Abstract
To test the hypothesis that a Th2 response to Helicobacter pylori is necessary for protection and to address the possibility that humoral and Th2 cellular responses may compensate for each other, we generated mice deficient in both interleukin-4 (IL-4) and antibodies. The immunized double-knockout mice were protected from H. pylori challenge, as were the parental strains and wild-type C57BL/6 mice. Neutralization of IL-4 in B-cell-deficient mice did not prevent protection. Immunized IL-5-deficient mice were also protected. Thus, IL-4 and IL-5 are not essential for protection.
Helicobacter pylori is a gram-negative curved bacterium that colonizes the human stomach. Infection can lead to gastritis, peptic ulcer disease, and gastric cancer (6, 39, 47). Although the natural immune response in patients rarely eliminates infection, mice immunized against H. pylori can clear or greatly reduce their bacterial load (10, 16, 26, 40). The requirement of major histocompatibility complex class II for protection in mice implies a role for CD4+ T cells (12), but the exact mechanism of bacterial clearance in mice is unknown.
Gastric inflammation in H. pylori-infected patients is characterized by production of interleukin-1β (IL-1β), IL-6, IL-12, tumor necrosis factor alpha, and gamma interferon (IFN-γ) (3, 11, 32, 49), and the adjuvants commonly used in mice polarize to a Th2 response (34), suggesting that a Th1 response leads to disease (13, 23) and a Th2 response promotes protection (10). Some data from the mouse-Helicobacter felis model appear to support this hypothesis. BALB/c mice that are predisposed to a Th2 response showed little inflammation when infected with H. felis, while C57BL/6 mice with a Th1 predisposition developed severe gastritis (37, 42). In vivo neutralization of IFN-γ reduced inflammation in infected C57BL/6 mice (35), and adoptive transfer of a Th2 cell line specific for H. felis enabled C57BL/6 mice to reduce their bacterial load (36). Also, immunized IL-4-deficient mice were not protected from H. felis infection (F. J. Radcliff, A. J. Ramsay, and A. Lee, abstr., Gastroenterology 110(Suppl.):A997, 1996). Finally, in a therapeutic immunization study using H. felis-infected BALB/c mice, a decrease in bacterial colonization was associated with increased production of IL-4 by CD4+ spleen cells stimulated in vitro (43). Collectively, these studies suggested that IL-4 production was important for protection, but most of the evidence was indirect.
In contrast, analysis of stomachs from protected immunized mice did not demonstrate elevated levels of mRNA for Th2 cytokines (IL-4, IL-5, and IL-13) at 4 weeks after challenge (15). Thus, if IL-4 were required for protection, it would most likely be effective during the induction phase of the immune response. The fact that mice lacking antibodies are protected by immunization from Helicobacter infection (5, 12, 45) suggests that the putative Th2 response is cellular rather than humoral. We also considered the possibility that clearance mechanisms may be redundant and that either a humoral response or a Th2 cellular response could reduce bacterial load. To determine whether IL-4 is necessary for protection in the absence of antibodies, we generated mice genetically deficient in production of both IL-4 and antibodies. We also neutralized IL-4 in vivo in μMT mice. Finally, mice deficient in IL-5 (IL-5 knockout [KO]) were immunized and challenged.
Generation of mice lacking both IL-4 and antibodies.
Mice genetically deficient in both IL-4 and antibodies (double knockout [DKO]) were generated by breeding female C57BL/6-Igh-6tm1Cgn mice (μMT) (25) (Jackson Laboratory, Bar Harbor, Maine) with male C57BL/6J-Il4tm1Cgn mice (IL-4 KO) (29) (Jackson Laboratory). All procedures involving mice were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Mice were specific pathogen free, housed in autoclaved static microisolator cages, and provided with water and sterile Teclad chow ad libitum. Pairs of the F1 offspring, heterozygous for both μ chain (chromosome 12) and IL-4 (chromosome 11), produced 295 F2 pups, which were typed by using genomic DNA from tail tips (15). Two PCRs per mouse were performed (14). Primers to distinguish the disrupted and wild-type (WT) μ genes were designed with the program Amplify (University of Wisconsin, Madison, Wis.) and the sequence of genomic Igh-1a (GenBank accession no. 202416). The primers TCCGTCTAGCTTGAGCTATTA and ACAGTGTGAATTGCTGT flanked the M1 exon, which is disrupted in μMT mice (25), and amplified a 360-bp product from the wild-type gene and sometimes an ∼1,200-bp product from the disrupted gene. The neomycin primer TCAGGACATAGCGTTGGC (IMR079 [www.jax.org/resources/documents/imr/protocols/]) primed within the insert to produce a 400-bp product from the disrupted μ gene. Primer sequences used to identify the IL-4 genotype of the F2 mice were obtained from the Jackson Laboratory web site. IMR077 and IMR078 amplified a 446-bp product from the wild-type IL-4 gene, and IMR078 and IMR079 produced a 576-bp product from the disrupted gene.
