Abstract
Some Lyme disease spirochete isolates can bind complement regulatory protein factor H (fH), a process that may allow evasion of complement-mediated killing. Here we demonstrate significant differences in the fH binding capabilities of species of the Borrelia burgdorferi sensu lato complex. The percentages of B. burgdorferi, B. afzelii, and B. garinii bacteria that bound fH in either enzyme-linked immunosorbent assays or affinity ligand binding immunoblot assays were 100, 83, and 29%, respectively. The fH binding protein profiles were examined and found to exhibit variability among isolates and to form two distinct classes. Differences in fH binding ability may contribute to the differences in pathogenesis and clinical course observed upon infection with different species of the B. burgdorferi sensu lato complex.
The Borrelia burgdorferi sensu lato complex is composed of closely related species, including those associated with the chronic infection Lyme disease (B. burgdorferi, B. afzelii, and B. garinii). Lyme disease spirochetes use several mechanisms for immune evasion (14, 16, 21, 24). Factor H (fH) binding to the cell surface with subsequent cleavage of C3b has been demonstrated (2), indicating that fH binding is of biological relevance and potentially important in vivo. OspE is one of several fH binding proteins (FHBPs) produced by Lyme disease spirochetes (7). In B. burgdorferi B31MI, the OspE paralogs BBL39 and BBN38 (also referred to as ErpA and ErpP, respectively) have both been demonstrated to bind fH (3, 17) in a conformation-dependent manner (17). The OspE paralogs are the only two FHBPs identified at the sequence level. The potential for OspE to interact with fH in vivo is supported by strong evidence that it is a surface protein (4, 6, 13) and expressed by spirochetes in both the tick and mammalian environments (1, 5, 16, 18, 19, 21).
Data published to date indicate that the fH binding phenotype is not universal among Lyme disease spirochete isolates and that the phenotype may correlate with individual species of the B. burgdorferi sensu lato complex (2, 11, 12, 23). The ability or inability to bind fH and cleave C3b could be an important determinant that influences the different pathogenic properties of B. burgdorferi sensu lato complex species. However, since only a limited number of isolates of each species have been analyzed to date, a conclusive correlation between fH binding and individual species has not been established. The goals of this study were to conduct a comprehensive analysis of the fH binding capabilities of B. burgdorferi sensu lato complex species to determine if a correlation between specific Borrelia species and fH binding exists.
In this study, fH binding to a group of 69 diverse Lyme disease spirochete isolates was assessed. Of these, 59 were tested with an enzyme-linked immunosorbent assay (ELISA) format (17). Briefly, cells were immobilized in microtiter plate wells and incubated (4°C, 15 h) with human fH (hfH; 10 ng μl−1; Calbiochem), goat anti-fH serum was added (Calbiochem; 1:800; 4 h, 4°C), and binding was detected by incubation (1 h, 4°C) with horseradish peroxidase-conjugated rabbit anti-goat immunoglobulin G (1:40,000; Calbiochem). All assays were conducted in triplicate. The percentages of B. burgdorferi, B. afzelii, and B. garinii isolates that bound hfH by this approach were 100% (22 of 22), 46% (5 of 11), and 31% (5 of 16), respectively (Fig. 1; Table 1). Regarding other species of the B. burgdorferi sensu lato complex, one or more isolates of B. valaisiana, B. japonica, B. turdi, and B. tanukii bound fH while B. andersonii, B. bissettii, and B. miyamotoi isolates did not. Recombinant BBL39, an OspE paralog of isolate B31MI and a demonstrated FHBP (3, 7, 10, 17), served as the positive control and bound fH at a high level. When hfH was omitted from the ELISAs (Fig. 1), fH-Borrelia complexes were still detected with some isolates. This likely results from binding of endogenous fH present in the goat anti-fH sera used in the binding assay. To verify this, a 1-μl aliquot of the goat anti-fH antiserum was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), immunoblotted, and screened with goat anti-hfH sera. An immunoreactive band of ∼150 kDa, consistent with the size of fH, was detected, demonstrating that fH is present in the antiserum and recognized by the anti-human fH antibodies (data not shown). However, a possible alternative interpretation of the ELISA data presented above is that the anti-hfH serum cross-reacted with the cells in an fH-independent fashion. This issue has been addressed in a separate study in which hfH binding directly to borreliae was demonstrated with an hfH monoclonal antibody (17). Lastly, Alitalo and colleagues unequivocally demonstrated the direct binding of hfH to some Borrelia isolates with radiolabeled fH (2).
