol, 20 g of agar) (12) was used to prevent P. mirabilis from swarming. When necessary, plates and media were supplemented with ampicillin (100 μg/ml).
P. mirabilis HI4320(pBAC001) fluoresces brightly in both swimmer and swarmer morphotypes.
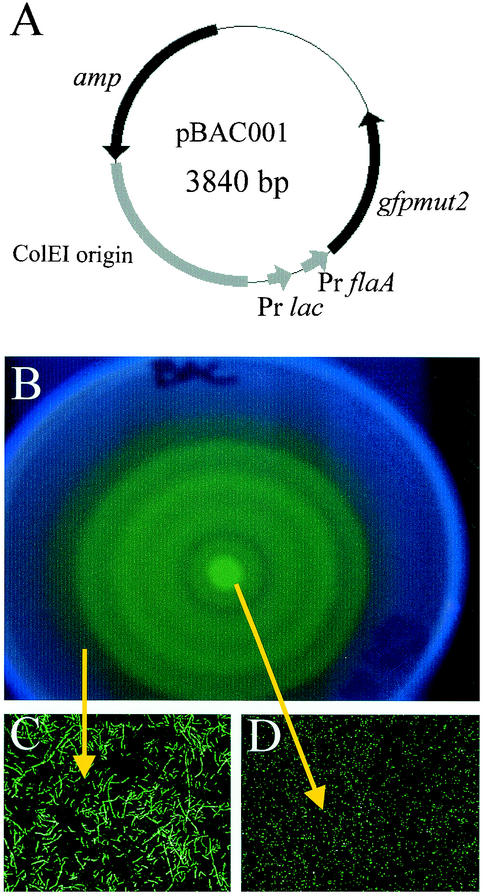
To accurately assess the relative presence of both swimmer and swarmer morphotypes in the urinary tract, it was necessary to construct a plasmid that expressed GFP in both morphotypes. The gene gfpmut2 was generously provided by Brendan Cormack (Johns Hopkins School of Medicine). PCR was used to amplify gfpmut2 sequence from plasmid pKEN-Gfpmut2 (14) using VENT DNA polymerase (New England Biolabs, Beverly, Mass.). PCR was performed in an MJ Research Minicycler thermal cycler as previously described (43). Plasmid pBAC001 was constructed so that both the lac promoter and the flaA promoter drive expression of gfpmut2 (Fig. 1A). The flaA promoter is contained within a 148-bp region upstream of the start codon for the flagellin gene (flaA) of pflaDAB, which was generously provided by Robert Belas (University of Maryland Center of Marine Biotechnology) (11). P. mirabilis was transformed with this plasmid using standard electroporation protocols (36). GFP is produced constitutively in P. mirabilis HI4320 transformed with this plasmid (Fig. 1B).
FIG. 1.
pBAC001 and transformed GFP-expressing swarmer and swimmer cells. (A) Schematic of pBAC001. The148 bases upstream of the start codon for the flagellin gene (flaA) were amplified by PCR, and this fragment was ligated 19 bases upstream of the gene coding for GFP and 57 bases downstream of the lac promoter (Pr lac) in pXL7301. (B) Fluorescent P. mirabilis bull's-eye colony. Five microliters of a culture of P. mirabilis HI4320(pBAC001) grown overnight was spotted onto the center of a swarming plate and incubated for 8 h at 37°C. P. mirabilis(pBAC001) swarmed normally and is fluorescent. (C and D) Swarmer (C) and swimmer (D) morphotypes expressed GFP. Bacteria from several regions of the bull's-eye colony were sampled and viewed by confocal microscopy.
Short-form swimmer cells isolated from the center of the bull's-eye (Fig. 1C) and the elongated swarmer cells taken from the leading edge of the outermost terrace (Fig. 1D) fluoresced bright green when bacteria were visualized by laser-scanning confocal microscopy. Thus, both swimmer and elongated swarmer cells can be easily detected by fluorescence microscopy.
