Abstract
Dendritic cells (DCs) discriminate different microbial pathogens and induce T-cell responses of appropriate effector phenotypes accordingly. Microbial recognition and differentiation are mediated in part by pattern recognition receptors such as Toll-like receptors (TLRs), whereas the development of T-cell effector functions is critically dependent on DC-derived cytokines such as interleukin-12 (IL-12) and IL-10. However, it is not entirely clear to what extent various microbial TLR activators could induce different functional states of DCs that favor different T-cell effector phenotypes. Toward a better understanding of this issue, we examined IL-10 and IL-12 production and T-cell-polarizing potentials of murine bone marrow-derived DCs after stimulation by three microbial TLR activators, namely, lipopolysaccharide (LPS), peptidoglycan (PGN), and zymosan. We found that the three stimuli induced drastically different profiles of IL-10 and IL-12 production in DCs. Further, these stimuli differentially conditioned CD40-dependent IL-10 and IL-12 production by DCs. Finally, LPS-, PGN-, and zymosan-stimulated DCs primed distinct T-cell cytokine profiles. Our results support the notion that microbe-specific information sensed through different TLRs by DCs is linked to differential Th priming through DC-derived cytokines.
To detect microbial infection, the immune system utilizes pattern recognition receptors such as Toll-like receptors (TLRs) to recognize invariant molecular structures of related microbes (8, 16). TLR activation results in rapid induction of innate defense programs and ultimately the initiation of adaptive immunity (2). Dendritic cells (DCs) are critically involved in this process. Upon stimulation by microbial TLR ligands, DCs undergo a maturation process characterized by upregulation of major histocompatibility complex and costimulatory molecules and by homing to the secondary lymphoid organ. When matured, DCs are potent antigen-presenting cells able to prime naive T cells and direct T-cell differentiation (4). Therefore, TLRs expressed by DCs constitute a critical link between pathogen recognition and the induction of T-cell immunity (3, 17).
The efficient control of microbial infections not only requires immune activation upon pathogen invasion but also demands the generation of appropriate types of immune responses tailored to a particular group of pathogens. For example, certain infections require Th1 responses, whereas others may be best countered by Th2 immunity (1, 27). Accumulating evidence indicates that DCs can shape the Th1-Th2 balance according to the outcome of their microbial interactions (18, 25). DCs achieve this at least in part through differential production of interleukin-10 (IL-10) and IL-12 (14), since IL-10 is implicated in priming Th2 responses (10, 30) while IL-12 potently induces gamma interferon (IFN-γ)-producing Th1 cells (32). However, it is not clear how the stimulation of various TLRs by microbes is connected to this process. Specifically, do microbial TLR activators differentially stimulate IL-10 and IL-12 production from DCs? Interestingly, a recent study shows that Escherichia coli lipopolysaccharide (LPS) and Porphyromonas gingivalis LPS, being TLR4 and TLR2 agonists, respectively, induce distinct profiles of inflammatory genes in murine macrophages (7). Furthermore, the two types of LPS differentially stimulate DC IL-12 production and have diverse impacts on Th differentiation in vivo (22). Thus, triggering different TLRs by various microbial stimuli might drive DCs to assume distinct phenotypes and functions. To further investigate this issue, we examined DC interactions with three microbial TLR stimuli: a TLR4 agonist, gram-negative bacterial LPS (9, 21), and two TLR2 agonists—gram-positive bacterial peptidoglycan (PGN) and yeast zymosan (31, 33). Specifically, we examined IL-10 and IL-12 production by DCs in response to these stimuli and tested their abilities to polarize T-cell responses.
MATERIALS AND METHODS
Mice.
Female BALB/c (H-2d) mice and C57BL/6 (H-2b) mice (6 weeks old) were purchased from Jackson Laboratory (Bar Harbor, Maine) and maintained under specific-pathogen-free conditions. All experimental protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston).
Reagents and antibodies.
