Abstract
We identified a cytolethal distending toxin (cdt) gene cluster in 87, 6, and 0% of sorbitol-fermenting (SF) enterohemorrhagic Escherichia coli (EHEC) O157:H−, EHEC O157:H7, and E. coli O55:H7/H− strains, respectively. The toxin was expressed by the wild-type EHEC O157 strains and by a cdt-containing cosmid from a library of SF EHEC O157:H− strain 493/89. The cdt flanks in strain 493/89 were homologous to bacteriophages P2 and lambda. Our data demonstrate that cdt, encoding a potential virulence factor, is present in the EHEC O157 complex and suggest that cdt may have been acquired by phage transduction.
Sorbitol-fermenting (SF) enterohemorrhagic Escherichia coli (EHEC) O157:H− strains have emerged as causes of diarrhea, hemorrhagic colitis, and the hemolytic-uremic syndrome (HUS) in continental Europe (11). Multilocus enzyme electrophoresis and sequence typing (5, 16) demonstrated that SF EHEC O157:H− is closely related to EHEC O157:H7 and evolved from a common enteropathogenic E. coli O55:H7-like ancestor or a similar, closely related organism (5). Whereas the complete genome sequences of two EHEC O157:H7 strains (EDL933 and the Sakai outbreak strain) have been published (6, 14), the genome of SF EHEC O157:H− remains mostly uncharacterized. In this study, a cosmid library of SF EHEC O157:H− reference strain 493/89 was used to characterize a novel gene cluster encoding cytolethal distending toxin (CDT) (8) in SF EHEC O157:H−.
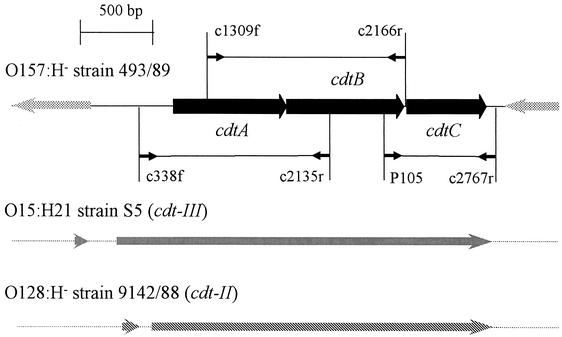
SF EHEC O157:H− strain 493/89 (9, 10) genomic DNA was partially digested with Sau3AI (Gibco BRL, Eggenstein, Germany), and the fragments were dephosphorylated with shrimp alkaline phosphatase, ligated into the arms of a cosmid (SuperCos I; Stratagene, Heidelberg, Germany), and packaged into phage heads with the Gigapack III XL-4 system (Stratagene). E. coli XL1-Blue MR transductants were selected on Luria-Bertani agar containing ampicillin (100 μg/ml) and sorted into a cosmid library. Plasmid DNA from randomly selected clones was isolated (Qiagen Midi Kit; Qiagen, Hilden, Germany) and sequenced with primers supercos1fwd (5′-CGGCCGCAATTAACCCTCAC-3′) and supercos1rwd (5′-GCGGCCGCATAATACGACTCACT-3′) by AGOWA GmbH, Berlin, Germany. Sequences were analyzed with the DNASIS program (Hitachi Software). Homology searches were performed with the EMBL GenBank database. PCRs with three different primer pairs (Fig. 1), i.e., c338f (5′-AGCATTAAATAAAAGCACGA-3′) and c2135r (5′-TACTTGCTGTGGTCTGCTAT-3′), c1309f (5′-AGCACCCGCAGTATCTTTGA-3′) and c2166r (5′-AGCCTCTTTTATCGTCTGGA-3′), and P105 (5′-GTCAACGAACATTAGATTAT-3′) and c2767r (5′-ATGGTCATGCTTTGTTATAT-3′), which are specific for cdtA, cdtB, and cdtC (12), respectively, were applied to DNA from 175 EHEC O157:H7 or H− and 26 E. coli O55:H7 or H− clinical isolates (Table 1). The primers were designed on the basis of the cdt sequence of SF EHEC O157:H− strain 493/89 and from the published cdt-III sequence (accession number U89305). The PCRs included 30 cycles of denaturing (94°C, 30 s), annealing (52, 54, and 