Abstract
Secreted aspartyl proteinases (Saps) contribute to the ability of Candida albicans to cause mucosal and disseminated infections. A model of vaginal candidiasis based on reconstituted human vaginal epithelium (RHVE) was used to study the expression and role of these C. albicans proteinases during infection and tissue damage of vaginal epithelium. Colonization of the RHVE by C. albicans SC5314 did not cause any visible epithelial damage 6 h after inoculation, although expression of SAP2, SAP9, and SAP10 was detected by reverse transcriptase PCR. However, significant epithelial damage was observed after 12 h, concomitant with the additional expression of SAP1, SAP4, and SAP5. Additional transcripts of SAP6 and SAP7 were detected at a later stage of the artificial infection (24 h). Similar SAP expression profiles were observed in three samples isolated from human patients with vaginal candidiasis. In experimental infection, secretion of antigens Sap1 to Sap6 by C. albicans was confirmed at the ultrastructural level by using polyclonal antisera raised against Sap1 to Sap6. Addition of the aspartyl proteinase inhibitors pepstatin A and the human immunodeficiency virus proteinase inhibitors ritonavir and amprenavir strongly reduced the tissue damage of the vaginal epithelia by C. albicans cells. Furthermore, SAP null mutants lacking either SAP1 or SAP2 had a drastically reduced potential to cause tissue damage even though SAP3, SAP4, and SAP7 were up-regulated in these mutants. In contrast the vaginopathic potential of mutants lacking SAP3 or SAP4 to SAP6 was not reduced compared to wild-type cells. These data provide further evidence for a crucial role of Sap1 and Sap2 in C. albicans vaginal infections.
Although normally a commensal habitant of mucosal surfaces, Candida albicans frequently causes surface infections when certain host factors are imbalanced. Under certain circumstances these superficial infections may disseminate to cause serious systemic infections. Key virulence factors of C. albicans that appear to play major roles in the pathogenesis of this opportunistic fungus are the secreted aspartyl proteinases (Saps), which are encoded by 10 SAP genes. These genes are regulated differentially in vitro and in vivo during infection of three-dimensional models for oral and cutaneous candidiasis in infected tissue of mice and in patient samples (3, 10, 15). Therefore, it has been concluded that different SAP genes may have distinct roles at different times of the infection process and during different types of infection (15). For example, by use of SAP-deficient mutants, it has been shown that SAP1, SAP2, and SAP3 contribute significantly to tissue damage and invasion of oral epithelium and cutaneous epidermis (23, 25), while SAP4, SAP5, and SAP6 are important for systemic infections (12, 22). Several studies dealing with proteinase secretion and proteinase activity have shown a clear correlation between the ability of C. albicans strains to secrete Saps and to cause disease (4, 5, 7, 9). The expression and importance of SAP1, SAP2, and SAP3 during murine vaginal candidiasis were also demonstrated by using reverse transcriptase PCR (RT-PCR) and SAP-deficient mutants (6, 8, 10, 28). It is not clear whether the murine model is representative of proteinase expression during human vaginal infection. Since it is known that vaginal candidiasis is an extremely common fungal disease affecting nearly three-quarters of all healthy women (27), we studied the expression and role of the SAP gene family in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium by using RT-PCR, immunoelectron microscopy, Sap-deficient mutants, and proteinase inhibitors. Furthermore, we compared the SAP expression profile observed in our in vitro studies to that found in samples from patients suffering from vaginal candidiasis. The data obtained from our studies suggest that Sap1 and Sap2 play a crucial role in C. albicans vaginal infections.
(This article is a part of the doctoral thesis written by Matthias Bein to be submitted to the medical faculty of the University of Munich.)
MATERIALS AND METHODS
Candida strains.
The clinical C. albicans wild-type strain SC5314 (14), the URA3/ura3 heterozygote CAF2-1 (13), the SAP null mutant strains sap1, sap2, sap3 (16), and sap4 to sap6 (16), the SAP2 reconstituted strain M40 (17), and a SAP1 reconstituted strain were used in the study. To reconstitute the sap1 mutant, a 2,384-bp PCR fragment containing the open reading frame of SAP1, 986 bp of the SAP1 promoter region, and 202 bp of the SAP1 3′ untranslated region was cloned into the HindIII-XhoI sites of pCIp (19) to give pSB1. Primers used to amplify this SAP1 fragment were 5′-ATCAAGCTTTAAAAAGAAGTGGGGATTGAAGAG-3′ and 5′-GATCCTCGAGAGTT TATTATTTGGTAGAGATTG-3′. The RP10 region of pSB1 was then digested with NcoI and transformed into the Ura− sap1 mutant. Integration of the plasmid into the RP10 locus was confirmed by Southern analysis. Only transformants that had growth rates similar to those of the wild type were chosen for further experiments. For further comparisons, we also used the Ura− sap1 mutant carrying the empty pCIp10 plasmid.
Culture media and growth conditions.
For the infection of the reconstituted vaginal epithelium, inocula were prepared by culturing C. albicans for 24 h at 37°C on Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.). Cells were washed three times in 0.9% NaCl, and approximately 2 × 105 cells were then suspended in 10 ml of yeast extract-Peptone-dextrose medium (Difco). The suspension was cultured for 16 h at 25°C through orbital shaking. A suspension of 4 × 106 cells from this culture was incubated through shaking in fresh medium for a further 24 h at 37°C. After washing three times with phosphate-buffered saline (PBS), the final inoculum was then adjusted to the desired density with PBS solution.
