Abstract
Background
Otitis media is one of the most common infections of early childhood. Surfactant protein A functions as part of the innate immune response, which plays an important role in preventing infections early in life. This prospective study utilized a candidate gene approach to evaluate the association between polymorphisms in loci encoding SP-A and risk of otitis media during the first year of life among a cohort of infants at risk for developing asthma.
Methods
Between September 1996 and December 1998, women were invited to participate if they had at least one other child with physician-diagnosed asthma. Each mother was given a standardized questionnaire within 4 months of her infant's birth. Infant respiratory symptoms were collected during quarterly telephone interviews at 6, 9 and 12 months of age. Genotyping was done on 355 infants for whom whole blood and complete otitis media data were available.
Results
Polymorphisms at codons 19, 62, and 133 in SP-A1, and 223 in SP-A2 were associated with race/ethnicity. In logistic regression models incorporating estimates of uncertainty in haplotype assignment, the 6A4/1A5haplotype was protective for otitis media among white infants in our study population (OR 0.23; 95% CI 0.07,0.73).
Conclusion
These results indicate that polymorphisms within SP-A loci may be associated with otitis media in white infants. Larger confirmatory studies in all ethnic groups are warranted.
Background
Otitis media is one of the most common infections of early childhood: 60%-80% of children will have at least one episode during their first year of life [1,2]. Rates of early-onset (before 6 months of age) and recurrent otitis media are increasing in the United States [3,4]. Recurrent otitis media may increase a child's risk for hearing loss and delays in speech development [1,5].
Acute otitis media is an infectious disease resulting from complex interactions between microbes, the environment, and the host immune response. The majority of episodes are thought to be bacterial, but acute otitis media is most often associated with, or preceded by, a viral respiratory infection [6,7]. Epidemiologic studies have consistently highlighted the importance of daycare attendance and the number of siblings as predictors of acute otitis media [6,7]. Genetic factors play an important role in otitis media susceptibility. Monozygotic twins have higher concordance in otitis media rates than dizygotic twins, and estimates of heritability range from 0.45–0.74 [8-10]. While studies have provided strong evidence of a genetic predisposition to otitis media, only a few studies have identified specific genes related to otitis media risk [11,12].
The innate immune response may be critical for otitis media susceptibility in early life before the acquisition of specific immunity. Surfactant protein A (SP-A) is the most abundant surfactant protein in the lung, and is an important component of the innate immune system [13]. SP-A is a member of the collectin protein family; these proteins recognize carbohydrates on the surface of pathogens via the carbohydrate recognition domain [14]. As a pattern recognition receptor, SP-A functions in the first line of defense in the absence of specific antimicrobial antibodies. SP-A knockout mice show delayed clearance of otitis media associated pathogens Haemophilus influenzae and respiratory syncitial virus (RSV) [15]. The gene locus for SP-A is in chromosome 10q21 through q24 and has two functional and highly homologous SP-A genes (SP-A1 and SP-A2) [16-18].
SP-A has been detected in the Eustachian tube [19] and may play a role in the pathogenesis of otitis media [20]. Recurrent otitis media has previously been linked to the 6A4/1A5 haplotype in SP-A [11]. This study took place in Finland, and the distribution of SP-A haplotypes in the Finnish population has been shown to differ from frequencies found within the United States [21]. Among a prospective cohort of infants at risk for developing asthma, we used a candidate gene approach to determine whether polymorphisms within the SP-A1 and SP-A2 genes were associated with any otitis media during the first year of life.
Methods
Cohort
Data used in this report were obtained from infants enrolled in a longitudinal cohort to study the impact of environmental exposures on asthma development and morbidity. Between September 1996 and December 1998, women delivering babies in six hospitals were invited to participate if they had at least one other child at home younger than 12 years of age with physician-diagnosed asthma. Because of the asthmatic sibling, these infants were considered at risk for this disease. A full description of the methods is provided elsewhere [22-24]. Our Human Investigations Committee as well as institutional review boards at each participating hospital reviewed and approved the study.
Of the initial 1,002 infants enrolled, data were available on otitis media during the first year of life for 889. Between the third and fifth year of participation, a second visit was made to the home at which time an effort was made to collect a blood sample from the cohort subjects. The analyses in this report include a convenience sample of 355 infants for whom whole blood and complete quarterly otitis media data were available.
Data collection
Trained research assistants described the study to the infant's mother, obtained written informed consent, and administered standardized questionnaires to study participants during home interviews within 4 months of the infant's birth. We collected household demographic data such as maternal race and ethnicity, education, and number of children. We also collected detailed information regarding infant care such as use of daycare, any breastfeeding during the first year of life, and whether the infant was exposed to environmental tobacco smoke (ETS) in the home. Mothers were asked to report on their history of allergies or physician-diagnosed asthma. Infant respiratory symptoms were collected during quarterly telephone interviews at 6, 9 and 12 months of age. During these telephone interviews, we asked the mother to report her infant's respiratory symptoms and doctor or clinic visits (month and year of visit, reason for visit, and mother's report of the diagnosis). When the infant reached one year of age, each mother completed an extensive phone questionnaire covering her infant's health status during the previous year.