LPS (Salmonella enterica serovar Typhimurium) and PGN (Staphylococcus aureus), monensin, phorbol myristate acetate, ionomycin, and saponin were purchased from Sigma (St. Louis, Mo.). Zymosan A from Saccharomyces cerevisiae was purchased from Molecular Probes (Eugene, Oreg.). For intracellular staining of T-cell cytokines, phycoerythrin-conjugated anti-IL-4 (BVD4-1D11), anti-IL-10 (JES5-16E3), anti-IFN-γ (XMG1.2), and their respective isotype-matched control antibodies were purchased from BD Pharmingen (San Diego, Calif.). Tri-Color-conjugated anti-CD4 (TC-CD4) was from Caltag (Burlingame, Calif.).
DC culture.
Bone marrow-derived DCs (BM-DCs) were generated as previously described (15, 23) with certain modifications. Briefly, BM cells were cultured in a petri dish (Fisher Scientific, Houston, Tex.) at 2 × 106 per 10 ml of 10% fetal bovine serum-supplemented Iscove’s modified Dulbecco medium. Culture supernatants of J558L cells that had been transfected with the murine gm-csf gene were used as the source of granulocyte-macrophage colony-stimulating factor (the transfected cell line was a kind gift from Charles Janeway, Yale University). Nonadherent cells were harvested at day 7 and further cultured in a six-well plate overnight. Resultant nonadherent cells were typically >80% CD11c+ cells as judged by fluorescence-activated cell sorting (FACS) analysis. Sometimes, CD11c+ cells were directly purified from a day 7 culture to >95% purity with microbeads according to the manufacturer's protocol (Miltenyi Biotec, Auburn, Calif.).
Microbial stimulation and anti-CD40 treatment of DCs.
DCs were stimulated with different concentrations of LPS, PGN, or zymosan in 96-well plates at 1.25 × 105 in 200 μl or in 24-well plates at 6.25 × 105 in 0.5 ml. The stimulation culture was not supplemented with any cytokines including granulocyte-macrophage colony-stimulating factor. For some experiments, an agonistic anti-CD40 antibody (FGK45 [26]) was added, together with microbial stimuli, and supernatants were harvested 24 h later. For other experiments, DCs were stimulated with microbial stimuli for 12 h, washed twice, and then cultured in the presence of anti-CD40 antibody for additional 24 h before the supernatants were harvested. The anti-CD40 antibody was used in the form of FGK45 hybridoma supernatants at a 1:10 dilution, an optimal titration as determined previously (23). For the T-cell priming assay, DCs were harvested at 12 h, washed twice with complete medium, and then cocultured with T cells. Aliquots of these DCs were used to isolate total RNA with Tri-Reagent (Sigma) for measuring mRNA levels of IL-10 and IL-12p40 (see below).
Isolation of CD4+ CD45RBhigh T cells and T-DC coculture.
For each isolation, three to five female C57BL/6 mice were used to minimize individual variation. Total CD4+ T cells were purified from pooled spleens and lymph nodes with Dynabeads mouse CD4 and DETACHaBEAD (Dynal, Inc., Lake Success, N.Y.) to >99% purity as assayed by FACS. The CD45RBhigh fraction was then purified from the total CD4+ T-cell preparation as previously described with certain modifications (5). Briefly, cells were consecutively labeled with biotin-CD45RB monoclonal antibody (BD Pharmingen) and streptavidin-conjugated microbeads and then passed through a positive-selection column on a magnetic separator (Miltenyi Biotec). The unbound CD45RBlow fraction was discarded. The bound fraction was eluted and reapplied to a new column and the column-retained fraction was harvested as a CD45RBhigh fraction. CD4+ CD45RBhigh T cells (2 × 105/well), together with 2 × 104 DCs that were stimulated for 12 h, were seeded into 96-well plates in 200 μl of Iscove’s modified Dulbecco’s medium supplemented with 1 ng/ml of recombinant IL-2 (BD Pharmingen). The T-DC coculture was maintained in 96-well plates for 4 days, transferred to 24-well plates with supplementation of 0.5 ml of plain medium, and further cultured for an additional 6 days. Primed T cells were then assayed for the cytokine profile by intracellular staining after restimulation with 20 ng of phorbol myristate acetate/ml, 500 ng of ionomycin/ml, and 2 μM monensin for 5 h.