Reconstituted human vaginal epithelium (RHVE) and model of vaginal candidiasis.
The human epithelium for the in vitro model of vaginal candidiasis was supplied by Skinethic Laboratory (Nice, France). It was obtained by culturing transformed human keratinocytes of the cell line A 431 derived from a vulval epidermoid carcinoma (21). Keratinocytes were incubated in serum-free conditions in a defined medium based on the MCDB-153 medium (Clonetics, San Diego, Calif.), containing 5 μg of insulin/ml, on a 0.5-cm2 microporous polycarbonate filter for 7 days at the air-liquid interface. A431 cells form a three-dimensional epithelial tissue resembling human vaginal mucosa in vivo. The in vitro model and all culture media were prepared without antibiotics and antimycotics.
Triplicate infection experiments were performed for each C. albicans strain. RHVE was infected with 2 × 106 C. albicans cells of the SC5314 and the CAF2-1 parental strains, the mutants sap1, sap2, sap3, and sap4 to sap6, and the SAP1 and SAP2 revertant strains in 50 μl of PBS for 6, 12, and 24 h. Controls contained 50 μl of PBS alone.
For inhibition of Saps, amprenavir (Glaxo/Wellcome, Bad Oldeslohe, Germany), pepstatin A (Sigma, Deisenhofen, Germany) and ritonavir (Abbott, Wiesbaden, Germany) were dissolved in absolute methanol and administered to 50 μl of PBS, containing 2 × 106 C. albicans SC5314 cells at final concentrations of 0.1, 15, and 32 μM, respectively. The same concentrations of the inhibitors were also applied for the maintenance media (1 ml) of the epithelial cultures. Controls contained 50 μl of PBS supplemented with pepstatin A, amprenavir, or ritonavir but without C. albicans cells. Incubation periods were 6, 12, and 24 h.
All tissue cultures were incubated at 37°C with 5% CO2 at 100% humidity.
Assay of LDH activity.
The release of lactate dehydrogenase (LDH) from epithelial cells into the surrounding medium was monitored as a measure of epithelial cell damage. LDH release in the maintenance media of the cultures from uninfected and infected epithelial cells was measured at 6, 12 and 24 h. LDH activity was analyzed spectrophotometrically by measuring the NADH disappearance rate at 340 nm during the LDH-catalyzed conversion of pyruvate to lactate, according to the Wróblewski-La Due method (30). The LDH activity is given as units per liter at 37°C.
Light and immunoelectron microscopy.
Light microscopy studies were performed to evaluate histological changes during infection. Part of each specimen was fixed, postfixed, embedded in glycide ether, and cut by using an ultramicrotome (Ultracut; Reichert, Vienna, Austria). Semithin sections (1 μm) were studied with a light microscope after staining with 1% toluidine blue and 1% pyronine G (Merck, Darmstadt, Germany). The histological changes of the mucosa were evaluated on the basis of 50 sections from five different sites for each infected epithelium.
Postembedding immunogold labeling was carried out for intracellular detection of Sap antigen in SC5314-infected samples. A part of each specimen was fixed in Karnovsky solution for 1 h at room temperature and was embedded in glycide ether. Sections, 80 to 100 nm thick, were mounted on nickel grids. The grids were rinsed on drops of distilled water for 10 min and were floated on drops of PBS containing 5% (vol/vol) normal goat serum for 2 × 10 min. Grids were then incubated with the anti-Sap polyclonal rabbit antibodies, directed against Sap1 to Sap3 or against Sap4 to Sap6 (2), diluted 1:100 in PBS supplemented with 1% ovoalbumin, 0.1% Tween 20, and 0.015 M Na-azide for 3 h at room temperature. After washing overnight with PBS, grids were incubated with 10-nm gold-conjugated goat anti-rabbit immunoglobulin G (Auroprobe EM Immunogold reagents; Amersham, Little Chalfont, United Kingdom) diluted 1:50 in 0.02 M Tris hydrochloride acid buffer, 0.15 M NaCl, 0.015 M Na-azide, 1% ovoalbumin, and 0.1% Tween 20, adjusted to pH 8.2 (TBS-OT), for 1.5 h at room temperature. In control samples the anti-Sap polyclonal antibodies were omitted. After several washing steps with TBS-OT, grids were fixed with 2% glutaraldehyde in PBS and were washed again in water. Grids were stained with 0.5% uranyl acetate for 10 min and with 2.7% lead citrate for 5 min (Ultrastainer; LKB, Bromma, Sweden) at 20°C. Grids were examined with a Zeiss EM 902 transmission electron microscope (Zeiss, Oberkochen, Germany) operating at 80 kV, at magnifications between ×3,000 and ×85,000.
Patients and samples.
Samples of pseudomembrane were removed from the vaginal mucosa of three volunteer, untreated human immunodeficiency virus-negative female patients. The patients were 19 (patient 1), 16 (patient 2), and 37 (patient 3) years old and had suffered from a vaginal and/or vulvar itching and burning sensation with discharge for at least 6 days. Some of the clinical material from all three patients was used for microbiological investigations to identify the infecting Candida species. The remaining material was shock frozen for gene expression studies. After inoculation on Kimmig's agar (Merck), the specimens were incubated for 27 h at 37°C. Biochemical identification of C. albicans was based on the use of the Ready-Made System ATB 32 C (API System; bioMérieux, La Balme-les-Grottes, Montalieu Vercieu, France).