Definitions of otitis media
Otitis media was ascertained during each quarterly survey by mother's report of an episode of clinician-diagnosed otitis media with the month and year of diagnosis. Additional physician diagnoses of otitis media occurring in the same month or next consecutive month were counted as a single episode.
Genotyping of SP-A genes
DNA from whole blood samples was extracted using the QIAamp DNA blood minikit (Qiagen) according to the manufacturer's instructions. Genotyping for the single nucleotide polymorphisms (SNPs) at aa19, aa50, aa62, aa133, and aa219 in SP-A1 and aa9, aa91, aa223 in SP-A2 were done using a sequence specific primer-PCR methodology described by Pantelidis et al.[25]. This method involves high stringency PCR amplification using allele-specific primers designed with the final 3' end nucleotide complementary to the nucleotide variants within a particular SNP locus. In our hands, this method did not give reproducible results for the CT polymorphism at aa140 within SP-A2. To detect polymorphisms in SP-A2 at aa140, we used PCR based cRFLP as described by DiAngelo et al. [26].
Data analysis
Unadjusted associations between otitis media episodes and selected study characteristics, SNPs, and individual SP-A1 or -A2 alleles were initially evaluated by χ2 tests. Observed SNP frequencies were tested for Hardy-Weinberg equilibrium by χ2 analysis. Allele haplotypes for SP-A1 and for SP-A2 were examined for linkage disequilibrium and haplotype frequencies were estimated using PROC HAPLOTYPE in the SAS/Genetics module of SAS version 9.1 (Cary, NC). Logistic regression was used to model the association between SP-A1 and SP-A2 haplotypes and otitis media. Note that specific haplotypes can only be inferred since each individual has two chromosomes, and the methods of genotyping provide only the two bases at each site without a chromosomal assignment. For example, homozygous SNPs result in one and only one haplotype assignment. In the presence of heterozygous SNPs, the allele haplotype could be one of several, each occurring with a probability based on observed SNP frequencies. Therefore, we made an effort to estimate the effect of uncertainty in haplotype assignment on model variability. We used the set of potential haplotypes (PROC HAPLOTYPE) and a regression calibration technique similar to that described by Carroll et al. [27] to create 100 separate data sets by randomly assigning a haplotype for a particular subject based on the probability of each of the possible haplotypes given for that subject. Next, 100 separate logistic regression models with coefficient and variance estimates for the ith model of bi and , respectively, were fit for each haplotype of interest. The estimate of the association () between otitis media and each particular haplotype was calculated as the mean of the 100 logistic regressions. The variability of the estimate was calculated as
var () = mean () + var (bis)
where mean () is defined as the mean of the 100 logistic regression variances and var (bis) is defined as the variance of the 100 logistic regression b coefficients.
Results
Participant characteristics
The majority of the 355 infants in our study experienced otitis media during their first year. Thirty-eight percent of the infants experienced only one episode of otitis media, 24% experienced two, and 10% had three or more episodes during their first year of life (Table 1). Male infants were more likely to experience otitis media during their first year of life: 78% of males experienced otitis media compared to 67% of female infants (P = 0.02). Black infants tended to have less otitis media than white or Hispanic infants during their first year of life. For example, 58% of black infants experienced otitis media compared to 77% of white infants and 73% of Hispanic infants (P = 0.06). We did not find significant associations between maternal education, maternal allergies, maternal asthma, daycare before six months of age, breastfeeding, exposure to ETS in the home, or season of birth and otitis media during the first year of life.
Table 1.
Unadjusted associations between personal characteristics and otitis media episodesa for 355 infants at risk for developing asthma. (CT and MA, 1998 – 2000)
| Otitis Media Episodes | |||||
| 0 | 1 | 2 | 3+ | ||
| Characteristic | n (%) | % | % | % | % |
| All subjects N (%) | 355 | 97 (27.3) | 137 (38.6) | 87 (24.5) | 34 (9.6) |
| Gender | |||||
| Male | 171 (48.2) | 21.6 | 42.7 | 24.6 | 11.1 |
| Female | 184 (51.8) | 32.6 | 34.8 | 24.5 | 8.2 |
| Ethnicity | |||||
| White | 226 (63.7) | 23.4 | 38.0 | 28.8 | 9.7 |
| Black | 48 (13.5) | 41.7 | 37.5 | 14.6 | 6.2 |
| Hispanic | 62 (17.5) | 27.4 | 41.9 | 22.6 | 8.1 |
| Other | 19 (5.4) | 36.8 | 36.8 | 5.3 | 21.0 |
| Maternal education | |||||
| < HS | 38 (10.7) | 29.0 | 36.8 | 29.0 | 5.3 |
| Some college | 179 (50.4) | 29.0 | 39.7 | 20.1 | 11.2 |
| College degree | 138 (38.9) | 24.6 | 37.7 | 29.0 | 8.7 |
| Maternal allergies | |||||
| No | 146 (41.1) | 30.8 | 39.7 | 24.0 | 5.5 |
| Yes | 209 (58.9) | 24.9 | 37.8 | 24.9 | 12.4 |
| Maternal asthma | |||||
| No | 246 (69.3) | 30.1 | 38.2 | 22.0 | 9.8 |
| Yes | 109 (30.7) | 21.1 | 39.4 | 30.3 | 9.2 |
| Daycare before 6 mo | |||||
| No | 279 (78.6) | 29.0 | 39.4 | 23.3 | 8.2 |
| Yes | 76 (21.4) | 21.0 | 35.5 | 29.0 | 14.5 |
| Breast feeding | |||||
| No | 113 (31.8) | 27.4 | 40.7 | 22.1 | 9.7 |
| Yes | 242 (68.2) | 27.3 | 37.6 | 25.6 | 9.5 |
| ETS | |||||
| No | 304 (86.6) | 26.0 | 38.5 | 26.3 | 9.2 |
| Yes | 47 (13.4) | 34.0 | 40.4 | 14.9 | 10.6 |
| Season of birth | |||||
| Winter | 75 (21.1) | 28.0 | 37.3 | 26.7 | 8.0 |
| Spring | 101 (28.4) | 25.7 | 41.6 | 24.8 | 7.9 |
| Summer | 97 (27.3) | 26.8 | 39.2 | 27.8 | 6.2 |
| Fall | 82 (23.1) | 29.3 | 35.4 | 18.3 | 17.1 |
aOtitis media determined by mother's report. More than one episode reported in a month or in the next consecutive month counted as a single episode.