A total of 27 DKO, 16 μMT, 20 IL-4 KO, and 21 WT F2 mice were obtained. The distribution of genotypes fit the predicted Mendelian ratio for a double heterozygous breeding (χ square analysis not shown). Breeding pairs of DKO, μMT, IL-4 KO, and WT F2 mice generated additional mice for the immunization challenge experiments.
Phenotype confirmation of DKO mice.
The phenotypes of representative DKO mice were verified by attempting to detect antibodies in the serum and IL-4 from stimulated splenocytes. Splenocytes (5 × 106 cells per ml) from five DKO and three WT mice were incubated for 72 h in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL) containing 10% fetal bovine serum (FBS) (Gibco BRL), 20 μg of gentamicin per ml (Gibco BRL), 2.0 mM l-glutamine (Gibco BRL), 0.1 mM minimal essential medium (MEM) nonessential amino acids (Gibco BRL), and 50 μM 2-mercaptoethanol (Sigma, St. Louis, Mo.) ± concanavalin A (Vector Laboratories, Burlingame, Calif.) at 4 or 8 μg/ml. For detection of IL-4, media with 4 μg of concanavalin A per ml also contained anti-mouse IL-4 receptor antibody (anti-IL-4R) (M1; Immunex, Seattle, Wash.) at a final concentration of 5 μg/ml to prevent IL-4 sequestration by soluble receptors (4). The supernatants were assayed for IL-4 and IFN-γ by enzyme-linked immunosorbent assay (ELISA) with antibody pairs and standards purchased from BD-Pharmingen (San Diego, Calif.) (21).
No IL-4 was detectable in supernatant from DKO splenocytes. WT splenocytes produced both IL-4 (0.82 ± 0.19 ng/ml [mean ± standard error]) in the presence of anti-IL-4R) and IFN-γ (2.56 ± 0.24 and 7.56 ± 0.25 ng/ml when stimulated with 4 and 8 μg of concanavalin A per ml, respectively). The DKO splenocytes produced more IFN-γ (5.67 ± 1.69 ng/ml) than WT splenocytes at the lower concentration of concanavalin A, but the higher concentration of concanavalin A increased production of IFN-γ by DKO cells only slightly (6.78 ± 1.62 ng/ml). The spleens of DKO mice were smaller than those of WT mice and yielded fewer splenocytes (11 to 35 million cells per mouse) than WT spleens (115 to 139 million cells per mouse).
Endpoint titers (16) for total serum immunoglobulin G (IgG) were measured on plates coated with goat anti-mouse Ig (Southern Biotechnology, Birmingham, Ala.). Antibodies were not detectable in sera from DKO and μMT mice, but were present at titers of 10−4 to 10−5.5 in naive IL-4 KO and WT mice.
Bacteria and bacterial products.
H. pylori Sydney strain (SS1) (31) cells were cultured on Columbia agar containing 7% horse blood in a microaerophilic environment as described previously (16). Soluble sonicate made from plate-cultured H. pylori was used for all immunizations (15). Liquid cultures of H. pylori were passaged in vitro no more than four times before challenge, and H. pylori was isolated from stomachs on plates containing antibiotics that suppress the growth of gastric flora (16).
Protection against H. pylori in immunized mice lacking IL-4 and/or antibodies.