RNA isolation, cDNA synthesis (RT), and primers.
RT-PCR was used for analysis of SAP1 to -10 and EFB1 gene expression during epithelial and in vivo infection. For the detection of mRNA, samples were rapidly removed and shock frozen in liquid nitrogen. Total RNA from shock-frozen samples was isolated with RNAPureTM (Peqlab, Erlangen, Germany) according to the manufacturer's instructions. RNA concentrations were measured by absorbance at 260 nm. cDNA synthesis was performed with Superscript II RT (Gibco, Eggenstein, Germany) following the manufacturer's instructions. For an internal mRNA control and for detection of genomic DNA, we used primers for the EFB1 gene (Table 1) (18). The DNA amplification fragment of EFB1 contained a 364-bp intron. Absence of genomic DNA was verified by a single 564-bp-long PCR product of EFB1. The absence of genomic DNA was further verified by omitting RT. Only samples that failed to amplify SAP gene products when RT was omitted were used for further experiments. For detection of SAP1 to -10 transcripts, SAP-specific pair primers were designed (Table 1). The specificity of each set of primers was confirmed with genomic DNA. Similar sensitivity of the primer pairs for DNA amplification was determined by testing dilutions of genomic DNA. The cDNA samples were subjected to 35 cycles of denaturation for 1 min at 95°C, annealing for 1 min at 60°C, extension for 1 min at 72°C, and 10 min at 72°C (final).
TABLE 1.
SAP and EFB1 PCR primers used in this study and expected fragment lengthsa
| Primer (length in bp) | Orientation | Sequence |
|---|---|---|
| SAP1 (783) | Fwd | 5′-GAT GTC ATT AAA ACT CCT GTT AAT G-3′ |
| Rev | 5′-CCA GTT TCA ATT CAG CTT GG-3′ | |
| SAP2 (915) | Fwd | 5′-CTC CTA AAG CAT TCC CAG TTA C-3′ |
| Rev | 5′-CAT CAT CAC CTT GTA AAG AAG C-3′ | |
| SAP3 (802) | Fwd | 5′-CAT GTC AAG CTG GTC AAG GAC-3′ |
| Rev | 5′-ATA GGC TGA TCT CAA GAA ATT ATC-3′ | |
| SAP4 (870) | Fwd | 5′-GTT CCA GAT TCA AAT GCC G-3′ |
| Rev | 5′-CTT GAG CCA TGG AGA TCT TTC-3′ | |
| SAP5 (902) | Fwd | 5′-TGA GAC TGG TAG AGA TGG TG-3′ |
| Rev | 5′-GGT TTA CCA CTA GTG TAA TAT GT-3′ | |
| SAP6 (605) | Fwd | 5′-AAA CCA ACG AAG CTA CCA GAA C-3′ |
| Rev | 5′-TAA CTT GAG CCA TGG AGA TTT TC-3′ | |
| SAP7 (866) | Fwd | 5′-GAT AAG GCA TCA GGT ACT ATG G-3′ |
| Rev | 5′-AGG AAC AAC GGC ATG GTT ATC-3′ | |
| SAP8 (903) | Fwd | 5′-CTG TTA TTG TTG ACA CAG GTT C-3′ |
| Rev | 5′-GTA GAA ATA CTT GAA GAA GTA GTG-3′ | |
| SAP9 (898) | Fwd | 5′-CAC CAT AAG CAA CGT GAC TG-3′ |
| Rev | 5′-GCG AAA GCA ACA ACC CAT AC-3′ | |
| SAP10 (938) | Fwd | 5′-ACG TCA GAA GAC TTT TCC ATT G-3′ |
| Rev | 5′-ATA TGG CGA TCC ATG AAC GTG-3′ | |
| EFB1 (564/928 [cDNA/DNA]) | Fwd | 5′-AGT CAT TGA ACG AAT TCT TGG CTG-3′ |
| Rev | 5′-TTC TTC AAC AGC AGC TTG TAA GTC-3′ |
Fwd, forward; Rev, reverse.
RESULTS
Morphology of uninfected and infected RHVE.
Uninfected RHVE consisted of stratified keratinocytes with no stratum corneum. Incubation of RHVE with PBS alone over the time course of our infection experiments for up to 24 h led to an increasing number of cell layers.
In all experiments, histological alterations of the RHVE induced by SC5314 or by CAF2-1 were similar, indicating that the URA3/ura3 heterozygote C. albicans strain is not attenuated in its potential to cause tissue damage in this model. Colonization of the RHVE by C. albicans SC5314 did not cause any major histological alterations of the host cells during the first 6 h (Fig. 1A). After 12 h, vaginal epithelium infected with SC5314 showed tissue damage characterized by edema, vacuolization, and detachment of the keratinocytes of the upper layers (Fig. 1B). Similar histological alterations of deeper parts of the vaginal epithelium were also observed at 24 h (Fig. 1C).
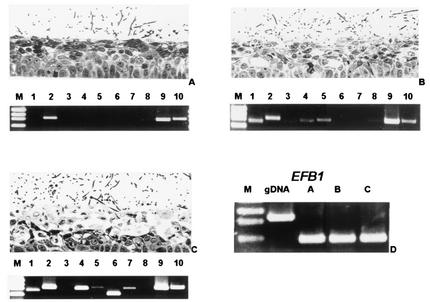
FIG. 1.