There were socioeconomic differences between the group from the study cohort who chose to participate in the blood draw (355 out of 889 [40%]) and those who did not (n = 554): whole blood samples were obtained from more white infants (64% vs. 57%) and fewer Hispanic infants (18% vs. 26%) (P = 0.04), more of the mothers in the blood draw group had college degrees (39% vs. 31%, P = 0.03) and chose to breastfeed their infants (68% vs. 60%, P = 0.02). The rates of otitis media between the two blood draw groups did not differ: 28% compared to 26% for otitis media in the first 6 months; 73% vs. 71% for any otitis media in the first year; and 10% vs. 9% for 3 or more episodes of otitis media in the first year for the blood draw participants vs. non-participants, respectively.
Analysis of single nucleotide polymorphisms
Table 2 contains unadjusted associations between each of the nine candidate SNPs and otitis media for all 355 infants in our sample. Each of the individual SNPs was in Hardy-Weinberg equilibrium in the full sample of 335 subjects and within each racial/ethnic category (P > 0.05). Polymorphisms in SP-A1 at codon 19 were associated with otitis media during the first year of life. Infants with an alanine (CT) at codon 19 were also more likely to have a greater number of otitis media episodes during their first year of life. The percentage of infants with an alanine at codon 19 and 0, 1, and 2 or more episodes of otitis media during their first year were 10%, 49%, and 41% respectively. In contrast, the percentage of infants with valine (homozygote TT) at codon 19 and 0, 1, and 2 or more episodes of otitis media during their first year were 31%, 36%, and 33% respectively (P = 0.01).
Table 2.
Unadjusted associations between SNPs from surfactant protein A alleles (SP-A1, SP-A2) and otitis media (OM) episodes before 12 months of age for 355 infants at risk for developing asthma. (CT and MA, 1998 – 2000)
| OM Episodes | ||
| SNP | n (%) | % |
| All subjects N (%) | 355 | 258 (72.7) |
| SP-A1a | ||
| aa 19b | ||
| CC | 1 (0.3) | |
| CT | 56 (15.8) | 89.5 |
| TT | 294 (83.8) | 69.0 |
| aa 50 | ||
| CC | 75 (21.2) | 80.0 |
| CG | 171 (48.3) | 69.0 |
| GG | 108 (30.5) | 74.1 |
| aa 62 | ||
| GG | 5 (1.4) | |
| AG | 88 (25.2) | 74.2 |
| AA | 256 (73.4) | 71.9 |
| aa 133 | ||
| GG | 2 (0.6) | |
| AG | 51 (14.4) | 83.0 |
| AA | 302 (85.1) | 70.9 |
| aa 219 | ||
| TT | 1 (0.3) | |
| CT | 46 (13.0) | 68.1 |
| CC | 308 (86.8) | 73.4 |
| SP-A2a | ||
| aa 9 | ||
| CC | 73 (20.6) | 74.0 |
| AC | 171 (48.2) | 71.9 |
| AA | 111 (31.3) | 73.0 |
| aa 91 | ||
| CC | 6 (1.7) | |
| CG | 85 (24.2) | 73.6 |
| GG | 261 (74.2) | 72.4 |
| aa 140 | ||
| TT | 25 (7.1) | 64.0 |
| CT | 142 (40.2) | 71.1 |
| CC | 186 (52.7) | 75.3 |
| aa 223 | ||
| AA | 18 (5.1) | 77.8 |
| AC | 129 (36.3) | 72.9 |
| CC | 208 (58.6) | 72.1 |
aEach of the SNPs was in Hardy-Weinberg equilibrium (P > 0.05).
bP = 0.02, χ2-test.