Two immunization challenge experiments were performed with the F2 and F3 mice with five to eight mice of each strain per treatment group. The three treatments were as follows: unimmunized but not challenged (U/NC), immunized and challenged (I/C), and unimmunized and challenged (U/C). Mice were immunized intranasally four times at weekly intervals with 100 μg of H. pylori sonicate mixed with 5 μg of cholera toxin (CT) and challenged with >107 CFU of H. pylori 2 weeks after the final immunization (16). Four weeks after challenge, a strip from the greater curvature of the stomach was fixed in 10% buffered formalin for histology, and half of the remaining glandular stomach was cultured quantitatively (16). Bacterial load was expressed as log CFU per gram of stomach tissue. Challenged mice that were culture negative were assigned a value of 1 CFU/g in order to calculate the log CFU per gram and geometric means. No H. pylori colonies were detected in any negative control (U/NC) mice.
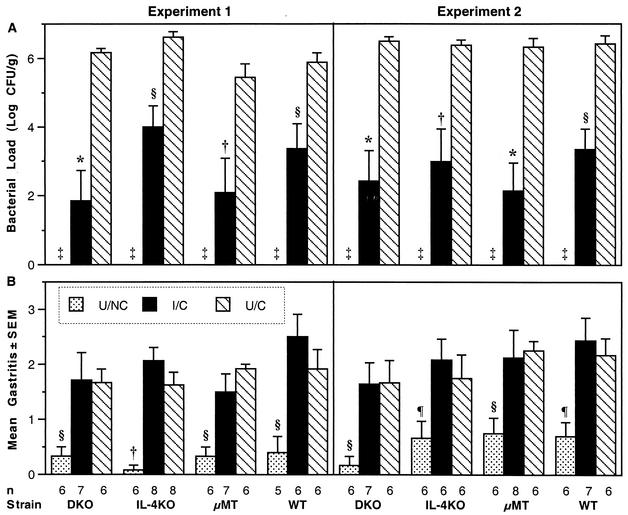
The mean bacterial load for each group of mice is shown in Fig. 1 A. For these studies, we have defined protection as a reduction in bacterial load of at least 2 logs (99% decrease) from the colonization in U/C mice of the same genotype (15, 16). By this criterion, the I/C groups of all strains, DKO, IL-4 KO, μMT, and WT, were protected in both experiments (Fig. 1A). Comparisons between groups were made by analysis of variance with StatView 4.5 (Abacus, Berkeley, Calif.) with Fisher's protected least significant difference as the post hoc test. In both experiments, the colonization of each I/C group was significantly less than that of its respective U/C group (P < 0.01), and the bacterial loads in the I/C groups of DKO and μMT mice were not different from the colonization in the I/C group of WT mice. In the first experiment, the colonization in the I/C IL-4 KO group was higher than that in the I/C DKO and the I/C μMT groups (P < 0.05), but it was not different from that in the I/C WT group. In the second experiment, there were no significant differences in mean colonization among the I/C groups. In both experiments, the U/C groups were not different from one another. These results indicate that IL-4 is not necessary for protection and suggest that IL-4 does not influence the bacterial load in C57BL/6 mice. In addition, protection in the DKO mice demonstrates that protection in mice lacking IL-4 is not dependent on antibodies and protection in mice lacking antibodies is not dependent on IL-4.
FIG. 1.
Mice genetically lacking IL-4 and/or antibodies were protected by intranasal immunization and had H. pylori-induced gastritis similar to that of WT mice at 4 weeks after challenge. (A) Quantitative culture results (log CFU per gram of stomach) are shown as the geometric mean ± standard error for each group. Each I/C group had a >2-log decrease in mean colonization compared to its respective U/C group. ‡, all unchallenged mice H. pylori negative. (B) Gastric inflammation was graded in the worst ×10 field on a scale of 0 to 5. The I/C and U/C groups of each strain were not different, but both challenged groups had significantly more inflammation than the respective U/NC group. *, P < 0.0001; †, P < 0.001; §, P < 0.01; ¶, P < 0.05.
In contrast to our findings, others have found impaired mucosal immune responses, both humoral and T-cell mediated, in IL-4 KO mice (38, 46). The impaired T-cell responses in IL-4 KO mice were attributed to lack of germinal center development in the Peyer's patches (46), and it is possible that intranasal immunization may be more effective than intragastric immunization in IL-4 KO mice because the induction site is in the upper respiratory mucosa rather than in Peyer's patches (30).