P. mirabilis HI4320(pBAC001) causes ascending UTI in an experimental mouse model.
To visualize fluorescent bacteria in urinary tract tissue, CBA mice were inoculated transurethrally with 109 CFU of P. mirabilis HI4320(pBAC001) bacteria expressing GFP in both swimmer and swarmer morphotypes. Six 8-week-old female CBA mice (Jackson Laboratory, Bar Harbor, Maine) were inoculated transurethrally via a Harvard pump in a modified version (21) of the Hagberg method (20). Two or four days postinoculation, an overdose of methoxyfluorane was given to the mice, and the bladders, ureters, and kidneys of the mice were aseptically removed. Bladders and kidneys were weighed and then sliced in half longitudinally. To determine the number of viable bacteria per gram of tissue, half of each bladder and kidney was homogenized by mortar and pestle and then plated onto Luria agar containing ampicillin (100 μg/ml). To visualize fluorescent bacteria, frozen 10- to 30-μm-thick tissue sections from infected mice with bacterial counts of >105/g of tissue were observed by confocal microscopy. Organs were fixed in 4% (vol/vol) paraformaldehyde containing 20% (wt/vol) sucrose at 4°C for 24 h. Tissues were embedded in Tissue-Tek optimal cutting temperature compound (Miles, Elkhart, Ind.), frozen in a mixture of dry ice and ethanol, and held at −80°C until used. The 10-, 15-, and 30-μm-thick sections were cut with a cryostat and stored at −20°C until stained. Tissues were permeabilized by 30-min incubation in 0.1% Triton X-100. Surface carbohydrates on the host epithelium were stained with biotinylated Lycopersicon esculentum (tomato) lectin (Vector Laboratories, Eugene, Oreg.) for 20 min at room temperature; the tissue was rinsed with copious amounts of phosphate-buffered saline (PBS) and incubated three times for 6 min each time with PBS. Streptavidin conjugated to Alexa-Fluor 568 (Molecular Probes, Eugene, Oreg.) was then applied to the tissue and incubated for 20 min at room temperature. After three 6-min washes in PBS, the sections were mounted with Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI) (Vectashield Laboratories Inc., Burlington, Calif.).
Actin staining was performed by incubating sections with a monoclonal antibody against actin, AC-40, conjugated to 5,5′-disulfo-1,1′-(-α-carbopentynyl)-3,3,3′,3′-tetramethylindolocarbocyanin-N-hydroxysuccinimidester (Cy3) Sigma Chemical Company, St. Louis, Mo.) at 37°C for 1 h. After three 6-min washes in PBS, the sections were mounted with Vectashield mounting medium with DAPI (Vectashield Laboratories Inc.).
Four sections from each organ from eight mice (four mice at day 2 postinoculation and four mice at day 4 postinoculation) were viewed. Slides were examined with a Zeiss LSM410 confocal laser-scanning microscope with a 63× lens objective and a numerical aperture of 1.4. GFP and Alexa-Fluor 568 signals and Cy3 were excited with the 488- and 568-nm-wavelength lines of a 50-mW KrAr laser and detected through 515- to 540-nm-wavelength band-pass and 590-nm-wavelength long-pass filters, respectively. The 100-mW argon laser (Coherent Enterprise) with laser lines with wavelengths of 351 and 364 nm excited the DAPI-stained nuclei and Alexa-Fluor 350-conjugated MR/P fimbriae. Signals were detected through a 460-nm-wavelength long-pass filter.