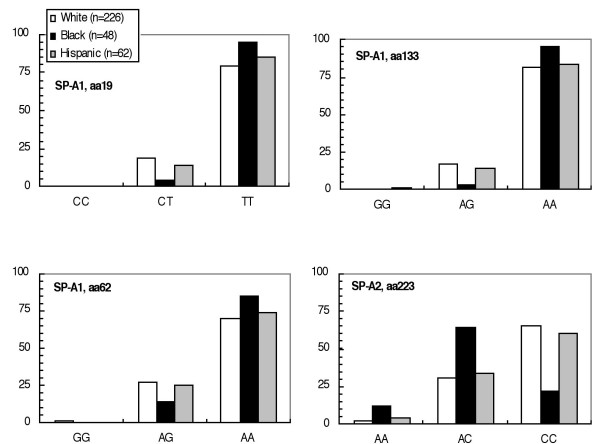
We did not find associations between polymorphisms within any of the SNPs and gender, maternal education, maternal allergies, maternal asthma, daycare before six months, exposure to ETS, or season of birth. Codons 19, 62, and 133 in SP-A1, and 223 in SP-A2 were associated with race/ethnicity (Figure 1). Given the association of otitis media with both race/ethnicity and specific polymorphisms within our study population, we restricted our haplotype analyses to the ethnic group with the largest number of subjects (226 white infants).
Figure 1.
Ethnic distribution of surfactant protein A (SP-A) SNPs among infants at risk for developing asthma (P-values from χ2 tests).
The unadjusted relationship between polymorphisms in each of the SNPs and any episode of otitis media was examined in logistic regression models in the white infants. White infants having an alanine (CT) at SP-A1 codon 19 were more than 3.8 times more likely (95% CI 1.3, 11.2) than those with a valine (TT) to have otitis media during their first year of life.
Allele and haplotype analyses
We designated the SP-A1 and SP-A2 alleles by 6An and 1Am respectively as has been previously described [21,26]. The associated nomenclature as well as the nucleotide and amino acid changes in the major SP-A1 and SP-A2 alleles are given in Table 3. The five SNPs in SP-A1 were in linkage disequilibrium. The most common SP-A1 allele haplotypes among the white infants in our study population were 6A2, 6A3, 6A4, and 6A in decreasing order of frequency. The four SNPs in SP-A2 were also in linkage disequilibrium (P < 0.0001). 1A0 was the most common SP-A2 allele among the white infants in our study followed by 1A1, 1A2, 1A, 1A5, 1A6, and 1A3. All others combined made up 4% of the SP-A2 alleles.
Table 3.
Surfactant protein A (SP-A1 and SP-A2) allele haplotypesa and unadjusted odds ratios (OR) and 95% confidence intervals (CI) from logistic regression modelsb of any episodes of otitis media before 12 months of age. (CT and MA, 1998 – 2000)
| Allele, haplotypec | Nucleotide/amino acidd | Estimated Frequency Distributione % (95% CI) | Any OM Episodes OR (95% CI) | ||||
| SP-A1 | aa 19 | aa 50 | aa 62 | aa 133 | aa 219 | ||
| 6A | C/Ala | C/Leu | G | G | C/Arg | 6.1% (4.0 – 8.3) | 1.96 (0.63, 6.10) |
| 6A2 | T/Val | G/Val | A | A | C/Arg | 54.0% (49.3 – 58.7) | 0.95 (0.44, 2.04) |
| 6A3 | T/Val | C/Leu | A | A | C/Arg | 25.6% (21.6 – 29.6) | 0.85 (0.45, 1.60) |
| 6A4 | T/Val | C/Leu | G | A | T/Trp | 6.2% (4.1 – 8.4) | 0.34 (0.15, 0.79) |
| all others | 8% | ||||||
| SP-A2 | aa 9 | aa 91 | aa 140 | aa 223 | |||
| 1A | C/Thr | C/Pro | C | C/Gln | 8.4% (6.0 – 10.8) | 3.03 (0.88, 10.39) | |
| 1A0 | A/Asn | G/Ala | C | C/Gln | 54.2% (49.5 – 58.8) | 1.04 (0.48, 2.25) | |
| 1A1 | C/Thr | G/Ala | T | A/Lys | 13.7% (10.7 – 16.7) | 0.69 (0.34, 1.39) | |
| 1A2 | C/Thr | G/Ala | C | C/Gln | 10.2% (7.6 – 12.7) | 0.98 (0.43, 2.22) | |
| 1A3 | A/Asn | G/Ala | T | A/Lys | 1.8% (0.7 – 2.9) | 0.80 (0.15, 4.35) | |
| 1A5 | C/Thr | C/Pro | T | C/Gln | 4.6% (2.7 – 6.5) | 0.38 (0.14, 1.05) | |
| 1A6 | C/Thr | G/Ala | T | C/Gln | 3.2% (1.7 – 4.7) | 0.77 (0.20, 2.92) | |
| all others | 4% | ||||||
aN = 219 white infants with complete data for SP-A1 alleles and N = 222 white infants with complete data for SP-A2 alleles.
bSeparate logistic regression analyses were performed for each outcome measure and each haplotype with all other haplotypes serving as the reference group in each model. All model results include estimates of model variability due to ambiguity in allele haplotype assignment (see "Data analysis" in text).
cBy convention [ref Lofgren et al JID 2002;185:283-289], SP-A1 allele haplotypes are denoted by 6An and SP-A2 alleles by 1Am.
dCodons aa62, aa133, and aa140 are silent.
eEstimated frequency distribution of SP-A1 and SP-A2 haplotypes (mean, 95% CI) for white infants.