Gastritis in mice without IL-4 and/or antibodies.
Histologic grading was performed by one pathologist (C.A.G.) on sections stained with hematoxylin and eosin using a scale from 0 to 5 (16). In both experiments, the mean gastritis scores of the I/C mice were not significantly different from those of the U/C mice of the same genotype (Fig. 1B). In the first experiment, the mean gastritis score in the I/C WT group was higher than that in the I/C μMT group (P = 0.0175), but in the second experiment, the I/C groups of the four strains were not significantly different from one another. Within each strain, both challenged groups had significantly more inflammation than the respective U/NC group, and within each experiment, the U/NC groups were not different from one another. These gastritis results were consistent with the 4-week time point in our kinetic study (16). The peak gastritis in the kinetic study occurred 1 to 2 weeks after challenge in immunized mice, but time points earlier than 4 weeks were not studied for the DKO, IL-4 KO, and μMT mice, because our main interest was the reduction of bacterial load, which was greater at 4 weeks than at 2 weeks (16).
Within each group of challenged mice, inflammation varied from mild to moderate or severe (Fig. 1 B). The inflammatory infiltrate comprised neutrophils, macrophages, plasma cells, eosinophils, and lymphocytes, as described previously (16), and did not appear to differ qualitatively among the different genotypes. Since the ranges of inflammation in each treatment group were similar for the four strains and the mean gastritis scores of the groups lacking IL-4 were not different from those of the WT groups, IL-4 did not appear to play a role in the severity of H. pylori-induced gastritis.
It is noteworthy that the presence or absence of endogenous IL-4 did not alter the level of colonization or severity of the gastric inflammation in our U/C mice. There are conflicting reports concerning Helicobacter colonization and gastritis in mice lacking IL-4. In an H. felis study, IL-4−/−mice had less severe gastric inflammation but heavier colonization than the IL-4-expressing mice of the same genetic background (36). In contrast, more severe inflammation in IL-4 KO mice than in WT mice was reported 5 weeks after infection with H. pylori (44). However, our results in U/C IL-4 KO mice are consistent with those of two recent studies. Kamradt et al. found no difference in colonization of IL-4 KO mice on either the C57BL/6 or BALB/c genetic background when compared to WT mice of the same background (24). Also, Chen et al. found no differences in either colonization or gastritis between IL-4 KO or IL-4 transgenic mice and WT mice (8). The targeted mutation of IL-4 used by Chen et al. was derived from Kopf et al. (28), while the mice used in our work were derived from the mutation made by Kühn et al. (29). Thus, endogenous IL-4 does not have a measurable effect on the ability of H. pylori to colonize or cause gastric inflammation in C57BL/6 mice.
Protection from H. pylori in μMT mice after in vivo neutralization of IL-4.
One argument against using mice genetically deficient in a specific cytokine to determine the role of that cytokine is that mice lacking a cytokine since birth may have developed alternate pathways not normally used in cytokine-replete mice. As an alternative approach to study the requirement for IL-4 in protection from H. pylori, μMT mice were depleted of IL-4 during immunization or during challenge with anti-IL-4 monoclonal antibody. Neutralizing anti-IL-4 and irrelevant isotype control antibodies (clones 11B11 and Y13-259, respectively; American Type Culture Collection, Manassas, Va.) were purified from culture supernatant with a protein G column and tested for antigen specificity by a modified IL-4 ELISA. Three groups of six μMT mice were immunized intranasally four times at weekly intervals with 100 μg of H. pylori sonicate and 5 μg of cholera toxin. Twenty-four hours prior to each immunization, one group received 2 mg of anti-IL-4 intraperitoneally, and one group was treated with the irrelevant rat IgG1. The remaining group of immunized mice was treated with anti-IL-4 24 h before challenge with H. pylori and on postchallenge days 6, 13, and 20. Six unimmunized mice were given the control rat IgG1 during the challenge period. The dose of antibody was based on a study of oral tolerance inhibition (7) and was four times that used in other published studies (20, 41). Four weeks after challenge, the stomachs were quantitatively cultured as described above.