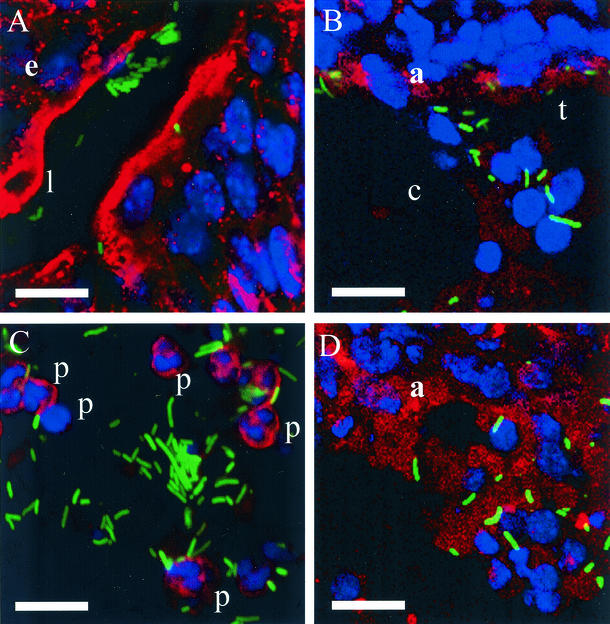
Numerous fluorescent bacteria were observed in the bladder, ureter, and kidney samples after both 2 and 4 days. Bacteria (green) colonized along the lumen of the bladder and within the mucous coat covering the bladder umbrella cells (Fig. 2A). Bladder mucosa is stained red with biotinylated lectin-streptavidin-conjugated Alexa-Fluor 568 (Fig. 2A). Kidney cells are stained red with a Cy3-conjugated antiactin antibody (Fig. 2B and D), and nuclei are blue (DAPI). Polymorphonuclear cells are stained red with the biotinylated lectin-streptavidin-conjugated Alexa-Fluor 568. Three areas of the kidney are shown: proximal tubules and capillaries (Fig. 2B), medulla (Fig. 2D), and pus in the renal medulla (Fig. 2C). Again, the bacteria are green, while the kidney tubular epithelial cells, endothelial cells (Fig. 2B), and polymorphonuclear cells (Fig. 2C) are red, and the host nuclei are blue (Fig. 2). In these examples, bacterial length varied from 1.5 to 9.5 μm.
FIG. 2.
GFP-expressing bacteria colonize the urinary tract. Confocal micrographs showing typical fields (seen with a 63× lens objective) of bladder (A) and kidney parenchyma (B and D) sections and pus in kidney (C). Host cells and actin are red, and host cell nuclei are blue. GFP-expressing bacteria are green. e, epithelium; l, lumen; c, capillary; t, tubule; p, polymorphonuclear cell; a, actin. Bars, 10 μm.
Swimmer cells were the predominant morphotype; swarmer cells were rarely observed in the urinary tract.
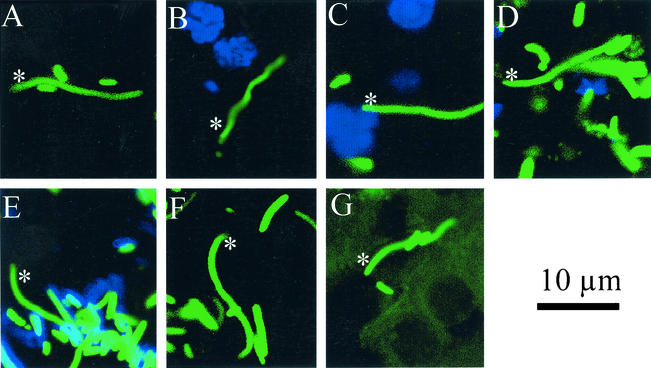
Of 5,087 bacteria measured in the bladders, ureters, and kidneys of eight mice, only 7 (0.14%) bacteria met our criteria of the swarmer cell morphotype. Bacteria were categorized as swimmer or swarmer cells according to their length. We defined a swarming cell based on bacterial length in accordance with the observations of Matsuyama and colleagues in published reports (30). In their paper focusing on the specific factors of the cyclic swarming behavior of P. mirabilis, they measured the length of the cells from seven distinct areas of a bull's-eye colony. At the outermost terrace, >80% of the 100 bacteria measured were >10 μm in length. We chose to use this criterion of >10 μm in length as our definition of a swarming cell. The overall prevalence of swarmers on both days and in all organs examined was 1.38/1,000. There was no significant statistical variability between days or sites. Bladder, ureter, and kidney sections from eight mice (four mice from day 2 postinoculation and four mice from day 4 postinoculation) were examined for bacterial morphotype. All seven of these rare morphotypes are depicted in Fig. 3. The swarmer cells varied in length between 12 and 19 μm. All swarmers but two (Fig. 3B and C) were in close contact with surrounding swimmer cells. There were no groups of swarmer cells. Swarmers were found both in tissue and in pus. However, the vast majority of fields studied using confocal microscopy exclusively showed all swimmer morphotypes (Fig. 2). To ensure that we were not missing elongated swarmer cells that had cured themselves of the plasmid and thus were not fluorescent, we examined several sections of tissue stained by hematoxylin and eosin (H&E). After measuring ≈600 bacteria, we determined that the percentage of swarmer cells observed in the H&E-stained tissues was consistent with the negligible numbers found by confocal microscopy (data not shown) (17).