We examined associations between any otitis media during the first year of life and specific allele haplotypes in SP-A1 and SP-A2 (Table 3). The 6A4 allele of SP-A1 was protective for otitis media in white infants (OR 0.34; 95% CI 0.15, 0.79). The 1A5 allele in SP-A2 was underrepresented among white infants with otitis media (OR 0.38; 95% CI 0.14, 1.05). White infants with the 1A allele in SP-A2 were over 3 times more likely to have otitis media during the first year of life (OR 3.03; 95% CI 0.88, 10.39), but with wide confidence intervals that included unity.
SP-A1 and SP-A2 alleles are in close proximity and are known to be in strong linkage disequilibrium [28,29]. Within our population of white infants, the SP-A1 and SP-A2 allele haplotypes were also in linkage disequilibrium (P < 0.0001). Since the alleles at these two loci cosegregate as one unit, we examined the distribution of SP-A1 and SP-A2 alleles together. Unadjusted relationships between the SP-A haplotypes and any episode of otitis media are shown in Table 4. We did not adjust for other known otitis media risk factors (e.g. daycare, breastfeeding) because these were not associated with polymorphisms in SP-A. Consistent with our allele analysis, white infants with the 6A4/1A5 haplotype experienced decreased risk for otitis media during their first year of life (OR 0.23; 95% CI 0.07,0.73).
Table 4.
Estimated frequency distribution of surfactant protein A (SP-A) haplotypes among infants at risk for the development of asthma. Unadjusted odds ratios (OR) and 95% confidence intervals (CI) from logistic regression modelsa of any episodes of otitis media before 12 months of age. (N = 226 white infants, CT and MA, 1998 – 2000.)
| SP-A Haplotypeb | Estimated Frequency Distributionc % (95% CI) | Any OM Episodes OR (95% CI) |
| 6A/1A | 5.4% (3.2 – 7.2) | 2.13 (0.59, 7.68) |
| 6A2/1A0 | 49.0% (44.4 – 53.6) | 1.10 (0.53, 2.30) |
| 6A2/1A2 | 1.8% (0.6 – 2.9) | 1.71 (0.20, 14.4) |
| 6A2/1A3 | 1.0% (0.2 – 1.9) | 0.90 (0.09, 8.83) |
| 6A3/1A0 | 4.7% (2.8 – 6.6) | 0.87 (0.29, 2.58) |
| 6A3/1A1 | 11.3% (8.5 – 14.1) | 0.70 (0.33, 2.47) |
| 6A3/1A2 | 5.6% (3.6 – 7.6) | 0.90 (0.33, 2.47) |
| 6A3/1A6 | 1.7% (0.5 – 2.8) | 0.73 (0.13, 4.01) |
| 6A4/1A5 | 2.9% (1.4 – 4.4) | 0.23 (0.07, 0.73) |
| 6A4/1A6 | 1.8% (0.6 – 3.0) | 0.46 (0.10, 2.09) |
| Others | 14.9% |
aSeparate logistic regression analyses were performed for each outcome measure and each haplotype with all other haplotypes serving as the reference group in each model. All model results include estimates of model variability due to ambiguity in allele haplotype assignment (see "Data analysis" in text).
bN = 217 infants with complete data for both SP-A1 and SP-A2 alleles.
cEstimated frequency distribution of SP-A haplotypes (mean, 95% CI) for white infants.
Discussion
We found that specific SNPs, alleles, and haplotypes of SP-A were associated with otitis media risk among a cohort of infants at risk for asthma. The 6A4 allele haplotype was protective for otitis media in white infants during their first year. Correspondingly, white infants with the 6A4/1A5 haplotype experienced a 76% decreased risk for otitis media during the first year of life.
These data are in contrast to a Finnish study of the association between SP-A polymorphisms and otitis media [11]. That study reported that the 6A4/1A5 haplotype was overrepresented among children with both acute otitis media before 6 months of age and recurrent otitis media. Under our definition of otitis media, which did not distinguish between acute otitis media and otitis media with effusion, otitis media before 6 months was highly correlated with season of birth. Among infants born in the summer or fall, one-third or more (33% and 42%, respectively) had otitis media before 6 months of age compared to less than one-quarter (23% and 18%, respectively) of the infants born in winter or spring (χ2, P = 0.002). Otitis media has been shown to undergo seasonal variation in parallel with respiratory virus infections [30]: rates are highest in the fall and winter months. We did not have enough power (i.e., too few subjects to stratify analysis by season of birth) to detect associations with otitis media before 6 months and specific SP-A haplotypes. In addition, by definition, our episode count for the first year of life was conservative. We identified a small number of white infants (n = 25) with 3 or more otitis media episodes during their first year, which perhaps accounts for our finding no association between recurrent otitis media and SP-A haplotypes. The population in the Finnish study was different from ours: children in the Finnish study were older (1–10 years of age), and cases were children with recurrent otitis media who were admitted to the hospital for adenoidectomy, tympanostomy, or both. The controls for the Finnish study were consecutive infants born in the hospital.