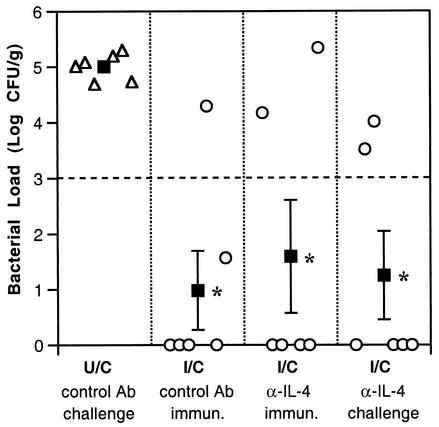
Each group of immunized μMT mice had a mean reduction in bacterial load of ≥2 logs (99% decrease) compared to the U/C mice (P ≤ 0.0015) regardless of whether anti-IL-4 was administered during immunization or challenge (Fig. 2). Neither anti-IL-4-treated group was significantly different from the group that received the control rat IgG during immunization.
FIG. 2.
Depletion of IL-4 during immunization or challenge does not block protective immunity in B-cell-deficient mice. Open symbols show bacterial colonization in individual mice, and solid squares indicate the geometric mean ± standard error. The threshold for protection (dashed line) is a 2-log decrease from the mean of the U/C mice. Control or anti-IL-4 antibodies (Ab) were administered during immunization (immun.) or challenge periods. Colonization in the three groups of immunized mice is significantly less than that in unimmunized mice, but the three immunized groups are not different from one another. *, P < 0.005.
Vaccine-induced protection in mice genetically deficient in IL-4 and in IL-4-depleted μMT mice indicates that IL-4 is not essential for reduction of bacterial load. Our data are consistent with reports in mice lacking the IL-4 receptor α (IL-4Rα) chain (1, 33). BALB/c mice deficient in IL-4Rα and unable to respond to IL-4 and IL-13 had approximately 1-log reductions in H. pylori colonization after immunization with either urease plus cholera toxin or a recombinant Salmonella enterica strain expressing urease (1). Also, adoptive transfer of H. pylori urease-specific CD4+ T cells into IL-4Rα−/− mice was followed by a reduction of bacterial load that was similar in magnitude to the reduction following transfer of cells into WT BALB/c mice, indicating that signaling by IL-4 and IL-13 was not required for reduction in bacterial load (33). Finally, a recent report with orally immunized IL-4 KO mice also showed a reduction in bacterial load (2).
The finding that IL-4 is not required for protection is not irreconcilable with previous studies. CT does induce IL-4 production by splenocytes (34, 48), but protection of IL-4−/− mice following immunization with CT and sonicate demonstrates that the adjuvant effect of CT is not solely dependent on IL-4 induction. In addition to Th2 cytokines, CT induces IL-2, IFN-γ, and IL-6 (9, 22, 30). In the therapeutic immunization study by Saldinger et al., the increased IL-4 production by spleen cells was most likely due to the use of CT, but clearance of H. felis, although occurring at the same time, was not necessarily a result of IL-4 production (43). The fact that IL-4 is not essential for protection does not mean that a Th2 polarized response cannot be protective. The adoptive transfer of a Th2 cell line decreased bacterial load in mice infected with H. felis (36). Also, mice immunized with H. pylori antigens and alum (AlOH) showed a Th2 response (greater numbers of IL-5-producing T cells than IFN-γ-positive cells) and were protected from H. pylori infection (17). Mixed Th1 and Th2 responses have elicited protection from H. pylori (18, 19), and induction of a Th1 response with complete Freund's adjuvant protected mice from both H. pylori and H. felis (17).
Vaccine-induced protection in IL-5-deficient mice.