FIG. 1.
Whole-cell ELISA analysis of fH binding by B. burgdorferi sensu lato complex isolates. All of the methods used are briefly described in the text and presented in detail in reference 17. Bacteria were harvested, gently washed with PBS, and immobilized in triplicate in the wells of microtiter plates. hfH was added to one set of wells (black bars) and omitted from a second set (open bars). Goat anti-hfH serum was added, and antibody binding was detected as described in the text and expressed as A450 values. Recombinant BBL39 (OspE paralog of B. burgdorferi B31MI) served as a positive control, and bovine serum albumin served as a negative control. The low degree of fH binding to bovine serum albumin represents the baseline. The baseline value was subtracted to obtain the data presented.
TABLE 1.
B. burgdorferi sensu lato isolates used in this study
| Species and isolate designation | Biological source | Geographic origin | ELISA resulta | Molecular mass(es) (kDa) of fH binding protein(s) | Detection of OspE proteins by immunoblottingd |
|---|---|---|---|---|---|
| B. burgdorferi | |||||
| B31M1 | Ixodes scapularis | New York | +++ | 19, 26.5, 27 | + |
| 272 | Human skin | United States | +++ | 22, 24, 26.5, 27 | + |
| 1352 | Amblyomma americanum | Texas | +++ | 22, 26 | + |
| 20004 | I. ricinus | France | NDc | 27, 27.5 | ND |
| 21343 | Mouse skin | Texas | +++ | 20, 26.5, 27 | + |
| 21721 | I. scapularis | Wisconsin | +++ | 22, 27 | + |
| 25015 | I. scapularis | New York | +++ | 23, 30, 31 | + |
| 27985 | I. scapularis | Connecticut | +++ | 19, 22, 27, 28 | + |
| 297CH | Human CSFb | Connecticut | +++ | 18, 27.5 | + |
| CA2-87 | I. pacificus | California | +++ | 20, 26.5 | + |
| CA3 | I. pacificus | California | +++ | 20, 26.5 | + |
| CA4 | I. pacificus | California | +++ | 21, 22, 26.5, 27 | − |
| CA8 | I. pacificus | California | +++ | 16, 20, 27, 27.5, 30 | + |
| CA12 | I. pacificus | California | ND | 19, 27.5 | + |
| HB19 | Human blood | California | +++ | 23, 26.7 | − |
| JD1 | I. scapularis | Massachusetts | +++ | 21, 26.5 | ND |
| LP3 | Human skin | Connecticut | +++ | 19, 24, 26 | + |
| LP4 | Human skin | Connecticut | +++ | 19, 22, 23, 28 | + |
| LP5 | Human skin | Connecticut | +++ | 19, 26.5, 27 | + |
| LP7 | Human skin | Connecticut | ND | 20, 21 | + |
| N40CH | I. scapularis | New York | +++ | 22, 27 | + |
| NY13-87 | Human skin | New York | + | 19, 27 | ± |
| SH2-82 | I. scapularis | New York | +++ | 18, 23, 29 | + |
| Veery | Veery bird | Connecticut | +++ | 19, 26.5, 27 | + |
| VS219 | I. ricinus | Germany | +++ | 22, 26, 26.5, 27 | + |
| B. garinii | |||||
| 153 | I. ricinus | France | − | —e | − |
| 20047 | I. ricinus | France | − | — | − |
| AO1 | Human CSF | The Netherlands | − | — | − |
| B-4/91 | Human skin | Norway | − | 22, 26.5 | − |
| B-5/92 | Human skin | Norway | + | 22, 26.5 | + |
| FRG | I. ricinus | Germany | − | — | ND |
| G1 | Human CSF | Germany | − | ND | ND |
| G2 | Human CSF | Germany | ND | — | − |
| G25 | I. ricinus | Sweden | − | — | ND |