RSV infection has been strongly linked to the pathogenesis of acute otitis media [6] and polymorphisms in SP-A have been linked to susceptibility to bronchiolitis caused by this virus [31]. Lofgren et al. [31] found that 6A/1A was protective for severe RSV infection in infants (OR 0.17; 95% CI 0.04, 0.80), and the 6A2/1A3 haplotype was associated with increased risk of disease (OR 10.4; 95% CI 1.3, 83.2). Neither of these haplotypes was associated with otitis media in our study population. Interestingly, the frequency of infants with 6A4/1A5, our protective otitis media-associated haplotype, was slightly lower among infants with RSV infections when compared to the control group (4% vs 7%) [31].
Our results indicate that SNPs at aa19 in the SP-A1 gene are associated with otitis media risk. Codon 19 is located in the N terminal domain of SP-A1. Further experiments are needed to identify whether amino acid changes in this region impact biological properties such as gene expression levels or interactions with pathogens. Infants with an alanine at codon 19 were more likely to have otitis media during their first year of life. Infants with the 6A4/1A5 haplotype have a valine at codon19.
While the association of SP-A with otitis media is biologically plausible, otitis media is a multigenic, multifactorial disease, and there are likely other susceptibility genes involved. A genome scan mapped recurrent otitis media to chromosome q10 at marker D10S212 and to chromosome 19q at marker D19S254 [32]. SP-A is contained on chromosome 10, but is in a separate region from D10S212.
In our study, black infants experienced less otitis media than white infants. The potential problems associated with population stratification related to ethnicity have been addressed with regard to surfactant proteins [33]. Seven ethnic groups from three races (Caucasian, Black and Hispanic) were examined, and SP-A allele and genotype frequencies were shown to differ between ethnic groups from different races. We did not have enough subjects in the black (n = 47 with complete data) or Hispanic (n = 62 with complete data) groups to examine the associations of specific haplotypes with otitis media.
As stated previously, 60–80% of infants in the United States experience otitis media during the first year of life. Our finding that 73% of infants experienced otitis media during the first year is on the high end of this range. This may be due in part to the special nature of our population. All of the infants in our cohort have an older sibling in the home. Exposure to other children has been shown to be associated to otitis media [1,2]. In contrast to other studies [1-4], we did not find a strong association between otitis media and attendance in daycare outside the home. Infants in our study experienced a greater number of otitis media episodes, but the difference was not statistically significant. Another study, of 806 infants from the same cohort, identified an association between early otitis media and attendance in daycare before six months of age [24]. Therefore, the lack of statistical significance is likely due to the small number of white infants (76 total) attending daycare before six months of age.
One limitation of this study was that the cohort examined was not specifically recruited to study otitis media. We collected data by asking mothers whether their infants had a clinician-diagnosed medical condition. The diagnostic criteria may have differed between clinicians. Furthermore, mothers reported diagnosis of an ear infection but were not asked to distinguish between acute otitis media and otitis media with effusion. Different genetic polymorphisms may influence different otitis media phenotypes. Since we were unable to distinguish between different types of otitis media, grouping all types together may have diluted our findings. Since accuracy in episode counts was dependent on maternal report, we attempted to limit recall bias by asking mothers about their child's medical conditions every three months. Parents have been shown to accurately report the number of otitis media episodes (kappa = 0.94) [34]. Our study used a conservative count of otitis media episodes: two episodes occurring within the same month or consecutive months counted as one episode.
The generalizability of our results may also be limited given that our cohort contains children at risk for asthma. Since SP-A is part of the innate immune system in the lungs, infants in our cohort could differ in SP-A1 and SP-A2 hapylotype distributions compared to the general population. Liu et al. examined SP-A1 and SP-A2 allele haplotype frequencies in over 300 white Americans [33]. Their results indicate that SP-A1 allele haplotype frequencies were 56.2% 6A2, 24.3% 6A3, 9.3% 6A, 7.6% 6A4 and 2.6% other. Approximate allele haplotype frequencies for SP-A2 were 53% 1A0, 10.2% 1A, 14.3% 1A1, 7.6% 1A2, and 14.9% all others. With the exception of allele haplotype 6A (SP-A1), the distribution of SP-A1 and SP-A2 haplotypes in our population (Table 3) is similar to that of white Americans in the general population.
Strengths of this study include the prospective study design and well characterized demographic and illness information for the infant cohort. Adjustments for uncertainty in haplotype assignment are rarely incorporated into association studies of genetic haplotypes and disease; instead these studies often use the most likely haplotype assignment [35]. We utilized logistic regression models that incorporate estimates of the effect of this uncertainty to more accurately model the association between polymorphisms in SP-A and otitis media.