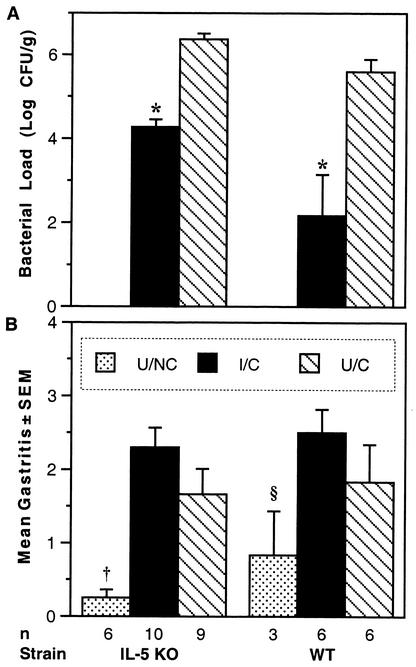
Although IL-5 is not necessary for the development of a Th2 response, it is secreted by Th2 cells and activated mast cells. Possibly, IL-5 could play a role in vaccination-induced clearance of H. pylori by inducing differentiation and activation of eosinophils. IL-5-deficient mice have low basal levels of eosinophils, but they failed to develop eosinophilia when infected with a helminth parasite (27). To study the requirement for IL-5 in H. pylori clearance, we immunized and challenged IL-5 KO mice donated by Eric Pearlman (Case Western Reserve University). Since the IL-5 KO mice were evaluated at the same time as IFN-γ KO and IL-12 KO mice in a related study, they share the same WT controls previously reported (15). Mice lacking IL-5 were protected by intranasal immunization (Fig. 3 A). The mean bacterial load in the I/C IL-5 KO mice was ≥2 logs lower that of the U/C IL-5 KO mice (P = 0.0005). In the same experiment, WT mice were also protected by immunization (P < 0.0001). The bacterial load in the U/C IL-5 KO mice was higher than that in corresponding U/C WT group, and the colonization in the I/C IL-5 KO mice was higher than the level in the I/C WT group (P = 0.0014). One possible explanation for higher colonization in IL-5 KO mice than in WT mice is that endogenous IL-5 may suppress colonization. However, genetic background can influence colonization, and the WT mice were from Harlan (Indianapolis, Ind.), while the IL-5 KO mice were derived from C57BL/6 mice in Germany and had been interbred for many generations. The fact that immunized IL-5 KO mice reduced their bacterial load by >2 logs indicates that IL-5 is not essential for protection, although it could still play a roll.
FIG. 3.
IL-5 KO mice were protected 4 weeks after challenge. (A) Colonization by H. pylori is expressed as the geometric mean ± standard error. Both immunized groups showed a >2-log decrease from their respective U/C group. *, P ≤ 0.0005. (B) Gastritis was not statistically different in the I/C and U/C groups. Untreated IL-5 KO mice had significantly lower scores than I/C and U/C groups of both strains, and untreated WT mice had less inflammation than both I/C groups. †, P < 0.006; §, P ≤ 0.02.
The mean gastritis scores of the I/C and U/C treatment groups were not statistically different (Fig. 3B). Also, challenged IL-5 KO and WT mice did not have significantly different levels of gastric inflammation, but both had more inflammation than U/NC mice. Eosinophils were counted at the corpus-antrum junction in hematoxylin-and-eosin-stained sections (data not shown). Within each treatment group, the IL-5 KO mice had fewer eosinophils than the WT mice. The number of eosinophils correlated more closely with the level of inflammation than with the bacterial load, but for similar gastritis scores or similar reductions in bacteria, WT mice had five- to sixfold more eosinophils than IL-5 KO mice. These results demonstrated that IL-5 was not essential for development of gastritis and suggested that eosinophils were not major effector cells in H. pylori clearance.
In summary, many immunization regimens can induce protection against H. pylori in mice. The mechanism of protection remains unknown. Here we have demonstrated that a Th2 response is not necessary for protection and that protection in the absence of IL-4 is not dependent on antibodies or vice versa. In related studies, we have found that proinflammatory cytokines are associated with protection and that IL-12 p40 appears to be important in the development of protective immunity (15). Hopefully, elucidation of the mechanisms of protection and identification of effector cells in the mouse model may yield clues for modulating the nonprotective immune response in human H. pylori infection.
Acknowledgments
This work was supported in part by Public Health Service grants CA-73515 (C.A.G.), DK-46461 (S.J.C. and J.G.N.), AI-40701 (J.G.N.), and AI-36359 (J.G.N. and S.J.C.). F.P.H. is supported by grants AI-45602 and AI-35979 and by the VA Medical Research Service.