Cytokine assays.
To measure the levels of IL-10, IL-12p70, and TNF-α in DC cultures, enzyme-linked immunosorbent assay was performed with OptEIA Kits (BD Pharmingen). The multiprobe template set mCK2b from RiboQuant RPA system (BD Pharmingen) was used to measure mRNA levels of IL-10 and IL-12p40 in an RNase protection assay according to the manufacturer's instruction. To enumerate IL-4, IL-10, and IFN-γ producers in T cells primed by DCs, cells were first stained for CD4 and then fixed, permeablized, and stained with fluorochrome-conjugated monoclonal antibodies specific for cytokines. Stained cells were analyzed on a FACScan flowcytometer (BD Biosciences, Franklin Lakes, N.J.). Data were analyzed with the FlowJo software (TreeStar, San Carlos, Calif.).
RESULTS AND DISCUSSION
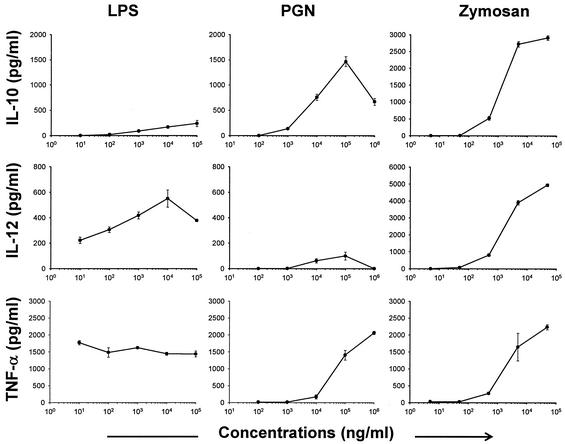
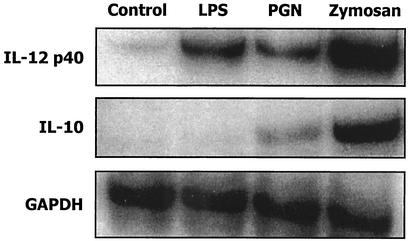
To examine potential differences of LPS, PGN, and zymosan in their stimulatory effects on DCs, efforts were first focused on DC production of IL-10 and IL-12. DCs were stimulated for 24 h, and cytokine concentrations in supernatants were measured. As shown in Fig. 1, when tested over a 10,000-fold dose range, LPS induced low-level IL-10 but high-level IL-12p70 production. In contrast, DCs exposed to PGN produced low levels of IL-12 but high levels of IL-10. Another profile was observed in zymosan-exposed DCs: high levels of both IL-10 and IL-12. This observation suggests that LPS, PGN, and zymosan have inherently distinct abilities to induce DC IL-10 and IL-12 production. Alternatively, this phenomenon might simply reflect different sensitivities of DCs to these microbial stimuli, since the molar concentrations of actual TLR-engaging ligands in these stimuli are not known. However, even at the highest concentration tested, LPS did not induce IL-10 to a level comparable to that induced by PGN or zymosan, whereas PGN failed to induce IL-12 to a level comparable to LPS or zymosan. This in fact argues for the first possibility. To further differentiate the two, we tested tumor necrosis factor alpha (TNF-α) induction in DCs exposed to LPS, PGN, and zymosan. Because all microbial TLR activators trigger the NF-κB activation leading to TNF-α production (16), different levels of TNF-α induction would reflect different DC sensitivities to these stimuli. As shown in the lower panel of Fig. 1, although LPS was the most efficient TNF-α inducer at lower concentrations, PGN and zymosan were able to stimulate similar levels of TNF-α at higher concentrations. Thus, at concentrations at which comparable levels of TNF-α were stimulated, LPS, PGN, and zymosan induced distinct profiles of IL-10 and IL-12. This was further confirmed with sorted DCs (>95% CD11c+) to exclude potential effects of contaminating non-DCs in the BM-DC preparation (data not shown). Finally, RNase protection assay was used to test levels of IL-10 and IL-12p40 mRNA in DCs stimulated with 1 μg of LPS, 10 μg of PGN, or 5 μg of zymosan/ml. At these respective concentrations, LPS, PGN, and zymosan induced similar levels of TNF-α (∼1,500 pg/ml, Fig. 1). As shown in Fig. 2, zymosan-exposed DCs expressed the highest level of IL-10 mRNA, followed by PGN-exposed DCs. The IL-10 level was not detectable in LPS-exposed DCs by this assay. For IL-12p40, zymosan-exposed DCs remained to be the highest producer, followed by LPS- and PGN-exposed DCs. Of note, all of the experiments described above were also done with C57BL/6 DCs with similar results obtained (data not shown). Thus, the three microbial TLR activators are inherently different in their abilities to induce IL-10 and IL-12 production from murine DCs.