FIG. 3.
Rare swarmer cells. Of 5,087 bacteria measured, 7 were swarmer cells. Swarmer cells are indicated in the micrographs by a white asterisk. These elongated bacteria were found in bladder (A) and kidney (B to G) tissue. The swarmer cells are all >12 μm long, and all but one (B) are in close contact with surrounding swimmer cells and have a gentle curve. The swarmer cell in panel E is not expressing MR/P fimbriae, as are most of the surrounding swimmer cells (blue). The host nuclei are stained blue with DAPI in panels B to D.
Because a swarmer cell synthesizes hundreds to thousands of flagella, it is easy to hypothesize that the swarmer morphotype is required for virulence in the urinary tract, where the bacterial population must colonize and multiply while combating the host's immune system, including the flushing action of urine. Other published data, however, bring into question the relevance of the swarmer morphotype. First, an aflagellate P. mirabilis isolate was obtained from a human with a UTI and UTI symptoms and used to assess whether flagella and hence swarming are necessary for infection (44). This aflagellate isolate was able to infect mice, albeit in reduced numbers, when inoculated intravenously and transurethrally. Second, follow-up studies demonstrated the ability of an isogenic nonflagellated P. mirabilis mutant to infect mice in numbers similar to those of the wild-type strain (23). Studies conducted in our laboratory found that a nonswarming, nonmotile flagellum mutant (34) was also able to infect the urinary tract but in numbers 100 times lower than those of wild-type P. mirabilis. Therefore, we sought to quantitatively determine the proportion of swarmer cells in vivo by tracking P. mirabilis expressing GFP during an ascending UTI.
Allison and colleagues arrived at very different conclusions (2) in their 1994 article examining P. mirabilis infection. Using H&E staining of tissue from mice infected with P. mirabilis, they showed that long differentiated cells (40 μm long) were the major cell type in renal tissue, while the inflammatory exudates primarily contained short vegetative cells. There are several possible explanations for the dissimilar results of our experiments. They used suckling mice injected intravenously with a highly virulent P. mirabilis strain obtained from a patient with a chronic UTI and renal stone. We infected adult female mice transurethrally with HI4320, a strain obtained from a woman with catheter-associated bacteriuria. Allison and colleagues allowed the infection to continue for 15 days, much longer than the 2- and 4-day time points we examined. They observed several elongated bacteria in the kidney parenchyma; these bacteria might have infected the kidney via the bloodstream (2). Perhaps the swarmer morphotype is significant for kidney colonization and persistence in intravenous infections of P. mirabilis. Therefore, these data are not necessarily mutually exclusive; they may just examine different aspects of P. mirabilis infection. In our experimental model of ascending UTI, the swarmer morphotype was rarely observed.
Bacteria in ureters were all swimmer cells.