Light micrographs of RHVE and SAP expression pattern as obtained by RT-PCR 6, 12, and 24 h after infection with C. albicans SC5314. (A) Colonization of RHVE without marked morphological alteration and expression of SAP2, SAP9, and SAP10 were observed 6 h after infection with SC5314. (B) Invasion of C. albicans with vacuolization and edema at 12 h was accompanied by additional expression of SAP1, SAP4, and SAP5. (C) Later stage of infection at 24 h with extensive edema, vacuolization, and additional SAP6 and SAP7 transcripts. (D) A 564-bp fragment size obtained by amplification with EFB1 primers demonstrates the cDNA origin of the templates used for panels A to C, whereas the same set of primers amplified an 928-bp intron-containing fragment when genomic (g) DNA was used as a template. In lanes 1 the molecular mass marker (M) XIII (PEQLAB, Erlangen, Germany) was used, giving fragments that were 1,353, 1,078, 872, and 603 bp long.
SAP and EFB1 gene expression by C. albicans during the course of infection.
To investigate the correlation between expression of distinct SAP genes and the onset of morphological alterations, we analyzed the SAP expression profile by RT-PCR in three independent infection experiments. In all samples, colonization of the superficial epithelial layers with no or only minor signs of epithelial damage at 6 h was accompanied by expression of SAP2, SAP9, and SAP10 (Fig. 1A). At 12 h, the first signs of tissue damage were visible and in these samples we always detected additional strong signals for SAP1 and SAP2 and weak signals for SAP4 and SAP5 transcripts (Fig. 1B). At 24 h damage of deeper epithelial parts as characterized by numerous, large vacuoles and damaged, light epithelial cells in the lower layers coincided with expression of SAP1, SAP2, SAP4, SAP5, SAP6, SAP7, SAP9, and SAP10 in all samples (Fig. 1C). Expression of SAP9 and of SAP10 was observed in all samples.
The cDNA origin of the templates and the absence of genomic DNA were demonstrated by amplification of a 564-bp fragment of the intron-containing gene EFB1 (Fig. 1D).
Influence of aspartyl proteinase inhibitors on the virulence of C. albicans in experimental vaginal candidiasis.
To investigate whether Sap activity contributed to tissue damage during the in vitro infection of vaginal epithelium, we analyzed the effects of several aspartyl proteinase inhibitors, including pepstatin A and the human immunodeficiency virus proteinase inhibitors amprenavir and retrovir. All three inhibitors clearly reduced virulence of the wild-type SC5314 (shown for pepstatin A [15 μM] in Fig. 2B) when compared to tissue damage and invasive potential in the absence of proteinase inhibitors (Fig. 2A).
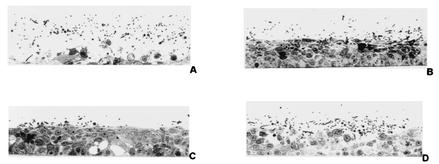
FIG. 2.
Appearance of RHVE 24 h after infection with C. albicans SC5314 (A), SC5314 in the presence of pepstatin A (15 μM) (B), sap1 (C), and sap2 (D) mutant cells. Severe vacuolization, edema, and detachment of all epithelial layers are only seen in SC5314 infection without the addition of pepstatin A (A). In contrast, a reduced-virulence phenotype was observed in the presence of 15 μM pepstatin A (B) and after infection with the sap1 (C) and the sap2 (D) mutants.
Morphology of RHVE and SAP expression after infection with sap mutants.
The ability of aspartyl proteinase inhibitors to reduce tissue damage of RHVE indicates that Saps may be involved in the pathogenesis of the artificial infection model. Since the expression of SAP1 and SAP2 was always detected when tissue damage was first observed, we analyzed the contribution of the corresponding Sap isoenzymes by first studying the virulence phenotype of the sap1 and sap2 null mutants in the RHVE model (Fig. 2C and D). Compared to the virulence of SC5314 (Fig. 2A), the invasive potential and the tissue damage induced by the sap1 and sap2 mutants were significantly reduced (Fig. 2C and D). Tissue damage was not prevented completely. We also compared the expression profile of other SAP genes in the sap1 and sap2 mutants with that of the wild type. Increased expression of SAP4 and SAP7 and the additional expression of SAP3 (which was never detected in wild-type cells) in the sap1 and sap2 mutants suggested an up-regulation of these genes due to the deletion of SAP1 or SAP2 (data not shown). In contrast, the virulence phenotypes of mutants lacking SAP3 (Fig. 3B) or SAP4 to SAP6 (Fig. 3C) were not attenuated compared to the phenotype of the wild type (Fig. 3A). In order to fulfill the molecular Koch's postulates (11), we reintroduced plasmids (pCIp10) carrying the SAP1 and SAP2 genes into the Ura− sap1 and sap2 mutants and compared the retransformant strains with these mutants, a mutant carrying the empty plasmid pCIp10 and the wild-type SC5314. The mutants carrying the empty plasmid had a phenotype similar to that of the mutants carrying the disruption cassette. Plasmid-borne expression of SAP1 and SAP2 in these mutants strains led to reconstitution of the pathogenic potential in experimental vaginal candidiasis (Fig. 4). Histological features were supplemented by measuring significant differences of LDH release as a marker of cell injury (Table 2).
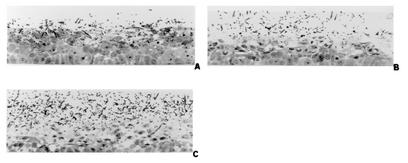
FIG. 3.