FIG. 1.
Cytokine production by LPS-, PGN-, and zymosan-stimulated DCs. BM-derived BALB/c DCs were stimulated with indicated concentrations of LPS, PGN, or zymosan, and 24-h supernatants were harvested to measure IL-10, IL-12p70, and TNF-α by enzyme-linked immunosorbent assay. None of these cytokines was measurable (<15 pg/ml) in untreated DCs (not shown). Representative results of five independent experiments for IL-10 and IL-12 and two for TNF-α are shown. Data are presented as means ± standard deviations.
FIG. 2.
IL-10 and IL-12p40 mRNA levels in LPS-, PGN-, and zymosan-stimulated DCs. DCs were stimulated for 12 h with 1 μg of LPS, 10 μg of PGN, or 5 μg of zymosan/ml, and then total RNA was isolated and subjected to RNase protection assay with the RiboQuant multiprobe template set mCK2b. The bands corresponding to IL-12p40, IL-10, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) are shown. The data represent two independent experiments.
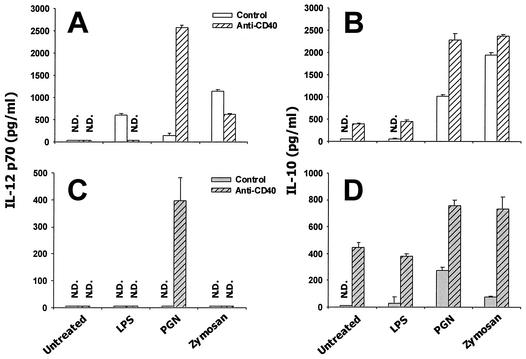
Upon interactions with T cells, microbe-exposed DCs may be further triggered to produce cytokines that are dependent on T-cell-derived signals. Conceivably, such cytokine production would significantly contribute to the cytokine milieu controlling the outcome of T-cell differentiation (19). Previous studies have shown that IL-12 production by DCs can be augmented upon ligation of their CD40 receptors by T-cell-derived CD40 ligands, which can be mimicked by using agonistic anti-CD40 antibodies (6, 12, 28). Therefore, given the result that LPS, PGN, and zymosan trigger distinct programs of innate cytokine production (Fig. 1 and 2), we further tested the impact of CD40 ligation on IL-10 and IL-12 production by DCs exposed to these stimuli. An agonistic anti-CD40 antibody (clone FGK45 [26]) was used to engage CD40 receptors on DCs. DCs that were stimulated with LPS, PGN, or zymosan were treated with the anti-CD40 antibody either immediately (Fig. 3A and B) or after being washed after a 12-h microbial stimulation (Fig. 3C and D). The 12-h time point was chosen because the initial wave of microbe-induced cytokine production was largely completed by this time (unpublished data), a finding similar to what was previously reported for human monocyte-derived DCs (13). As shown in Fig. 3A, LPS- or zymosan-induced IL-12p70 production was significantly reduced when DCs were simultaneously activated with anti-CD40. In sharp contrast, whereas PGN by itself did not induce a high level of IL-12p70, simultaneous triggering of CD40 led to an approximately 20-fold increase in IL-12p70 production. When DCs were stimulated with these stimuli for 12 h, washed, and then cultured for additional 24 h in the presence of anti-CD40 antibody, only PGN-stimulated DCs produced a significant level of IL-12p70 (Fig. 3C). Clearly, PGN is distinguished from LPS and zymosan with its ability of conditioning DCs to produce a high level of IL-12p70 in response to CD40 engagement. Of note, applied either immediately or 12 h after microbial stimulation, the anti-CD40 antibody treatment