49°C [cdtA, cdtB, and cdtC, respectively], 60 s), and extension (72°C, 60 s), followed by a final extension (72°C, 5 min), resulting in amplicons of 1,329, 1,363, and 748 bp for cdtA, cdtB, and cdtC, respectively. CDT was assayed with Chinese hamster ovary (CHO) cells and a modification of the procedure described by Scott and Kaper (18). Briefly, supernatants of overnight bacterial cultures grown with shaking (180 rpm) in cell culture medium (Ham's F12 with 10% fetal calf serum) were filter sterilized (0.22-μm-pore-size filters; Schleicher & Schuell GmbH, Dassel, Germany) and 1-ml portions of filtrates or their twofold dilutions were added in duplicate to 103 freshly seeded CHO cells in 1.5 ml of Ham's F-12 medium in six-well tissue culture plates (Falcon 3502; Becton Dickinson Labware, Franklin Lakes, N.J.). The assay mixtures were incubated for 5 days at 37°C in 5% CO2 and examined daily for a typical distending effect (8). The CDT titer was defined as the highest filtrate dilution that caused distension in 50% of CHO cells. Strain 6468/62, producing CDT-I (18) (gift from D. A. Scott, University of Maryland School of Medicine, Baltimore), and E. coli XL1-Blue MR, containing the SuperCos I vector, were used as the positive and negative controls, respectively. Statistical analysis was performed with the χ2 test (4); P values of <0.05 were considered significant.
FIG. 1.
PCR strategy used to detect the cdt cluster in EHEC O157 and design of cdt-flanking regions in SF EHEC O157:H− strain 489/93. The positions of the PCR primer pairs c338f-c2135r, c1309f-c2166r, and P105-c2767r within the cdt region of strain 493/89 are indicated by thin black arrows. The primer sequences and PCR conditions are specified in text. The gray bars in the 493/89 cdt-flanking regions demonstrate bacteriophage P2 sequences. The homologies of the cdt region of strain 493/89 to those of cdt-III of E. coli O15:H21 strain S5 and cdt-II of E. coli O128:H− strain 9142/88 are indicated by vertical and diagonal stripes, respectively.
TABLE 1.
Distribution of cdt cluster in EHEC O157:H− and O157:H7 and E. coli O55:H7/H− clinical isolates
| Serotypea | Total no. of isolatesb | No. (%) of isolates positive for:
|
No. of patients with:
|
||||
|---|---|---|---|---|---|---|---|
| cdtc | stxd | eae | SF | HUS | Diarrhea | ||
| O157:H− | 75 | 65 (87)e | 75 | 75 | 75 | 49 | 26 |
| O157:H7 | 100 | 6 (6)e | 100 | 100 | 0 | 70 | 30 |
| O55:H7/H− | 26 | 0e | 0 | 26 | 26 | 0 | 26 |
H−, nonmotile.
All isolates were from our strain collection and were characterized for genes encoding Shiga toxins (stx1, stx2, and stx2c) and intimin (eae) and for SF as previously described (10, 11).
Each of the three PCRs for cdtA, cdtB, and cdtC (Fig. 1) was positive in each strain.
SF EHEC O157:H− contained stx2 only; EHEC O157:H7 contained stx2 and/or stx2c.
SF EHEC O157:H− versus EHEC O157:H7, P < 0.000001 (χ2 [1 df], 115.66). SF EHEC O157:H− versus E. coli O55:H7/H−, P < 0.000001 (χ2 [1 df], 63.22).
Identification and characterization of the cdt cluster in SF EHEC O157:H−.