We thank Steven Emancipator, Raymond Redline, and Abram Stavitsky for advice and many helpful discussions. We appreciate the assistance of Thomas Blanchard and Brandon Zagorsky in preparing the monoclonal antibodies and Howard Carr for quantitative culture in the IL-4 neutralization experiment. We thank Mary Anne O'Riordan for consultation on the statistical analysis of the data.
Editor: F. C. Fang
REFERENCES
- 1.Aebischer, T., S. Laforsch, R. Hurwitz, F. Brombacher, and T. F. Meyer. 2001. Immunity against Helicobacter pylori: significance of interleukin-4 receptor α chain status and gender of infected mice. Infect. Immun. 69:556-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 3.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 4.Beckmann, M. P., K. A. Schooley, B. Gallis, T. Vanden Bos, D. Friend, A. R. Alpert, R. Raunio, K. S. Prickett, P. E. Baker, and L. S. Park. 1990. Monoclonal antibodies block murine IL-4 receptor function. J. Immunol. 144:4212-4217. [PubMed] [Google Scholar]
- 5.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell. Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 161:626-633. [DOI] [PubMed] [Google Scholar]
- 7.Burstein, H. J., and A. K. Abbas. 1993. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J. Exp. Med. 177:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, W., D. Shu, and V. S. Chadwick. 1999. Helicobacter pylori infection in interleukin-4-deficient and transgenic mice. Scand. J. Gastroenterol. 34:987-992. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, C. J., A. D. Wilson, N. A. Williams, and C. R. Stokes. 1991. Mucosal priming of T-lymphocyte responses to fed protein antigens using cholera toxin as an adjuvant. Immunology 72:323-328. [PMC free article] [PubMed] [Google Scholar]
- 10.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 11.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 12.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615-640. [DOI] [PubMed] [Google Scholar]
- 14.Garhart, C. A. 2002. Ph.D. thesis. Case Western Reserve University, Cleveland, Ohio.
- 15.Garhart, C. A., F. P. Heinzel, S. J. Czinn, and J. G. Nedrud. 2003. Vaccine-induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent, but gamma interferon and inducible nitric oxide synthase independent. Infect. Immun. 71:910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garhart, C. A., R. W. Redline, J. G. Nedrud, and S. J. Czinn. 2002. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottwein, J. M., T. G. Blanchard, O. S. Targoni, J. C. Eisenberg, B. M. Zagorski, R. W. Redline, J. G. Nedrud, M. Tary-Lehmann, P. V. Lehmann, and S. J. Czinn. 2001. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J. Infect. Dis. 184:308-314. [DOI] [PubMed] [Google Scholar]
- 18.Guy, B., C. Hessler, S. Fourage, J. Haensler, E. Vialon-Lafay, B. Rokbi, and M.-J. Q. Millet. 1998. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine 16:850-856. [DOI] [PubMed] [Google Scholar]
- 19.Guy, B., C. Hessler, S. Fourage, B. Rokbi, and M.-J. Q. Millet. 1999. Comparison between targeted and untargeted systemic immunizations with adjuvanted urease to cure Helicobacter pylori infection in mice. Vaccine 17:1130-1135. [DOI] [PubMed] [Google Scholar]
- 20.Heinzel, F., M. Sadick, B. Holaday, R. Coffman, and R. Locksley. 1989. Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzel, F. P., R. M. Rerko, F. Ahmed, and E. Pearlman. 1995. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J. Immunol. 155:730-739. [PubMed] [Google Scholar]
- 22.Hornquist, E., and N. Lycke. 1993. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur. J. Immunol. 23:2136-2143. [DOI] [PubMed] [Google Scholar]
- 23.Ibraghimov, A., and J. Pappo. 2000. The immune response against Helicobacter pylori—a direct linkage to the development of gastroduodenal disease. Microbes Infect. 2:1073-1077. [DOI] [PubMed] [Google Scholar]