augmented IL-10 production by murine BM-DCs regardless of microbial stimuli used (Fig. 3B and D). Further, in the absence of microbial costimulation, these DCs produced IL-10 but not IL-12p70 in response to CD40 engagement (Fig. 3). This result is in contrast to what was described for human monocyte-derived DCs (6) but is in agreement with observations made with isolated murine myeloid DCs (11). Taken together, these results suggest that microbial TLR activators such as LPS, PGN, and zymosan differentially modulate the potential of DCs to produce IL-12 in response to a T-cell-derived signal.
FIG. 3.
IL-10 and IL-12p70 production in microbe-exposed DCs in response to CD40 ligation. (A and B) DCs were stimulated for 24 h with 1 μg of LPS, 10 μg of PGN, or 5 μg of zymosan/ml, together with an anti-CD40 antibody (▨) or control rat immunoglobulin G (□). (C and D) DCs were stimulated with microbial stimuli for 12 h, washed, and then further cultured for 24 h in the presence of the anti-CD40 antibody (▨) or control antibody (□) At the end of these culture periods, supernatants were harvested to measure IL-12p70 (A and C) and IL-10 (B and D). N.D., not detectable.
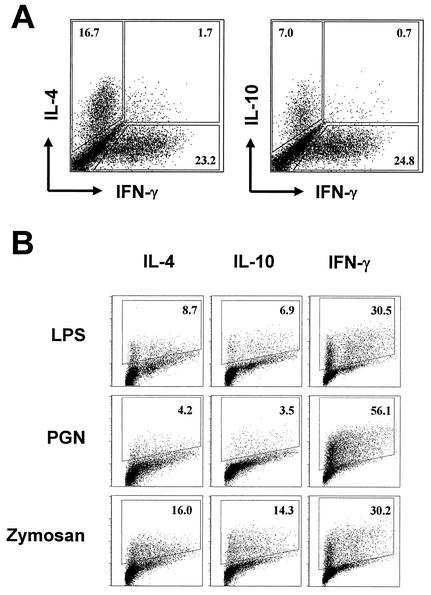
To test whether LPS-, PGN-, and zymosan-conditioned DCs would prime for distinct Th phenotypes in vitro, we used a mixed-leukocyte reaction, in which BALB/c DCs (H-2d) were used to activate C57BL/6 CD4+ T cells (H-2b). To minimize the influence of antigen-experienced memory cells, purified naive T cells (as defined by CD45RBhigh [see reference 5]) were used. In the absence of microbial stimulation, DCs primed T cells to exhibit a mixed cytokine profile as assayed by intracellular staining 10 days after the onset of coculture. As shown in Fig. 4A, ca. 23% the cells produced IFN-γ, whereas IL-4 and IL-10 producers were 17 and 7%, respectively. A negligible fraction of cells simultaneously produced IFN-γ and IL-4 or IFN-γ and IL-10. As shown in Fig. 4B, LPS exposure rendered DCs to prime much less IL-4 but more IFN-γ producers. PGN stimulation significantly diminished DC potentials to prime T cells producing IL-4 and IL-10 but evidently enhanced their ability to prime IFN-γ producers. Interestingly, while inducing more IFN-γ-producing T cells, zymosan-exposed DCs primed as many IL-4 producers and even more IL-10 producers compared to the untreated counterparts. When tested at stimulating concentrations that were 10-fold higher or 10-fold lower than those presented in Fig. 4, the three stimuli still exhibited similar differences in their relative potencies to polarize T-cell responses (data not shown). Importantly, PGN was the strongest Th1-favoring stimulus, a finding consistent with the finding that it potently potentiated DCs to produce high levels of CD40-dependent IL-12p70 (Fig. 3). Together, these results reveal that LPS, PGN, and zymosan differentially condition DCs to prime Th effector phenotypes, suggesting that distinct microbial TLR agonists can be a cue that DCs sense in order to differentially direct Th effector development.