| IP90 | I. persulcatus | Russia | − | — | − |
| N34 | I. ricinus | Germany | − | — | − |
| Pbi | I. ricinus | Germany | + | 20.5, 26 | ND |
| Pbo | I. ricinus | Germany | + | 21.5, 22, 26 | + |
| Pbr | I. ricinus | Germany | − | — | ND |
| PTrob | Human CSF | Slovenia | ND | — | − |
| PHoe | Human CSF | Germany | ND | — | − |
| PBaeII | Human CSF | Germany | ND | — | − |
| VSBP | I. ricinus | Switzerland | + | 20, 21, 28 | + |
| VS102 | I. ricinus | Switzerland | ND | — | ND |
| VS290 | Switzerland | − | — | − | |
| VS492 | Switzerland | + | — | − | |
| B. afzelii | |||||
| BO23 | Human skin | Germany | + | 22 | − |
| ECM1 | Human skin | Sweden | + | 22, 27 | − |
| HT10 | I. persulcatus | Japan | + | 19, 22, 27 | + |
| R-IP3 | I. persulcatus | Russia | − | — | − |
| R-IP21 | I. persulcatus | Russia | − | 22, 27 | − |
| IPF | I. persulcatus | Japan | ND | 18, 27 | − |
| J1 | I. persulcatus | Japan | + | 19, 26.5, 33 | − |
| Pgau | Human skin | Germany | + | 22, 26.5 | ND |
| PKo | Human skin | Switzerland | − | 20, 27 | + |
| UMO1 | Human skin | Sweden | − | 21, 27 | + |
| UO1 | Human skin | Sweden | − | — | − |
| VS461 | Tick | Switzerland | − | 22, 26.5 | ND |
| B. andersonii | |||||
| 19857 | Rabbit kidney | New York | − | — | + |
| 21038 | I. dentatus | New York | − | — | + |
| B. turdi | |||||
| Ya501 | I. turdus | Japan | − | 19, 20, 26.5 | + |
| B. tunukii | |||||
| HK512 | I. tanukii | Japan | + | 19, 21, 21.5, 25, 26.5, 27 | + |
| B. valaisiana | |||||
| AM501 | I. columnae | Japan | + | — | ND |
| VS116 | I. ricinus | Switzerland | − | — | + |
| B. bissettii DN127 | I. pacificus | California | ND | — | − |
| B. japonica | |||||
| IKA2 | I. ovatus | Japan | − | — | − |
| HO14 | I. ovatus | Japan | − | — | + |
| NT112 | I. ovatus | Japan | + | 20, 26 | + |
| B. miyamotoi FR64b | Apodemus argenteus (blood) | − | — | + |
For ELISAs, A450 readings were scored as follows: +, 0.15 to 0.3; +++, >0.3; −, <0.15.
CSF, cerebrospinal fluid.
ND, not done.
+, OspE proteins detected; ±, weak detection; −, no OspE proteins detected.
—, none detected.
FHBP profiles of each isolate were assessed with an affinity ligand binding immunoblot assay as previously described (17). Briefly, cell lysates were fractionated by SDS-PAGE, immunoblotted, incubated (2 h, 4°C) with or without hfH (10 ng μl−1), and screened with goat anti-fH sera (1:800; with a horseradish peroxidase-conjugated rabbit anti-goat immunoglobulin G secondary antibody at 1:40,000). Two to six FHBPs were detected in all isolates that bound fH, and differential binding along species lines was evident (Fig. 2). By this approach, the percentages of B. burgdorferi, B. afzelii, and B. garinii isolates that bound hfH were 100, 83, and 25%. Note that with a shorter exposure of the film, the 27-kDa FHBP could clearly be resolved as two proteins. These ∼27-kDa FHBPs appear to be the dominant FHBPs; however, it is unclear if this is due to a higher expression level of these proteins or a greater affinity for hfH.
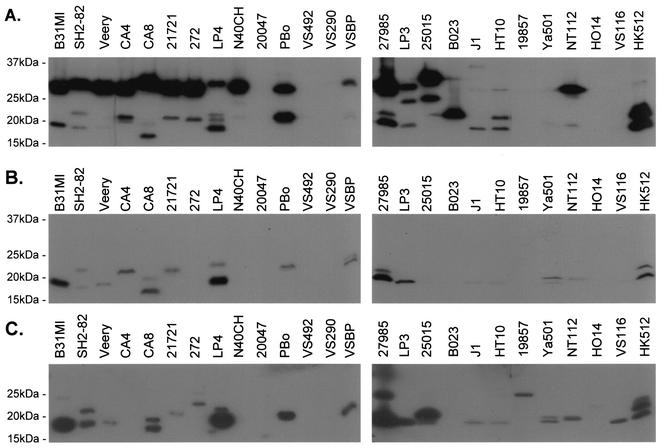
FIG. 2.