Appearance of RHVE 24 h after infection with C. albicans SC5314 (A), sap3 (B), and sap4-to-sap6 (C) mutant cells. Tissue damage caused by sap3 (B) and sap4-to-sap6 (C) mutants is not attenuated compared with SC5314 (A) infection. Histological damage after infection with the sap4-to-sap6 triple mutant (C) seemed to be increased compared with the parental strain (A).
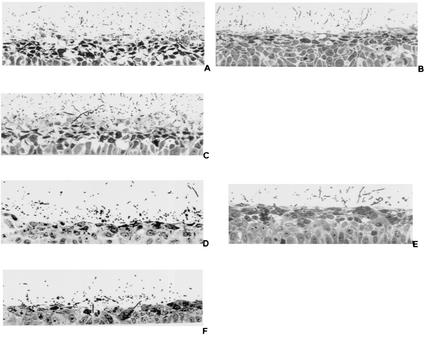
FIG. 4.
Appearance of RHVE 12 h after infection with C. albicans SC5314 (A and D), sap1 mutant (sap1::hisG/sap1::hisG::URA3::hisG) (B), SAP1 revertant (sap1::hisG/sap1::hisG/SAP1) (C), sap2 mutant (sap2::hisG/sap2::hisG::URA3::hisG) (E), and SAP2 revertant (sap2::hisG/sap2::hisG/SAP2) (F) cells. A similar degree of tissue damage was seen after infection with SC5314 (A and D) and the SAP1 (C) and SAP2 (F) reconstituted strains, while reduced tissue damage was observed for sap1 (B) and sap2 mutants (E).
TABLE 2.
Release of LDH by epithelial cells after infection with C. albicans parental and mutant strainsa
| Mutant (revertant) used | LDH release (U/liter) for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PBS
|
SC5314
|
Mutant
|
Revertant
|
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| sap1 (SAP1) | 8 | 1 | 83 | 6 | 36b | 4 | 88 | 9 |
| sap2 (SAP2) | 10 | 2 | 79 | 5 | 46b | 6 | 73 | 8 |
| sap3 | 25 | 4 | 105 | 8 | 88 | 5 | ||
| sap4 to sap6 | 25 | 4 | 105 | 8 | 148b | 9 | ||
Data represent analysis after 24 h of three independent experiments (mean plus or minus standard deviation).
Significantly different from values for SC5314 and SAP1 revertant, SC5314 and SAP2 revertant, or SC5314 and sap3 mutant (P < 0.005) as determined by the least-significant difference test.
Ultrastructural localization of Sap antigen.
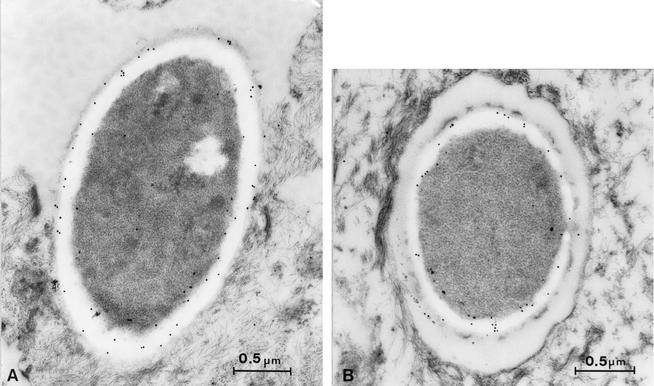
Initial lesions in the RHVE model correlated with high mRNA levels of SAP1 and SAP2 and low expression levels of SAP4 and SAP5 after 12 h. At 24 h expression levels of all these SAP genes increased concomitantly with damage of deeper epithelial parts. The fact that the virulence of the sap1 and sap2 mutants but not of the mutants sap3 and sap4 to sap6 was attenuated suggested that Sap1 and Sap2 but not Sap3 to Sap6 may contribute to this type of experimental candidiasis. To determine the ultrastructural localization of these different Saps in the in vitro model, we used two previously described polyclonal specific antibodies directed against Sap1 to Sap3 or Sap4 to Sap6 (2). Intensive immune reactivity to Sap1 to Sap3 was detected in the cell wall of all C. albicans cells after postembedding labeling with Sap1-to-Sap3 antibodies conjugated to 10-nm gold particles (Fig. 5A). Since expression of SAP3 was never observed in wild-type cells, we concluded that the polyclonal antibodies were detecting Sap1 and Sap2 proteins. Proteins Sap4 to Sap6 were also detected by immunogold labeling but at a significantly lower intensity (Fig. 5B). The number of Sap1-to-Sap3 gold particles was 75 ± 53 (mean plus or minus standard deviation), as opposed to 26 ± 17 particles for Sap4 to Sap6. These data were extracted from 10 randomly selected cells. The Sap4-to-Sap6 value was significantly different from the Sap1-to-Sap3 value as determined by the Fisher-Pitman test. Control experiments without the addition of the polyclonal antibodies showed the complete absence of any nonspecific immunogold labeling 12 h after infection with SC5314.
FIG. 5.
Electron microscopy with postembedding immunogold labeling, by use of 10-nm gold particles, in a sample of RHVE taken 12 h after infection with SC5314. Increased amounts of Sap1-to-Sap3 (A) compared to Sap4-to-Sap6 (B) antigen were detected within the cell wall of C. albicans yeast cells. The scale bar represents 0.5 μm.
SAP expression in human vaginal candidiasis.