FIG. 4.
In vitro priming of distinct Th effector phenotypes by LPS-, PGN-, and zymosan-stimulated DCs. BALB/c DCs were left untreated (A) or were stimulated with 1 μg of LPS, 10 μg of PGN, or 5 μg of zymosan/ml for 12 h (B) and then cocultured with 2 × 105 C57BL/6 CD4+ CD45RBhigh T cells (DC/T ratio = 1:10). T cells were harvested 10 days later to enumerate cytokine-producing cells after brief restimulation. (A) T cells primed by untreated DCs were double stained for IFN-γ and IL-4 or for IFN-γ and IL-10. (B) T cells primed by microbe-exposed DCs were stained for individual cytokines separately. Gates were drawn based on isolate controls for individual specific antibodies. The y axis is the fluorescent intensity in log10 scale, whereas the x axis is the linear-scale forward scatter. The data represent five independent experiments.
Results presented in this study have provided new information to our understanding of DC activation by microbial TLR ligands. Previously, using LPS as TLR4 and PGN as TLR2 ligand, Re and Strominger showed that human monocyte- derived DCs preferentially expressed IL-12 or IL-10 when stimulated through TLR4 or TLR2, respectively (24). Our study reported herein has confirmed their finding with murine DCs. Importantly, we tested the stimulants over a wide 10,000-fold dose range and with TNF-α as an internal control, and we still found LPS was a much stronger IL-12 inducer but weaker IL-10 stimulator than PGN (Fig. 1). This result strongly suggests that signaling programs induced by TLR4 and TLR2 are qualitatively rather than quantitatively different in DCs. This point is further supported by the interesting finding that simultaneous anti-CD40 treatment suppressed IL-12 production by DCs stimulated with LPS but enhanced IL-12 production by PGN-stimulated DCs (Fig. 3A). Interestingly, a recent study showed that as a immune-evading strategy, Yersinia pestis induced IL-10 through a TLR2-dependent pathway (29). This is very much in agreement with our observation that two TLR2 ligands, PGN and zymosan, directly induced high levels of IL-10 production from DCs (Fig. 1 and 2). The difference between TLR2 nd TLR4 signaling is probably not limited to DCs, as Hirschfeld et al. studied macrophage responses and found that TLR4 agonists were more potent in inducing proinflammatory cytokines and chemokines than TLR2 ligands (7). Collectively, these studies suggest that DCs may be induced to assume distinctive functions through activation of TLR4 or TLR2 and that TLR4 activation factors a stronger proinflammatory state than TLR2 activation. On the other hand, as TLR2 receptor has a large number of known ligands (16), all TLR2 ligands are not necessarily equal. For example, PGN and Zymosan, both being able to engage TLR2 and possibly TLR6 (20), dramatically differed in the ability to induce DC production of IL-12 (Fig. 1). We speculate that additional TLRs or other receptors are involved in DC activation by zymosan.