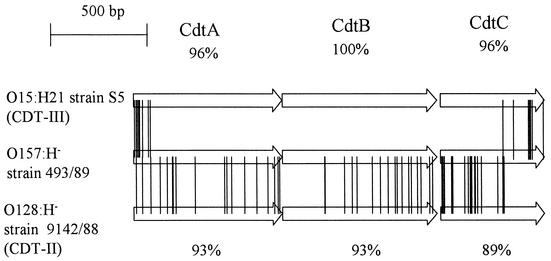
Three hundred 493/89 cosmid clones were sequenced and tested for homology to EHEC O157:H7 strain EDL933 (14) (accession number AE005174); 118 were homologous at both ends. Sequence data suggested an insertion of ca. 30 kb in one cosmid, compared to the E. coli O157:H7 sequence. Subcloning of this insert demonstrated cdt components cdtA, cdtB, and cdtC. Figure 2 depicts the differences among the deduced amino acid sequences of CDT from strain 493/89, CDT-II from E. coli O128:H− strain 9142/88 (15) (accession number U04208), and CDT-III from E. coli O15:H21 strain S5 (13) (accession number U89305). The 54 differences between CDT from 493/89 and CDT-II are distributed over each of the three open reading frames (ORFs), resulting in 93, 93, and 89% amino acid identity among CdtA, -B, and -C, respectively. In contrast, the 17 amino acid differences between CDT from 493/89 and CDT-III are clustered at the 5′ end of CdtA and the 3′ end of CdtC whereas the CdtB sequences are 100% identical (Fig. 2). The amino acid sequence homologies of CDT from 493/89 to CDT-I from E. coli O86:H34 strain 6468/62 (18) (accession number U03293) are only 39, 54, and 31% for CdtA, -B, and -C, respectively. CDT-III, which is the most similar to CDT from strain 493/89, has been reported to be encoded by a gene on a plasmid (13). However, the insert ends of the cdt-containing cosmid of the 493/89 library are homologous to the EDL933 chromosome.
FIG. 2.
Amino acid sequence homologies among the three CDT components of SF EHEC O157:H− strain 493/89, E. coli O15:H21 strain S5 (CDT-III), and E. coli O128:H− strain 9142/88 (CDT-II). The exchanges in the amino acids are marked by connecting lines between the ORFs. The amino acid sequence homologies between CDT from 493/89 and CDT-II (accession number U04208) and between CDT from 493/89 and CDT-III (accession number U89305) are given below and above the three depicted ORFs of CDT-II and CDT-III, respectively.
Shotgun cloning to assess the cdt flanks in strain 493/89 was performed and demonstrated homologues of a bacteriophage P2 (accession number AF063097) cohesive end and portal protein downstream of cdtA and bacteriophage P2 DNA replication protein upstream of cdtC (Fig. 1). This suggested that the 2,822-bp sequence containing the three cdt ORFs in strain 493/89 is located within the region of bacteriophage P2 late genes, where it replaces 3,255 bp of the phage genes that encode ORF91 and a hypothetical protein with no known essential function. The homology to the cdt-III sequence extends to the beginning of the phage homology (Fig. 1). In addition, shotgun cloning of the 493/89 cdt-containing insert demonstrated homology to bacteriophage lambda (accession number J02459) spanning the first half of the phage sequence that encodes DNA-packaging enzymes and tail and head components.
cdt in E. coli O157 and O55.
PCR targeting cdtA, cdtB, and cdtC (Fig. 1) was used to assess the conservation of these loci in 100 EHEC O157:H7, 75 SF EHEC O157:H−, and 26 E. coli O55:H7/H− strains isolated from patients (Table 1). Each of the three cdt genes was significantly more frequent in SF EHEC O157:H− (87%) than in EHEC O157:H7 (6%) (P < 0.000001) and was absent from all of the E. coli O55:H7/H− strains investigated (Table 1). Five of the 10 cdt-negative SF EHEC O157:H− strains originated from HUS patients, and five came from patients with diarrhea. Of the six cdt-positive EHEC O157:H7 strains, one was from an HUS patient and five were from patients with diarrhea.
CDT expression.