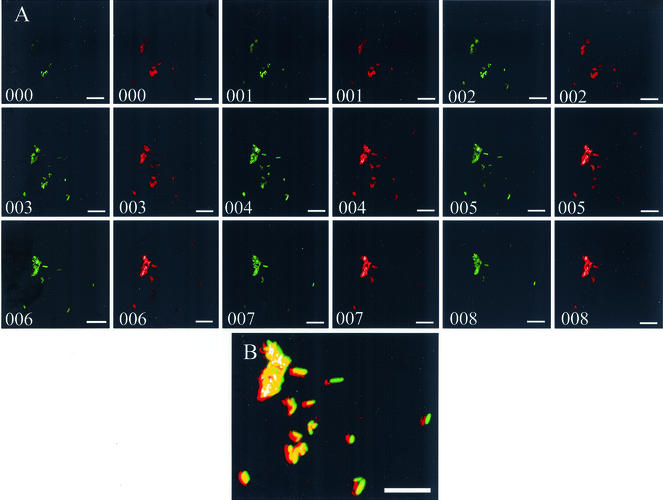
No bacterium viewed in the ureters, whether at 2 or 4 days postinoculation, was longer than 10 μm. A representative micrograph of ureter tissue shows that the majority of bacteria in the ureters expressed MR/P fimbriae (Fig. 4B). Tissue was stained with purified anti-MrpA. This antibody was created by subcutaneously injecting New Zealand White rabbits with purified MBP-MrpA fusion protein (100 μg) emulsified in Freund's complete adjuvant as previously described (24).
FIG. 4.
Confocal micrographs of MR/P fimbria-expressing swimmer cells in ureters. (A) Nine Z sections were taken, one every 0.5 μm through the ureter section. All the bacteria (green) are expressing MR/P fimbriae (red). (B) MR/P fimbriae are seen as red colocalizing with the green bacteria to yield yellow in this image in which the above 18 images are stacked together.
Bladder, ureter, and kidney tissues were permeabilized as described above, incubated for 60 min at 37°C with the antibody, and washed in PBS as described above. The tissue samples were treated with a secondary anti-rabbit antibody conjugated to Alexa-Fluor 350 (Molecular Probes) and washed three times in PBS as described above. Sections were mounted with fluorescent mounting medium (Vectashield). Negative controls for MR/P-binding specificity used the Alexa-Fluor 350-conjugated anti-rabbit antibody without the initial step of applying the MR/P antibody.
Z sections taken at 0.5-μm intervals of this cluster of bacteria show that all these bacteria express MR/P fimbriae (red) (Fig. 4A, right frame of each numbered pair of frames). Images were captured in the same focal plane with the laser exciting the Alexa-Fluor 350 MR/P fluorescence offset slightly left of the laser exciting GFP to show that the images are distinct. When the sections are merged into one image (Fig. 4B), one sees the colocalization (yellow) of the green bacteria with the red fluorescent MR/P fimbriae.
The swarmer cell does not express MR/P fimbriae.
One phenotypic characteristic of swimmer cells is the expression of MR/P fimbriae. MR/P fimbria expression is inversely regulated with swarming (27). The final gene of the MR/P fimbrial operon, mrpJ, is a repressor of motility in P. mirabilis. MrpJ represses transcription of the flagellar regulon and thus reduces flagellum synthesis when MR/P fimbriae are present. Therefore, it is logical to reason that the expression of MR/P fimbriae, as demonstrated by the anti-MrpA staining, indicates the hyperfimbriated swimmer cell, not the hyperflagellated swarmer cell. The section shown in Fig. 3E was treated with antibody to MrpA, the major pilin subunit of MR/P fimbriae. Note that the majority of the surrounding swimmer cells produce MR/P fimbriae (blue), while the swarmer cell does not (Fig. 3E).
A well-studied strain of P. mirabilis from a human case of catheter-associated bacteriuria was rendered fluorescent by transformation with a plasmid that expressed GFP in both swimmer and swarmer morphotypes. In an established murine model of ascending UTI, we identified by confocal microscopy that only 7 of 5,087 bacteria possessed the elongated swarmer morphotype. We conclude (despite the tempting hypothesis that the hypermotile swarmer cells are the cells that ascend the ureters to the kidneys) that the predominant morphotype in ascending UTIs is the short, motile, fimbriated swimmer cell.
Acknowledgments
This work was supported in part by Public Health Service grant DK47920 from the National Institutes of Health.
We thank Xin Li for helpful discussions and technical advice. We also thank Richard Hebel for statistical analysis, Gregg Davis for critical review of the manuscript, and Cinthia Drachenberg for assistance with evaluation of the H&E-stained sections.