Conclusion
We identified polymorphisms within SP-A loci that are associated with otitis media in white infants at risk for asthma. These polymorphisms may play a biological role in disease, or these polymorphisms may be in linkage disequilibrium with other unmeasured markers that may be the causal polymorphism. While we cannot definitively state whether the polymorphisms are causative or are neutral markers, the results of this study suggest a role for SP-A in otitis media susceptibility. Further studies are needed to replicate, in white and other ethnic groups, our observation of a relationship between otitis media in the first year of life and polymorphisms in SP-A.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
MMP conceived of the study, analysed and interpreted data, and drafted the manuscript. JFG participated in the analysis and interpretation of data, performed the statistical analysis, and helped revise the manuscript for important intellectual content. YZ was involved in the acquisition of data and provided technical support with the genotyping. EWT helped conceive the study, and was involved in the acquisition of data. KB conceived the study, participated in its design and coordination, and helped secure funding. TRH was involved in the critical revision of manuscript for important intellectual content and provided statistical expertise. MMB was involved in the study concept and design, study supervision, critical revisions of the manuscript for intellectual content, and helped obtain funding. BPL was involved in the study concept and design, study supervision, and obtained funding. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
Written consent was obtained from the mothers to participate in this study. We thank the original 1,002 original families who participated. We also thank Matthew P. York for excellent technical assistance. This studywould not have been possible without the following hospitals fromwhich our study population was selected: Yale-New Haven, Danbury, Bridgeport, Hartford (CT), and Bay State (MA). This study was supported by grants ES07456 and ES05410 from the National Institute of Environmental Health Sciences.
Contributor Information
Melinda M Pettigrew, Email: melinda.pettigrew@yale.edu.
Janneane F Gent, Email: janneane.gent@yale.edu.
Yong Zhu, Email: yong.zhu@yale.edu.
Elizabeth W Triche, Email: elizabeth.triche@yale.edu.
Kathleen D Belanger, Email: kathleen.belanger@yale.edu.
Theodore R Holford, Email: theodore.holford@yale.edu.
Michael B Bracken, Email: michael.bracken@yale.edu.
Brian P Leaderer, Email: brian.leaderer@yale.edu.
References
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective cohort study. Journal of Infectious Diseases. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- Paradise JL, Rockette HE, Colborn DK, Bernard BS, Smith CG, Kurs-Lasky M, Janosky JE. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–333. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Byrd RS, Auinger P, Hall CB. Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics. 1997;99:e1–7. doi: 10.1542/peds.99.3.e1. [DOI] [PubMed] [Google Scholar]
- Auinger P, Lanphear BP, Kalkwarf HJ, Mansour ME. Trends in otitis media among children in the United States. Pediatrics. 2003;112:514–520. doi: 10.1542/peds.112.3.514. [DOI] [PubMed] [Google Scholar]
- Daly KA, Rovers MM, Hoffman HJ, Uhari M, Casselbrant ML, Zielhuis G, Kvaerner KJ. Recent advances in otitis media. 1. Epidemiology, natural history, and risk factors. Annals of Otology, Rhinology, & Laryngology. 2005;194:8–15. doi: 10.1177/00034894051140s104. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. Pediatrics. 1999;340:260–264. doi: 10.1056/NEJM199901283400402. [DOI] [PubMed] [Google Scholar]
- Daly KA, Brown JE, Lindgren BR, Meland MH, Le CT, Giebink SG. Epidemiology of otitis media onset by six months of age. Pediatrics. 1999;103:1158–1166. doi: 10.1542/peds.103.6.1158. [DOI] [PubMed] [Google Scholar]
- Casselbrant ML, Mandel EM, Fall PA, Rockette HE, Kurs-Lasky M, Bluestone CD, Ferrell RE. The heritability of otitis media: a twin and triplet study. JAMA. 1999;282:2125–2130. doi: 10.1001/jama.282.22.2125. [DOI] [PubMed] [Google Scholar]
- Kvaerner KJ, Tambs K, Harris JR, Magnus P. Distribution and heritability of recurrent ear infections. Annals of Otology, Rhinology & Laryngology. 1997;106:624–632. doi: 10.1177/000348949710600802. [DOI] [PubMed] [Google Scholar]
- Rovers M, Haggard M, Gannon M, Koeppen-Schomerus G, Plomin R. Heritability of symptom domains in otitis media: a longitudinal study of 1,373 twin pairs. American Journal of Epidemiology. 2002;155:958–964. doi: 10.1093/aje/155.10.958. [DOI] [PubMed] [Google Scholar]
- Ramet M, Lofgren J, Alho OP, Hallman M. Surfactant protein-A gene locus associated with recurrent otitis media. Journal of Pediatrics. 2001;138:266–268. doi: 10.1067/mpd.2001.110133. [DOI] [PubMed] [Google Scholar]
- Joki-Erkkila VP, Puhakka H, Hurme M. Cytokine gene polymorphism in recurrent acute otitis media. Archives of Otolaryngology -- Head & Neck Surgery. 2002;128:17–20. doi: 10.1001/archotol.128.1.17. [DOI] [PubMed] [Google Scholar]