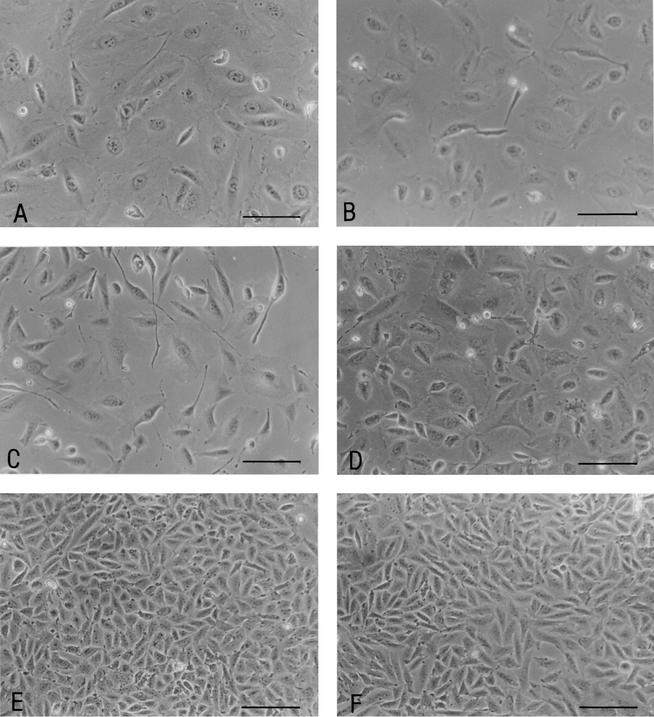
After 5 days of incubation, sterile filtrates from the 493/89 cdt-containing cosmid (Fig. 3B), wild-type SF EHEC O157:H− strain 493/89 (Fig. 3C), and EHEC O157:H7 strain 5791/99 (Fig. 3D) distended CHO cells to an extent comparable to that caused by CDT+ control strain 6468/62 (Fig. 3A). No morphological changes, compared to CHO cells incubated in Ham's F-12 medium only (Fig. 3F), were observed with E. coli XL1-Blue MR cells transduced with the SuperCos I vector (Fig. 3E). The CDT titer expressed by the 493/89 cdt-containing cosmid and by EHEC O157 wild-type strains 493/89 and 5791/99 was 1:4; strain 6468/62 produced a CDT titer of 1:8.
FIG. 3.
Photomicrographs of CHO cells after 5 days of incubation with sterile culture filtrates from CDT+ control strain 6468/62 (A), 493/89 cdt-containing cosmid (B), SF EHEC O157:H− strain 493/89 (C), EHEC O157:H7 strain 5791/99 (D), and E. coli strain XL1-Blue MR containing the SuperCos I vector (E). Panel F shows CHO cells incubated for 5 days in Ham's F-12 medium. Scale bars, 200 μm.
CDT, first described in 1987 (8), has been associated mainly with enteropathogenic and necrotoxigenic E. coli (3). We report the presence of cdt in EHEC strains. Although the cdt cluster of SF EHEC O157:H− strain 493/89 demonstrates the highest degree of homology to cdt-III, it differs from cdt-III by the presence of phage flanking regions. The juxtaposition of the cdt region of strain 493/89 with the bacteriophage P2 sequence (Fig. 1) suggests that cdt may have been acquired by phage transduction. In addition, the presence of bacteriophage lambda sequences in the cosmid insert containing cdt from strain 493/89 suggests possible recombination between these two phages. Mosaicism, resulting from multiple phage recombination events, has been well documented in Shiga toxin-converting bacteriophages (17). Furthermore, the conservation of all three cdt components in each of the cdt-positive EHEC O157 strains indicates that cdt may have been introduced by a single ancestral phage transduction into the O157 complex. Because cdt is present in most SF EHEC O157:H− strains but absent from most EHEC O157:H7 strains and from all of the E. coli O55:H7 strains tested, which are the proposed ancestors of EHEC O157 (5), we believe that the most parsimonious sequence of events is that a mobile element containing this cluster was acquired by SF EHEC O157:H− after this lineage diverged from E. coli O157:H7. This sequence contrasts with the situation that we believe happened with the efa1 (lifA) gene, which was lost by EHEC O157:H7 (7). The 10 cdt-negative SF EHEC O157:H− and 6 cdt-positive EHEC O157:H7 strains that represent exceptions to the evolutionary concept proposed above need to be investigated further to test the hypothesis that cdt was lost or acquired through a phage or other mobile element in these specific cases. For bacteriophage P2, such investigation is hampered by the fact that although spontaneous induction of the phage does occur (1), P2 phage lysogens cannot be artificially induced to release phage particles (1). Besides efa1 (lifA), which encodes a multifunctional virulence factor that can act as an adhesin or lymphostatin (7), and the sfp gene cluster, which encodes SF EHEC O157-specific fimbriae (2), the cdt cluster is a putative virulence gene that is typically associated with SF EHEC O157:H− but absent from EHEC O157:H7. In contrast, EHEC O157:H7 regularly harbors the pathogenicity island TAI (tellurite resistance- and adherence-conferring island) (19) and the espP and katP plasmid genes (11), which are absent from SF EHEC O157:H− (11, 19). In O157:H7, CDT does not appear to play a significant role in human diseases, because it is mostly absent from such strains. However, in SF EHEC O157:H−, CDT is commonly present and studies investigating its role in SF EHEC O157:H−-associated disease are under way.