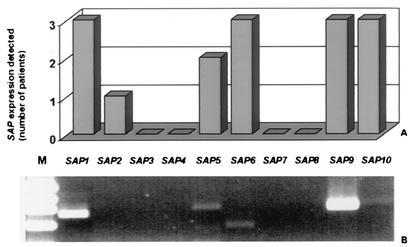
In order to evaluate whether the SAP expression pattern observed in the RHVE model reflects the expression pattern of SAP genes in C. albicans cells infecting human epithelia in vivo, we compared the experimental data to the SAP expression pattern in samples obtained from three patients with vaginal candidiasis. A smear of the lesions from each patient was shock frozen for RNA isolation. Another smear was cultured, and C. albicans was identified microbiologically in all patients. In all three samples, expression of SAP1, -6, -9, and -10 was observed. Samples from two patients were also positive for SAP5, and in one sample we also discovered transcripts for SAP2. No amplification products were detected for SAP3, -4, -7, and -8 in all three cases (Fig. 6).
FIG. 6.
Analysis of the in vivo expression of the SAP gene family in three patients with vaginal candidiasis (A) indicating the number of positive signals detected for each SAP gene. RT-PCR products of RNA (lanes 2 to 11) from patient 3 (B). Molecular mass marker pBR322 DNA/MvaI (M) (MBI Fermentas, St. Leon-Rot, Germany) with fragments that were 1,857, 1,058, 929, and 383 bp long (lane 1).
DISCUSSION
The pathogenesis of vaginal candidiasis is still unclear. As in the case for other types of Candida infections, both host and fungal attributes affect the development of this disease. Since Saps are known fungal virulence factors of mucosal infections, we investigated the expression of SAP genes in an in vitro model of vaginal candidiasis (RHVE) and in patient samples to study the pathogenic role of their gene products during epithelial tissue damage.
The 10 SAP genes were differentially expressed in patient samples and during the time course in our in vitro experiments. The predominant expressed SAP genes during late stages of in vitro infection were SAP1, SAP2, SAP4 to SAP7, SAP9, and SAP10. The onset of mucosal lesions always coincided with strong signals for SAP1, SAP2, and SAP9 and weaker SAP4, SAP5, and SAP10 transcripts. Analysis of the in vivo material from patients demonstrated that a similar set of SAP genes was expressed: SAP1, SAP6, SAP9, and SAP10 were identified in all and in additional SAP2 and SAP5 transcripts in two samples, while SAP3 was not detected, indicating that the expression profile of C. albicans cells in the in vitro model of vaginal candidiasis may also be found in cells infecting vaginal epithelium in vivo. Recent studies by Naglik et al. (J. Naglik, P. Shirlaw, C. Rodgers, G. Newport, D. Greenspan, J. Greenspan, N. Agabian, and S. Challacombe, Abstr. 6th Am. Soc. Microbiol. Conf. Candida Candidiasis, abstr. 48, 2002; and J. R. Naglik, C. A. Rodgers, P. J. Shirlaw, J. Dobbie, L. L. Fernandes-Naglik, D. Greenspan, N. Agabian, and S. J. Challacombe, unpublished data) analyzed expression of C. albicans SAP1-to-SAP8 expression during vaginal infection and colonization in humans. The data obtained in this study showed that SAP2 and SAP5 were the most commonly expressed genes during vaginal infection. The authors were able to demonstrate SAP2 expression during both vaginal colonization and infection, while SAP1 expression was found in the majority of patients but not in carriers. These results suggest a general importance of SAP2 for the viability of C. albicans and a distinct role of SAP1 for the infection process in vaginal candidiasis and are in agreement with our data. It is therefore possible that C. albicans strains from carriers with asymptomatic colonization express reduced levels of specific SAP genes in vivo, and it would be interesting to investigate whether there is a link between nonpathogenic isolates and the quantitative expression of certain SAP genes, e.g., SAP1. Immunoelectron microscopy studies with two specific polyclonal antibodies directed against Sap1 to Sap3 or Sap4 to Sap6 detected proteinase antigen at the early stage of experimental vaginal candidiasis in our study. Quantitative analysis confirmed the dominant secretion of proteinases for Sap1 to Sap3 in mucocutaneous infections (24, 25). Since the addition of different aspartyl proteinase inhibitors strongly reduced the virulence phenotype of SC5314, we concluded that Sap1 to -3 isoenzymes play a major role in these types of infections. Interestingly, we observed that epithelial lesions in the RHVE model were seen only at those time points when SAP1 transcripts were detectable, while high levels of SAP2, -9, and -10 transcripts were also detected in undamaged tissue. We concluded that SAP1 may play a dominant role in tissue damage in this model. Mutants lacking SAP1 were attenuated in their ability to damage the epithelial tissue. In addition, a reduced vaginopathogenic potential was also observed for a sap2 mutant, although SAP2 expression was also demonstrated during the early stages of colonization. This suggests that SAP2 may play a role during both colonization and infection in experimental vaginal candidiasis and that the activity of Sap2 contributes to tissue damage. The vaginopathic potential of both a sap3 mutant and a sap4-to-sap6 triple mutant was not attenuated compared to the virulence phenotype of the wild-type cells, suggesting a minor contribution of these SAP genes to virulence in experimental vaginal candidiasis. Surprisingly, the histological damage of the sap4-to-sap6 triple mutant seemed to be increased compared to that of the wild type, which could be explained by a compensatory up-regulation of SAP1 to SAP3 caused by the deletion of SAP4 to SAP6. This phenomenon was also seen previously in other virulence studies comparing the phenotype of the sap4-to-sap6 mutant with that of the wild type (24, 29). In fact, the SAP expression pattern of both the sap1 and the sap2 mutant in the RHVE model had an unusually high transcriptional level of other SAP genes (SAP3, SAP4, and SAP7) compared with the normal expression profile of the wild type, which may at least partially compensate the loss of SAP1 and SAP2. SAP9 and SAP10 expression was found in all samples investigated. Sequence comparisons showed that Sap9 and Sap10 differ from all other Sap isoenzymes not only by sequence homology but also by putative Glycosylphosphatidyl inositol anchor attachment sequences at their carboxy termini. Experiments studying the localization of these proteins suggest that these proteinases are in fact not secreted (A. Albrecht, M. Schaller, M. Monod, and B. Hube, unpublished results). These differences suggest a function of Sap9 and Sap10 that differs from the extracellular activity of Sap1 to -6. The exact role in pathogenesis of C. albicans infections remains to be investigated.