Editor: A. D. O'Brien
REFERENCES
- 1.Allison, C., N. Coleman, P. L. Jones, and C. Hughes. 1992. Ability of Proteus mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect. Immun. 60:4740-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, C., L. Emody, N. Coleman, and C. Hughes. 1994. The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J. Infect. Dis. 169:1155-1158. [DOI] [PubMed] [Google Scholar]
- 3.Allison, C., and C. Hughes. 1991. Closely linked genetic loci required for swarm cell differentiation and multicellular migration by Proteus mirabilis. Mol. Microbiol. 5:1975-1982. [DOI] [PubMed] [Google Scholar]
- 4.Allison, C., H.-C. Lai, D. Gygi, and C. Hughes. 1993. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol. Microbiol. 8:53-60. [DOI] [PubMed] [Google Scholar]
- 5.Allison, C., H.-C. Lai, and C. Hughes. 1992. Coordinate expression of virulence genes during swarming cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583-1591. [DOI] [PubMed] [Google Scholar]
- 6.Bahrani, F. K., S. Cook, R. A. Hull, G. Massad, and H. L. Mobley. 1993. Proteus mirabilis fimbriae: N-terminal amino acid sequence of a major fimbrial subunit and nucleotide sequences of the genes from two strains. Infect. Immun. 61:884-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahrani, F. K., G. Massad, C. V. Lockatell, D. E. Johnson, R. G. Russell, J. W. Warren, and H. L. Mobley. 1994. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 62:3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belas, R. 1994. Expression of multiple flagellin-encoding genes of Proteus mirabilis. J. Bacteriol. 176:7169-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belas, R. 1996. P. mirabilis swarming and UTI, p. 271-298. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, D.C.
- 10.Belas, R., D. Erskine, and D. Flaherty. 1991. Proteus mirabilis mutants defective in swarmer cell differentiation and multicellular behavior. J. Bacteriol. 173:6279-6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belas, R., and D. Flaherty. 1994. Sequence and genetic analysis of multiple flagellin-encoding genes from Proteus mirabilis. Gene 128:33-41. [DOI] [PubMed] [Google Scholar]
- 12.Belas, R., M. Goldman, and K. Ashliman. 1995. Genetic analysis of Proteus mirabilis mutants defective in swarmer cell elongation. J. Bacteriol. 177:823-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claret, L., and C. Hughes. 2000. Rapid turnover of FlhD and FlhC, the flagellar regulon transcriptional activator proteins, during Proteus swarming. J. Bacteriol. 182:833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 15.Fairley, K. F., N. E. Carson, R. C. Gutch, P. Leighton, A. D. Grounds, E. C. Laird, P. H. McCallum, R. L. Sleeman, and C. M. O'Keefe. 1971. Site of infection in acute urinary-tract infection in general practice. Lancet ii:615-618. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, G. M., L. Claret, R. Furness, S. Gupta, and C. Hughes. 2002. Swarming-coupled expression of the Proteus mirabilis hpmBA haemolysin operon. Microbiology 148:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frome, E. L. 1983. The analysis of rates using Poisson regression models. Biometrics 39:665-674. [PubMed] [Google Scholar]
- 18.Furness, R. B., G. M. Fraser, N. A. Hay, and C. Hughes. 1997. Negative feedback for a Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J. Bacteriol. 179:5585-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gygi, D., M. M. Rahman, H.-C. Lai, R. Carlson, J. Guard-Pettier, and C. Hughes. 1995. A cell surface polysaccharide that facilitated rapid population migration by differentiated swarm cells of Proteus mirabilis. Mol. Microbiol. 17:1167-1175. [DOI] [PubMed] [Google Scholar]
- 20.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. Svanborg-Eden. 1983. Ascending unobstructed urinary tract infection in mice caused by pyelonephritic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, D. E., R. G. Russell, C. V. Lockatell, J. C. Zulty, J. W. Warren, and H. L. Mobley. 1993. Contributions of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 61:2748-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, B. D., and H. L. Mobley. 1988. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J. Bacteriol. 170:3342-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legnani-Fajardo, C., P. Zunino, C. Piccini, A. Allen, and D. Maskell. 1996. Defined mutants of Proteus mirabilis lacking flagella cause ascending urinary tract infection in mice. Microb. Pathog. 21:395-405. [DOI] [PubMed] [Google Scholar]