Affinity ligand binding immunoblot analysis of fH binding of B. burgdorferi sensu lato complex isolates. Cell lysates were generated, fractionated by SDS-PAGE (15% gels), and immunoblotted to generate several identical immunoblots. All of the procedures used are briefly described in the text and in detail in reference 17. The immunoblots in panel A were incubated with hfH and screened with goat anti-hfH serum. HfH was not added to the immunoblots in panel B. The blots in panel C were screened with mouse anti-OspE sera. The isolates analyzed are indicated above the lanes. Note that blots A and B were exposed to the same piece of film for exactly the same amount of time. Note that detection of some fH binding proteins required longer exposure of the film.
To compare the expression profiles and fH binding properties of members of the OspE protein family in diverse isolates, an identical immunoblot was screened with anti-OspE sera (Fig. 2) generated as previously described (17). Recombinant OspE derived from the BBL39 locus of B. burgdorferi B31MI as part of a separate study (17) was used to produce the antisera. Immunoblot analyses (16) with various recombinant proteins and lysates of B. burgdorferi B31MI confirmed the specificity of the antisera. Extensive variation in the OspE expression patterns was observed, with each isolate expressing zero to four OspE paralogs. Sixty-three percent of the isolates tested produce at least one OspE paralog. Many B. garinii isolates did not produce OspE-related proteins, and in these isolates, ospE-related sequences were not detected by Southern hybridization (data not shown). This variability of OspE profiles among isolates is consistent with studies that demonstrated that OspE is part of a highly variable protein family (15, 21, 22). Comparison of the OspE immunoblot with the immunoblots used in the affinity ligand binding assays indicated that not all OspE paralogs can bind fH. Examples include the OspE paralogs of B. valaisiana VS116 and B. turdi Ya501 and one of the three produced by B. burgdorferi LP4 (Fig. 2). In a separate study, we demonstrated that conformational or structural determinants are important in fH binding (17). Future sequence analyses of OspE proteins that can or cannot bind fH may allow the identification of the sequence and structural determinants that convey fH binding.
As in the ELISA analyses, an hfH-negative control was included in the affinity ligand binding immunoblot assays. In this control, the only available fH would be the endogenous fH present in the goat anti-hfH sera. Goat fH bound readily to the OspE paralogs but not to other FHBPs (Fig. 2). Hence, this control yielded important information about the specificity of the fH binding properties of individual FHBPs. On the basis of antigenic relatedness to OspE and the differential binding of goat fH and hfH, two classes of FHBPs were delineated in this study. Class I FHBPs are related to OspE and bind both hfH and goat fH. Class II proteins are not related to OspE and bind only hfH. The identity of the class II FHBPs remains to be determined and is the subject of ongoing analyses. Regarding the fH-negative control, in an earlier study (20), the presence of high levels of endogenous fH in the goat anti-fH sera was apparently overlooked. This affected the interpretation offered in that report regarding the fH binding specificity of individual Borrelia proteins. For example, it was concluded that the recombinant OspE paralogs BBL39 and BBN38 (referred to as ErpA and ErpP in that report) bind fH from all of the mammals tested. However, the presence of endogenous goat fH in the goat-anti fH sera used to measure fH binding makes it impossible to reach conclusions about binding specificity.
It has been suggested that differences in serum sensitivity among B. burgdorferi sensu lato isolates (12, 23) may fall along species lines and reflect differences in fH binding capability (3, 7, 10). However, the numbers of isolates analyzed in earlier studies were limited, and as a result, the data were insufficient to correlate the fH binding phenotype with individual species (2, 12, 23). In addition, the abilities of less-studied species of the B. burgdorferi sensu lato complex to bind fH have not been investigated. This study, which builds upon work done by other groups (2, 3, 8-12), is the first comprehensive assessment of the correlation between individual species and fH binding. The fH binding phenotype was determined to be universal among B. burgdorferi isolates (100%), widespread among B. afzelii isolates (83%), and uncommon among B. garinii isolates (31%). These percentages correlate exceptionally well with the percentage of isolates of each species reported to be serum resistant (12, 23). The panel of isolates used here, which are now well characterized with regard to their fH binding abilities, can now be exploited to test hypotheses regarding the influence of fH on Borrelia serum sensitivity and pathogenesis. For example, the tropism of B. garinii for the central nervous system may reflect the inability of this species to bind fH. Residence within the central nervous system may provide some protection from complement attack. In contrast, B. burgdorferi, because of its fH binding capability, may be able to efficiently disseminate throughout the body. While fH binding by Lyme disease spirochetes is likely to be important in human disease, fH binding may also be important in spirochetal population maintenance in nature. The ability to circumvent complement-mediated killing would facilitate the maintenance of spirochetes in their mammalian hosts and ensure the completion of their enzootic cycle. In summary, on the basis of the data present here and our present understanding of the interaction of Lyme disease spirochetes with fH, we hypothesize that fH binding is an important pathogenic mechanism and an important determinant in the tropism of different Lyme disease spirochete species for specific anatomical niches.