The virulence potential of the sap1 and the sap2 mutants was fully restored by plasmid-borne expression of SAP1 and SAP2 in these strains, indicating that the observed reduced virulence of these mutants was in fact due to the disruption of SAP1 and SAP2. To exclude the possibility that the lack of a functional copy of the URA3 gene contributed to the attenuated virulence phenotype of the sap mutants (1), we compared the URA3/ura3 sap mutants with the URA3/ura3 strain CAF2-1 and with the URA3/URA3 parent SC5314. In all experiments, the virulence phenotype of CAF2-1 was similar to that of the SC5314 strain, suggesting that at least in this RHVE model the lack of one URA3 copy does not influence virulence.
Evidence for the expression and dominant role of SAP1 and SAP2 in an experimental rat vaginitis model was previously reported by De Bernardis et al. (6, 8). The expression pattern and role of SAP genes are likely to be similar in human and rat vaginal infections. However, there appear to be distinct differences between vaginal and oral infections. Recently we showed a dominant role for SAP1 and SAP3 in a model of oral candidiasis and evidence for SAP1 and SAP3 expression in samples from patients with oral infection (24, 26). Naglik et al. demonstrated that expression of SAP1 and SAP3 was predominantly found in patients with oral candidiasis as opposed to oral carriers (20; Naglik et al., Abstr. 6th ASM Conf. Candida Candidiasis, 2002; and Naglik et al., unpublished). Although this and other studies confirmed the importance of SAP1 to SAP3 during mucosal infections, SAP3 transcripts of wild-type cells were never detected in our study, suggesting that expression of SAP3 is not essential for tissue damage of vaginal epithelia. Since the constituents and the pH of the maintenance medium for the in vitro oral and vaginal epithelial infection models were identical, we concluded that the different expression profiles in these two models were regulated by direct contact or other interactions with the various epithelial cell types, either oral or vaginal keratinocytes. It is not clear how the proteinases contribute to the tissue damage, since the pH of the maintenance medium in the RHVE is higher (pH 6.5) than the pH optima of Sap1 (pH 4.5) and Sap2 (pH 3.5) (2). One possible explanation would be local pH differences within the tissue during infection.
In summary, our results suggest that a distinct set of SAP genes are expressed during infection of vaginal epithelial tissue and that a similar gene expression can be observed in patients suffering from vaginal candidiasis. The gene expression pattern was similar but not identical to that observed for oral Candida infections. In particular, Sap1 and Sap2, but not Sap3 to Sap6, seem to play an important role during tissue damage in the vaginal model as demonstrated by the use of proteinase inhibitors and of mutants sap1 to sap6.
Acknowledgments
We thank E. Januschke, University of Munich, for excellent technical assistance and W. Burgdorf, University of Munich, and J. Naglik, Guy's Hospital London, for critical reading of the manuscript.
This study was supported by the Deutsche Forschungsgemeinschaft for M.S. (Sch 897/1-2; 1-3), H.C.K. (KO 1106/4-1), and B.H. (Hu 528/8).