- 24.Li, X., D. E. Johnson, and H. L. Mobley. 1999. Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis. Infect. Immun. 67:2822-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, X., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2002. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol. Microbiol. 45:865-874. [DOI] [PubMed] [Google Scholar]
- 26.Li, X., and H. L. Mobley. 1998. MrpB functions as the terminator for assembly of Proteus mirabilis mannose-resistant Proteus-like fimbriae. Infect. Immun. 66:1759-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, X., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2001. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 20:4854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massad, G., J. Fulkerson, Jr., D. Watson, and H. Mobley. 1996. Proteus mirabilis ambient-temperature fimbriae: cloning and nucleotide sequence of the aft gene cluster. Infect. Immun. 64:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massad, G., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 1994. Proteus mirabilis fimbriae: construction of an isogenic pmfA mutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect. Immun. 62:536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuyama, T., Y. Takagi, Y. Nakagawa, H. Itoh, J. Wakita, and M. Matsushita. 2000. Dynamic aspects of the structured cell population in a swarming colony of Proteus mirabilis. J. Bacteriol. 182:385-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mobley, H. L., and R. Belas. 1995. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends Microbiol. 3:1-5. [DOI] [PubMed] [Google Scholar]
- 32.Mobley, H. L., and R. Hausinger. 1989. Microbial ureases: significance, regulation, and molecular characterization. Microbiol. Rev. 53:85-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mobley, H. L., and J. Warren. 1987. Urease-positive bacteriuria and obstruction of long term urinary catheters. J. Clin. Microbiol. 25:2216-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mobley, H. L. 1996. Virulence of Proteus mirabilis, p. 245-270. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, D.C.
- 35.Rubin, R., N. Tolkoff-Rubin, and R. Cotran. 1986. Urinary tract infection, pyelonephritis, and reflux neuropathy, p. 1085-1141. In B. Brenner and F. Rector (ed.), The kidney. W. B. Saunders, Philadelphia, Pa.
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Walker, K. E., S. Moghaddame-Jafari, C. V. Lockatell, D. Johnson, and R. Belas. 1999. ZapA, the IgA-degrading metalloprotease of Proteus mirabilis, is a virulence factor expressed specifically in swarmer cells. Mol. Microbiol. 32:825-836. [DOI] [PubMed] [Google Scholar]
- 38.Warren, J. W. 1991. The catheter and urinary tract infection. Med. Clin. N. Am. 75:481-493. [DOI] [PubMed] [Google Scholar]
- 39.Warren, J. W., D. Damron, J. H. Tenney, J. M. Hoopes, B. Deforge, and H. L. Muncie, Jr. 1987. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J. Infect. Dis. 155:1151-1158. [DOI] [PubMed] [Google Scholar]
- 40.Warren, J. W., H. L. Muncie, Jr., and M. Hall-Craggs. 1988. Acute pyelonephritis associated with bacteriuria during long-term catheterization: a prospective clinicopathological study. J. Infect. Dis. 158:1341-1346. [DOI] [PubMed] [Google Scholar]
- 41.Williams, F., and R. H. Schwarzhoff. 1978. Nature of the swarming phenomenon in Proteus. Annu. Rev. Microbiol. 32:101-122. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, H., X. Li, D. E. Johnson, I. Blomfield, and H. L. Mobley. 1997. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol. Microbiol. 23:1009-1019. [DOI] [PubMed] [Google Scholar]
- 43.Zhao, H., R. Thompson, V. Lockatell, D. Johnson, and H. Mobley. 1998. Use of green fluorescent protein to assess urease gene expression by uropathogenic Proteus mirabilis during experimental ascending urinary tract infection. Infect. Immun. 66:330-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zunino, P., C. Piccini, and C. Legnani-Fajardo. 1994. Flagellate and nonflagellate Proteus mirabilis in the development of experimental urinary tract infection. Microb. Pathog. 16:379-385. [DOI] [PubMed] [Google Scholar]