Acknowledgments
We thank Darrin Akins and Merri Seppo for freely sharing information regarding fH binding prior to its publication.
This study was supported in part by a grant from the National Institutes of Health (RO1AI37787-06). J. V. McDowell was supported in part by a molecular pathogenesis training grant from the National Institute of Allergy and Infectious Diseases and by a predoctoral award from the National Institute of Neurological Disorders and Stroke (F31NS43088).
Editor: V. J. DiRita
REFERENCES
- 1.Akins, D., S. F. Porcella, T. G. Popova, D. Shevchenko, S. I. Baker, M. Li, M. V. Norgard, and J. D. Radolf. 1995. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol. Microbiol. 18:507-520. [DOI] [PubMed]
- 2.Alitalo, A., T. Meri, L. Rämö, T. S. Jokiranta, T. Heikkilä, I. J. T. Seppälä, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alitalo, A., T. Meri, H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 4.El-Hage, N., K. Babb, J. A. Carroll, N. Lindstrom, E. R. Fischer, J. C. Miller, R. D. Gilmore, Jr., M. L. Mbow, and B. Stevenson. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147:821-830. [DOI] [PubMed] [Google Scholar]
- 5.Hefty, P. S., C. S. Brooks, A. M. Jett, G. L. White, S. K. Wikel, R. C. Kennedy, and D. R. Akins. 2002. OspE-related, OspF-related, and Elp lipoproteins are immunogenic in baboons experimentally infected with Borrelia burgdorferi and in human Lyme disease patients. J. Clin. Microbiol. 40:4256-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, and D. R. Akins. 2002. Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete Borrelia burgdorferi. Infect. Immun. 70:3468-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 8.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectiin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 9.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:3393-3401. [DOI] [PubMed] [Google Scholar]
- 10.Kraiczy, P., C. Skerka, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtenbach, K., S. M. Schafer, H. S. Sewell, M. Peacey, A. Hoodless, P. A. Nuttall, and S. E. Randolph. 2001. Differential survival of Lyme borreliosis in ticks that feed on birds. Infect. Immun. 70:5893-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtenbach, K., H.-S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam, T. T., T.-P. K. Nguyen, R. R. Montgomery, F. S. Kantor, E. Fikrig, and R. A. Flavell. 1994. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect. Immun. 62:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marconi, R. T., S. Y. Sung, C. N. Hughes, and J. A. Carlyon. 1996. Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J. Bacteriol. 178:5615-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDowell, J. V., S.-Y. Sung, G. Price, and R. T. Marconi. 2001. Demonstration of the genetic stability and temporal expression of select members of the Lyme disease spirochete OspF protein family during infection in mice. Infect. Immun. 69:4831-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metts, M. S., J. V. McDowell, M. Theisen, P. R. Hansen, and R. T. Marconi. 2003. Analysis of the OspE determinants involved in the binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection in mice. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed]
- 18.Nguyen, T.-P. K., T. T. Lam, S. W. Barthold, S. R. Telford III, R. A. Flavell, and E. Fikrig. 1994. Partial destruction of Borrelia burgdorferi within ticks that engorged on OspE- or OspF-immunized mice. Infect. Immun. 62:2079-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson, B., J. L. Bono, T. G. Schwan, and P. Rosa. 1998. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. 66:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung, S. Y., J. McDowell, J. A. Carlyon, and R. T. Marconi. 2000. Mutation and recombination in the upstream homology box flanked ospE related genes of the Lyme disease spirochetes results in the development of new antigenic variants during infection. Infect. Immun. 68:1319-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung, S.-Y., C. LaVoie, J. A. Carlyon, and R. T. Marconi. 1998. Genetic divergence and evolutionary instability in ospE-related members of the upstream homology box gene family in Borrelia burgdorferi sensu lato complex isolates. Infect. Immun. 66:4656-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Dam, A. P., A. Oei, R. Jaspars, C. Fijen, B. Wilske, L. Spanjaard, and J. Dankert. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65:1228-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, J.-R., J. M. Hardham, A. G. Barbour, and A. G. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of vmp like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]