- Sano H, Kuroki Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Molecular Immunology. 2005;42:279–287. doi: 10.1016/j.molimm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Eggleton P, Reid KB. Lung surfactant proteins involved in innate immunity. Current Opinion in Immunology. 1999;11:28–33. doi: 10.1016/S0952-7915(99)80006-5. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect. 2001;3:161–166. doi: 10.1016/S1286-4579(00)01363-0. [DOI] [PubMed] [Google Scholar]
- White RT, Damm D, Miller J, Spratt K, Schilling J, Hawgood S, Benson B, Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. Nature. 1985;317:361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- Katyal SL, Singh M, Locker J. Characterization of a second human pulmonary surfactant-associated protein SP-A gene. American Journal of Respiratory Cell & Molecular Biology. 1992;6:446–452. doi: 10.1165/ajrcmb/6.4.446. [DOI] [PubMed] [Google Scholar]
- Fisher JH, Kao FT, Jones C, White RT, Benson BJ, Mason RJ. The coding sequence for the 32,000-dalton pulmonary surfactant associated protein A is located on chromosome 10 and identifies two separate restiction-fragment length polymorphisms. American Journal of Human Genetics. 1987;40:503–511. [PMC free article] [PubMed] [Google Scholar]
- Paanenen R, Sormunen R, Glumoff V, van Eijk M, Hallman M. Surfactant proteins A and D in Eustachain tube epithelium. American Journal of Physiology Lung Cellular and Molecular Physiology. 2001;281:L660–L667. doi: 10.1152/ajplung.2001.281.3.L660. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Chun YM, Lee HY, Moon SK, Chang KH, Li JD, Andalibi A. Cell biology of tubotympanum in relation to pathogenesis of otitis media - a review. Vaccine. 2000;19 Suppl:S17–25. doi: 10.1016/S0264-410X(00)00273-5. [DOI] [PubMed] [Google Scholar]
- Ramet M, Haataja R, Marttila R, Hamalainen AM, Knip M, Hallman M. Human surfactant protein- A gene locus for genetic studies in the Finnish population. Disease Markers. 2000;16:119–124. doi: 10.1155/2000/914814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, Leaderer BP. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environmental Health Perspectives. 2002;110:A781–6. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, Jankun T, Ren P, McSharry JJE, Platts-Mills TA, Chapman MD, Bracken MB. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environmental Health Perspectives. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew MM, Gent J, Triche EW, Belanger KD, Bracken MB, Leaderer BP. Association of early-onset otitis media in infants and exposure to household mold. Paediatric and Perinatal Epidemiology. 2004;18:441–447. doi: 10.1111/j.1365-3016.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- Pantelidis P, Lagan AL, Davies JC, Welsh KI, du Bois RM. A single round PCR method for genotyping human surfactant protein (SP)-A1, SP-A2, and SP-D gene alleles. Tissue Antigens. 2003;61:317–321. doi: 10.1034/j.1399-0039.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- DiAngelo S, Lin Z, Wang G, Phillips S, Ramet M, Luo J, Floros J. Novel, non-radioactive, simple and multiplex PCR-cRFLP methods for genotyping human SP-A and SP-D marker alleles. Disease Markers. 1999;15:269–281. doi: 10.1155/1999/961430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RJ, Ruppert D, Stefanski LA. Measurement error in nonlinear models. Boca Raton, Florida, Chapman and Hall/CRC Press; 1995. [Google Scholar]
- Hoover RR, Floros J. Physical and radiation hybrid mapping reveal gene order and orientation. American Journal of Respiratory Cell & Molecular Biology. 1998;18:353–362. doi: 10.1165/ajrcmb.18.3.3035. [DOI] [PubMed] [Google Scholar]
- Floros J, DiAngelo S, Koptides M, Karinch AM, Rogan PK, Nielsen H, Spragg RG, Wattenberg K, Deiter G. Human SP-A locus: allele frequencies and linkage disequilibrium between the two surfactant protein genes. American Journal of Respiratory Cell & Molecular Biology. 1996;15:489–498. doi: 10.1165/ajrcmb.15.4.8879183. [DOI] [PubMed] [Google Scholar]
- Vesa S, Kleemola M, Blomqvist S, Takala A, Kilpi T, Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatric Infectious Disease Journal. 2001;20:574–581. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Lofgren J, Ramet M, Renko M, Marttila R, Hallman M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. Journal of Infectious Diseases. 2002;185:283–289. doi: 10.1086/338473. [DOI] [PubMed] [Google Scholar]
- Daly KA, Brown WM, Segade F, Bowden DW, Keats BJ, Lindgren BR, Levine SC, Rich SS. Chronic and recurrent otitis media: a genome scan for susceptibility loci. American Journal of Human Genetics. 2004;75:988–997. doi: 10.1086/426061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Bentley CM, Floros J. Study of human SP-A, SP-B and SP-D loci: allele frequencies, linkage disequilibrium and heterozygosity in different races and ethnic groups. BMC Genetics. 2003;4:13. doi: 10.1186/1471-2156-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly KA, Lindgren BR, Giebink SG. Validity of parental report of a child's medical history in otitis media research. American Journal of Epidemiology. 1994;139:1116–1121. doi: 10.1093/oxfordjournals.aje.a116955. [DOI] [PubMed] [Google Scholar]
- Kraft P, Cox DG, Paynter RA, Hunter D, De Vivo I. Accounting for uncertainty in matched association studies: a comparison of simple and flexible techniques. Genetic Epidemiology. 2005;28:261–272. doi: 10.1002/gepi.20061. [DOI] [PubMed] [Google Scholar]