Editor: T. R. Kozel
REFERENCES
- 1.Bain, J. M., C. Stubberfield, and N. A. Gow. 2001. Ura-status-dependent adhesion of Candida albicans mutants. FEMS Microbiol. Lett. 204:323-328. [DOI] [PubMed] [Google Scholar]
- 2.Borg-von Zepelin, M., S. Beggah, K. Boggian, D. Sanglard, and M. Monod. 1998. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol. Microbiol. 28:543-554. [DOI] [PubMed] [Google Scholar]
- 3.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 4.Cassone, A., F. De Bernardis, F. Mondello, T. Ceddia, and L. Agatensi. 1987. Evidence for a correlation between proteinase secretion and vulvovaginal candidosis. J. Infect. Dis. 156:777-783. [DOI] [PubMed] [Google Scholar]
- 5.De Bernardis, F., L. Agatensi, I. K. Ross, G. W. Emerson, R. Lorenzini, P. A. Sullivan, and A. Cassone. 1990. Evidence for a role for secreted aspartate proteinase of Candida albicans in vulvovaginal candidiasis. J. Infect. Dis. 161:1276-1283. [DOI] [PubMed] [Google Scholar]
- 6.De Bernardis, F., S. Arancia, L. Morelli, B. Hube, D. Sanglard, W. Schafer, and A. Cassone. 1999. Evidence that members of the secretory aspartyl proteinase gene family, in particular SAP2, are virulence factors for Candida vaginitis. J. Infect. Dis. 179:201-208. [DOI] [PubMed] [Google Scholar]
- 7.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bernardis, F., A. Cassone, J. Sturtevant, and R. Calderone. 1995. Expression of Candida albicans SAP1 and SAP2 in experimental vaginitis. Infect. Immun. 63:1887-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bernardis, F., F. Mondello, G. Scaravelli, A. Pachi, A. Girolamo, L. Agatensi, and A. Cassone. 1999. High aspartyl proteinase production and vaginitis in human immunodeficiency virus-infected women. J. Clin. Microbiol. 37:1376-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bernardis, F., P. A. Sullivan, and A. Cassone. 2001. Aspartyl proteinases of Candida albicans and their role in pathogenicity. Med. Mycol. 39:303-313. [DOI] [PubMed] [Google Scholar]
- 11.Falkow, S. 1988. Molecular Koch's postulates applied to microbial pathogenicity. Rev. Infect. Dis. 10:S274-S276. [DOI] [PubMed] [Google Scholar]
- 12.Felk, A., M. Kretschmar, A. Albrecht, M. Schaller, S. Beinhauer, T. Nichterlein, D. Sanglard, H. C. Korting, W. Schafer, and B. Hube. 2002. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70:3689-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 15.Hube, B., and J. Naglik. 2001. Candida albicans proteinases: resolving the mystery of a gene family. Microbiology 147:1997-2005. [DOI] [PubMed] [Google Scholar]
- 16.Hube, B., D. Sanglard, F. C. Odds, D. Hess, M. Monod, W. Schafer, A. J. Brown, and N. A. Gow. 1997. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun. 65:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim, A. S., S. G. Filler, D. Sanglard, J. E. Edwards, Jr., and B. Hube. 1998. Secreted aspartyl proteinases and interactions of Candida albicans with human endothelial cells. Infect. Immun. 66:3003-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maneu, V., A. M. Cervera, J. P. Martinez, and D. Gozalbo. 1996. Molecular cloning and characterization of a Candida albicans gene (EFB1) coding for the elongation factor EF-1 beta. FEMS Microbiol. Lett. 145:157-162. [DOI] [PubMed] [Google Scholar]
- 19.Murad, A. M., P. R. Lee, I. D. Broadbent, C. J. Barelle, and A. J. Brown. 2000. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16:325-327. [DOI] [PubMed] [Google Scholar]
- 20.Naglik, J. R., G. Newport, T. C. White, L. L. Fernandes-Naglik, J. S. Greenspan, D. Greenspan, S. P. Sweet, S. J. Challacombe, and N. Agabian. 1999. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect. Immun. 67:2482-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosdy, M., B. A. Bernard, R. Schmidt, and M. Darmon. 1986. Incomplete epidermal differentiation of A431 epidermoid carcinoma cells. In Vitro Cell. Dev. Biol. 22:295-300. [DOI] [PubMed] [Google Scholar]
- 22.Sanglard, D., B. Hube, M. Monod, F. C. Odds, and N. A. Gow. 1997. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect. Immun. 65:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaller, M., B. Hube, M. W. Ollert, W. Schafer, M. Borg-von Zepelin, E. Thoma-Greber, and H. C. Korting. 1999. In vivo expression and localization of Candida albicans secreted aspartyl proteinases during oral candidiasis in HIV-infected patients. J. Investig. Dermatol. 112:383-386. [DOI] [PubMed] [Google Scholar]
- 24.Schaller, M., H. C. Korting, W. Schafer, J. Bastert, W. Chen, and B. Hube. 1999. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol. Microbiol. 34:169-180. [DOI] [PubMed] [Google Scholar]
- 25.Schaller, M., C. Schackert, H. C. Korting, E. Januschke, and B. Hube. 2000. Invasion of Candida albicans correlates with expression of secreted aspartic proteinases during experimental infection of human epidermis. J. Investig. Dermatol. 114:712-717. [DOI] [PubMed] [Google Scholar]
- 26.Schaller, M., W. Schafer, H. C. Korting, and B. Hube. 1998. Differential expression of secreted aspartyl proteinases in a model of human oral candidosis and in patient samples from the oral cavity. Mol. Microbiol. 29:605-615. [DOI] [PubMed] [Google Scholar]
- 27.Sobel, J. D., S. Faro, R. W. Force, B. Foxman, W. J. Ledger, P. R. Nyirjesy, B. D. Reed, and P. R. Summers. 1998. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 178:203-211. [DOI] [PubMed] [Google Scholar]
- 28.Sobel, J. D., A. Hasegawa, F. Debernardis, D. Adriani, G. Pellegrini, A. Cassone, P. L. Fidel, C. G. Haidaris, F. Gigliotti, A. G. Harmsen, S. Fujita, K. Yamamoto, K. Makimura, K. Shibuya, K. Uchida, and H. Yamaguchi. 1998. Selected animal models: vaginal candidosis, Pneumocystis pneumonia, dermatophytosis and trichosporosis. Med. Mycol. 36:129-136. [PubMed] [Google Scholar]
- 29.Watts, H. J., F. S. Cheah, B. Hube, D. Sanglard, and N. A. Gow. 1998. Altered adherence in strains of Candida albicans harbouring null mutations in secreted aspartic proteinase genes. FEMS Microbiol. Lett. 159:129-135. [DOI] [PubMed] [Google Scholar]
- 30.Wróblewski, F., and S. L. John. 1955. Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biol. Med. 90:210-213. [DOI] [PubMed] [Google Scholar]