Nucleotide sequence accession number.
The sequence of the cdt-containing fragment from strain 493/89 has been deposited in the GenBank database and assigned accession number AJ508930.
Acknowledgments
This study was supported by grants from the BMBF Project Network of Competence Pathogenomics Alliance [Functional Genomic Research on Enterohaemorrhagic, Enteropathogenic and Enteroaggregative Escherichia coli (EHEC, EPEC, EAEC)], project group Schmidt/Karch, Universitätsklinikum Münster (BD numbers 119523 and 207800), and from the First European Graduate College (Gene Regulation in and by Microbial Pathogens).
The excellent technical assistance of Barbara Plaschke is greatly appreciated. We thank Phillip I. Tarr (Washington University School of Medicine, St. Louis, Mo.) for critical reading of the manuscript and helpful discussions.
Editor: A. D. O'Brien
REFERENCES
- 1.Birge, E. A. 1994. Genetics of temperate bacteriophages, p. 206-239. In E. A. Birge (ed.), Bacterial and bacteriophage genetics, 3rd ed. Springer-Verlag, New York, N.Y.
- 2.Brunder, W., A. Salam Khan, J. Hacker, and H. Karch. 2001. A novel fimbrial gene cluster encoded by the large plasmid of sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H−. Infect. Immun. 69:4447-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark, C. G., S. T. Johnson, R. H. Easy, J. L. Campbell, and F. G. Rodgers. 2002. PCR for detection of cdt-III and the relative frequencies of cytolethal distending toxin variant-producing Escherichia coli isolates from humans and cattle. J. Clin. Microbiol. 40:2671-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dicker, R. C. 1996. Analyzing and interpreting data, p. 92-131. In M. B. Greg, R. C. Dicker, and R. A. Goodman (ed.), Field epidemiology. Oxford University Press, Inc., New York, N.Y.
- 5.Feng, P., K. A. Lampel, H. Karch, and T. S. Whittam. 1998. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J. Infect. Dis. 177:1750-1753. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 7.Janka, A., M. Bielaszewska, U. Dobrindt, and H. Karch. 2002. Identification and distribution of the enterohemorrhagic Escherichia coli factor for adherence (efa1) gene in sorbitol-fermenting Escherichia coli O157:H−. Int. J. Med. Microbiol. 292:207-214. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, W. M., and H. Lior. 1987. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol. Lett. 43:19-23. [Google Scholar]
- 9.Karch, H., R. Wiss, H. Glonning, P. Emmrich, S. Aleksic, and J. Bockemühl. 1990. Hämolytisch-urämisches Syndrom bei Kleinkindern durch Verotoxin-produzierende Escherichia coli. Dtsch. Med. Wochenschr. 115:485-495. [DOI] [PubMed] [Google Scholar]
- 10.Karch, H., H. Bohm, H. Schmidt, F. Gunzer, S. Aleksic, and J. Heesemann. 1993. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H−. J. Clin. Microbiol. 31:1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karch, H., and M. Bielaszewska. 2001. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157:H− strains: epidemiology, phenotypic and molecular characteristics, and microbiological diagnosis. J. Clin. Microbiol. 39:2043-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lara-Tejero, M., and J. E. Galan. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 69:4358-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peres, S. P., O. Marches, F. Daigle, J. P. Nougayrede, F. Herault, C. Tasca, J. DeRycke, and E. Oswald. 1997. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol. Microbiol. 24:1095-1107. [DOI] [PubMed] [Google Scholar]
- 14.Perna, N. T., G. Plunkett, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 15.Pickett, C. L., D. L. Cottle, E. C. Pesci, and G. Bikah. 1994. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect. Immun. 62:1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid, S. D., C. J. Herbelin, A. C. Bumbaugh, R. K. Selander, and T. S. Whittam. 2000. Parallel evolution of virulence in pathogenic Escherichia coli. Nature 406:64-67. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt, H. 2001. Shiga toxin-converting bacteriophages. Res. Microbiol. 152:687-695. [DOI] [PubMed] [Google Scholar]
- 18.Scott, D. A., and J. B. Kaper. 1994. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect. Immun. 62:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarr, P. I., S. S. Bilge, J. C. Vary, S. Jelacic, R. L. Habeeb, T. R. Ward, M. R. Baylor, and T. E. Besser. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 68:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]