While activation through different TLRs leads to distinct cytokine production in DCs (Fig. 1 and 2) (24), it was not clear whether and how this would be linked to differential T-cell priming. Upon activation by microbe-exposed DCs, antigen-specific T cells rapidly upregulate CD40 ligands, which engage CD40 receptors on DCs to further modulate their cytokine production (34). Consistent with this notion, we found that the ability to produce IL-12 by LPS-, PGN-, and zymosan-stimulated DCs was significantly modulated by CD40 engagement (Fig. 3). Interestingly, LPS, PGN, and zymosan not only induced distinct cytokines in DCs directly but also differentially conditioned CD40-dependent IL-12 production by DCs. More importantly, the direct IL-12 induction by these TLR activators is not necessarily correlated with the conditioned CD40-dependent IL-12 production by DCs. For example, while failing to directly induce a high level of IL-12 (Fig. 1 and 2), PGN strongly potentiated DCs to produce this cytokine following CD40 ligation (Fig. 3C). While LPS and zymosan directly induced IL-12 from DCs (Fig. 1 and 2), they did not significantly potentiate CD40-dependent IL-12 production. Conceivably, the CD40-dependent cytokine production by microbe-exposed DCs would exert a greater impact on the outcome of T-cell differentiation than the innate cytokine response that microbes directly induce in DCs. Indeed, when tested for the ability to skew Th responses, PGN-stimulated DCs, which responded to CD40 engagement by producing a high level of IL-12 while LPS- and zymosan-stimulated DCs did not, were most potent in priming Th1 effectors (Fig. 4). Perhaps, the potential to orchestrate disparate innate and subsequent CD40-induced cytokine production provides DCs with certain flexibilities to sequentialy and differentially regulate the innate defense program and the adaptive T-cell response. On the other hand, for both of the innate and adaptive phases, differential signals channeled through various TLRs are likely to be the crucial cue.
Acknowledgments
This work was supported in part by a NIAID grant (AI43003) to L. Soong. H. Qi and T. L. Denning are supported by the James W. McLaughlin Fellowship Fund. T. L. Denning is also supported by an NIH training grant (AI07626).
We are grateful to Vivian L. Braciale for critical comments on the manuscript.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Boursalian, T. E., and K. Bottomly. 1999. Stability of naive and memory phenotypes on resting CD4 T cells in vivo. J. Immunol. 162:9-16. [PubMed] [Google Scholar]
- 6.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T-cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2001. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 10.Iwasaki, A., and B. L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki, A., and B. L. Kelsall. 2001. Unique functions of CD11b+, CD8α+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166:4884-4890. [DOI] [PubMed] [Google Scholar]
- 12.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311-316. [DOI] [PubMed] [Google Scholar]
- 14.Lanzavecchia, A., and F. Sallusto. 2001. The instructive role of dendritic cells on T-cell responses: lineages, plasticity and kinetics. Curr. Opin. Immunol. 13:291-298. [DOI] [PubMed] [Google Scholar]
- 15.Lutz, M. B., N. Kukutsch, A. L. J. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 17.Medzhitov, R., and C. A. Janeway, Jr. 1998. Innate immune recognition and control of adaptive immune responses. Semin. Immunol. 10:351-353. [DOI] [PubMed] [Google Scholar]
- 18.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 19.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275-283. [DOI] [PubMed] [Google Scholar]
- 20.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 22.Pulendran, B., P. Kumar, C. W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 24.Re, F., and J. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 25.Reis e Sousa, C., A. Sher, and P. Kaye. 1999. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr. Opin. Immunol. 11:392-399. [DOI] [PubMed] [Google Scholar]
- 26.Rolink, A., F. Melchers, and J. Andersson. 1996. The SCID but not the RAG-2 gene product is required for Sμ-Sɛ heavy chain class switching. Immunity 5:319-330. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani, S. 1994. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 12:227-257. [DOI] [PubMed] [Google Scholar]
- 28.Schulz, O., A. D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13:453-462. [DOI] [PubMed] [Google Scholar]
- 29.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stumbles, P. A., J. A. Thomas, C. L. Pimm, P. T. Lee, T. J. Venaille, S. Proksch, and P. G. Holt. 1998. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 188:2019-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 33.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 34.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2-17. [DOI] [PubMed] [Google Scholar]