- 24.Kamradt, A. E., M. Greiner, P. Ghiara, and S. H. Kaufmann. 2000. Helicobacter pylori infection in wild-type and cytokine-deficient C57BL/6 and BALB/c mouse mutants. Microbes Infect. 2:593-597. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura, D., J. Roes, R. Kühn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 26.Kleanthous, H., G. A. Myers, K. M. Georgakopoulos, T. J. Tibbitts, J. W. Ingrassia, H. L. Gray, R. Ding, Z.-Z. Zhang, W. Lei, R. Nichols, C. K. Lee, T. H. Ermak, and T. P. Monath. 1998. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect. Immun. 66:2879-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopf, M., F. Brombacher, P. D. Hodgkin, A. J. Ramsay, E. A. Milbourne, W. J. Dai, K. S. Ovington, C. A. Behm, G. Kohler, I. G. Young, and K. I. Matthaei. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15-24. [DOI] [PubMed] [Google Scholar]
- 28.Kopf, M., G. Le Gros, M. Bachmann, M. C. Lamers, H. Bluethmann, and G. Kohler. 1993. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 362:245-248. [DOI] [PubMed] [Google Scholar]
- 29.Kühn, R., K. Rajewsky, and W. Müller. 1991. Generation and analysis of interleukin-4 deficient mice. Science 254:707-710. [DOI] [PubMed] [Google Scholar]
- 30.Kurono, Y., M. Yamamoto, K. Fujihashi, S. Kodama, M. Suzuki, G. Mogi, J. R. McGhee, and H. Kiyono. 1999. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J. Infect. Dis. 180:122-132. [DOI] [PubMed] [Google Scholar]
- 31.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 32.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A.-M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas, B., D. Bumann, A. Walduck, J. Koesling, L. Develioglu, T. F. Meyer, and T. Aebischer. 2001. Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect. Immun. 69:1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, K. Fujihashi, and J. R. McGheee. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 35.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant TH1 phenotype and promote a DTH response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 36.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. Czinn. 1997. Murine CD4 T cell responses to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113:1848-1857. [DOI] [PubMed] [Google Scholar]
- 37.Mohammadi, M., R. Redline, J. Nedrud, and S. Czinn. 1996. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect. Immun. 64:238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okahashi, N., M. Yamamoto, J. L. Vancott, S. N. Chatfield, M. Roberts, H. Bluethmann, T. Hiroi, H. Kiyono, and J. R. McGhee. 1996. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect. Immun. 64:1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 40.Radcliff, F. J., S. L. Hazell, T. Kolesnikow, C. Doidge, and A. Lee. 1997. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect. Immun. 65:4668-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizzo, L. V., R. A. Morawetz, N. E. Miller-Rivero, R. Choi, B. Wiggert, C. C. Chan, H. C. Morse III, R. B. Nussenblatt, and R. R. Caspi. 1999. IL-4 and IL-10 are both required for the induction of oral tolerance. J. Immunol. 162:2613-2622. [PubMed] [Google Scholar]
- 42.Sakagami, T., M. Dixon, J. O'Rourke, R. Howlett, F. Alderuccio, J. Vella, T. Shimoyama, and A. Lee. 1996. Atrophic gastric changes in both H. felis and H. pylori infected mice are host dependent and separate from antral gastritis. Gut 39:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saldinger, P., N. Porta, P. Launois, J. Louis, G. Waanders, H. Bouzourene, P. Michetti, A. Blum, and I. Corthesy-Theulaz. 1998. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology 115:891-897. [DOI] [PubMed] [Google Scholar]
- 44.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 45.Sutton, P., J. Wilson, T. Kosaka, I. Wolowczuk, and A. Lee. 2000. Therapeutic immunization against Helicobacter pylori infection in the absence of antibodies. Immunol. Cell Biol. 78:28-30. [DOI] [PubMed] [Google Scholar]
- 46.Vajdy, M., M. H. Kosco-Vilbois, M. Kopf, G. Kohler, and N. Lycke. 1995. Impaired mucosal immune responses in interleukin 4-targeted mice. J. Exp. Med. 181:41-53. [DOI] [PubMed] [Google Scholar]
- 47.Warren, J. R. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i:1273. [PubMed]
- 48.Xu-Amano, J., H. Kiyono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai, and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 178:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, and J. Imanishi. 1996. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology 110:1744-1752. [DOI] [PubMed] [Google Scholar]