Abstract
Background
Breast spindle cell tumours (BSCTs), although rare, represent a heterogeneous group with different treatment modalities. This work was undertaken to evaluate the utility of fine needle aspiration cytology (FNAC), histopathology and immunohistochemistry (IHC) in differentiating BSCTs.
Methods
FNAC of eight breast masses diagnosed cytologically as BSCTs was followed by wide excision biopsy. IHC using a panel of antibodies against vimentin, pan-cytokeratin, s100, desmin, smooth muscle actin, CD34, and CD10 was evaluated to define their nature.
Results
FNAC defined the tumors as benign (n = 4), suspicious (n = 2) and malignant (n = 3), based on the cytopathological criteria of malignancy. Following wide excision biopsy, the tumors were reclassified into benign (n = 5) and malignant (n = 3). In the benign group, the diagnosis was raised histologically and confirmed by IHC for 3 cases (one spindle cell lipoma, one myofibroblastoma and one leiomyoma). For the remaining two cases, the diagnosis was set up after IHC (one fibromatosis and one spindle cell variant of adenomyoepithelioma). In the malignant group, a leiomyosarcoma was diagnosed histologically, while IHC was crucial to set up the diagnosis of one case of spindle cell carcinoma and one malignant myoepithelioma.
Conclusion
FNAC in BSCTs is an insufficient tool and should be followed by wide excision biopsy. The latter technique differentiate benign from malignant BSCTs and is able in 50% of the cases to set up the definite diagnosis. IHC is of value to define the nature of different benign lesions and is mandatory in the malignant ones for optimal treatment. Awareness of the different types of BSCTs prevents unnecessary extensive therapeutic regimes.
Background
BSCTs although rare, include benign and malignant lesions, necessitating different therapeutic approaches. Within the benign group that may mimics carcinoma clinically and radiologically, and are treated by conservative approach, one should differentiate between spindle cell lipoma (SCL) [1,2] myofiboblastoma (MFB) [3], fibromatosis [4,5], myoepithelioma (ME) [6,7], leiomyoma [8,9], solitary fibrous tumor (SFT) [10], nodular fasciitis [4,11], and benign nerve sheath tumor [12,13]. Noteworthy, although benign in nature, some these tumors such as fibromatosis and SFT have tendency for local recurrence [4,5,11]; therefore the distinction between them is of prognostic significance. In the malignant BSCTs, should be identified, malignant phyllode tumor (PT) [14,15], leiomyosarcoma [16,17] malignant myoepithelioma (MME) [6,7], malignant peripheral nerve sheath tumor [18], spindle cell carcinoma (SCC) [19,20] and metastatic spread from sarcomas.
This study emphasizes the combined role of FNAC and histopathology and the importance of IHC in BSCTs as the treatment modalities are different and crucial for patients' care.
Materials and methods
Patients and tissues
The study involved eight female cases complaining of breast masses and referred from surgeons for FNAC. Clinically, all lesions were highly suggestive of malignancy. The initial cytological diagnosis was that of BSCTs. Malignancy was ruled out in four cases due to [1] lack of cytologic atypia, [2] absence of necrosis, and [3] paucity of mitotic figures. Wide excision biopsy enabled to differentiate between benign and malignant BSCTs but was insufficient to reach a final definite diagnosis. Therefore, the precise nomination and the cell of origin or the nature of the lesions was based on IHC.
Immunohistochemistry
IHC was effectuated on 5 μm thick paraffin embedded tissue sections. The antibodies in the study, their sources, clones, the heat induced epitope antigen retrieval (HIER) and dilutions are illustrated in table 1. HIER was done by heating the slides in microwave (800 watts) for 15 minutes (3 cycles × 5 minutes).
Table 1.
The antibodies used in the study
| Antibodies | Source | Antibody | HIER | Dilution | Staining interpretation |
| Vimentin | DakoCytomation | Mo V9 | 2 | 1:50 | Cytoplasmic |
| Cytokeratin | DakoCytomation | Mo AE1/AE3 | 1 | 1: 50 | Cytoplasmic |
| S100 | DakoCytomation | Rabbit polyclonal | 1 | 1: 500 | Cytoplasmic +/-nuclear |
| Desmin | DakoCytomation | Mo D33 | 2 | prediluted | Cytoplasmic |
| SMA | DakoCytomation | Mo 1A4 | 2 | 1:50 | Cytopmasmic |
| CD34 | Biogenex | Mo QBEnd 10 | 2 | Prediluted | Membranous |
| CD10 | Novocastra | NCL-CD10-270 | 1 | 1:50 | Membranous |
Mo: mouse monoclonal.
1-Tris-EDTA pH9.0.
2-Citrate buffer pH6.0.
In brief, slides were hydrated in descending grades of alcohol followed by distilled water. Endogenous peroxidase activity was quenched by 0.3% hydrogen peroxide for 5 minutes followed by rinsing in distilled water and three times wash in phosphate buffer saline (PBS) Ph 7.4. The antibodies were incubated for 30 minutes at room temperature, and then the slides were rinsed in successive bathes of PBS. The revelation was done by the LSAB-2 detection kit (Dakocytomation) according to the manufacturer's instructions. Finally, diaminobenzidine tetrachloride (DAB) was applied for 5 minutes. Slides were counterstained in Harris haematoxylin (Hx), dehydrated, cleared in xyelene and coverslipped.
The control tissue for all antibodies was the normal breast tissue. In addition, blood vessels inside the tumors were used as an internal control tissue for all the antibodies except cytokeratin. Ommittment of the antibodies were used as negative control for the procedure. The staining interpretation is summarized in table 1.
IHC results were evaluated in semi-quantitative manner as following: 0, negative staining with positive control, -/+ when rare cells not exceeding 10% of the total population are positive, +/- when positivity is between 10 % and 50%, and + when more than 50% of the cells are positive.
Results
The age group for the BSCTs ranged from 37–69 years with a median age of 50.37 years. The common denominator on FNAC was the presence of spindle cells without ductal epithelial cells. Lesions diagnosed as benign BSCTs were formed by variable mixtures of spindle, oval and ovoid cells with bland nuclei, inconspicuous nucleoli and low mitotic rate (fig 1a). The presence of discohesive cells with mitotic activity, nuclear atypia and abnormal mitosis in a necrotic background favoured the diagnosis of malignant tumours (Fig 4a, b). In two controversial cases, suspicion of malignancy was raised. The reports were signed out as begnin, suspicious or malignant BSCTs. Cytological evaluation was followed by wide excision biopsy. Grossly, all the lesions were well circumscribed except two. Histologically, the main finding of all the lesions was the presence of spindle cells. Here also, the bland cellular morphology of the spindle cells, mitotic activity not exceeding 2 mitosis/10 high power fields (hpfs) and absence of necrosis, qualify the lesions as benign. Otherwise, the lesion was diagnosed as malignant. Depending on the different proportions of other cellular component, a proposed diagnosis or a differential diagnosis was based. Table 2 summarizes the patients' pathological data and table 3 the IHC profile of the diagnosed cases. Figures 1 through 5 illustrate the cytological, histopathological and IHC findings of some cases.
Figure 1.
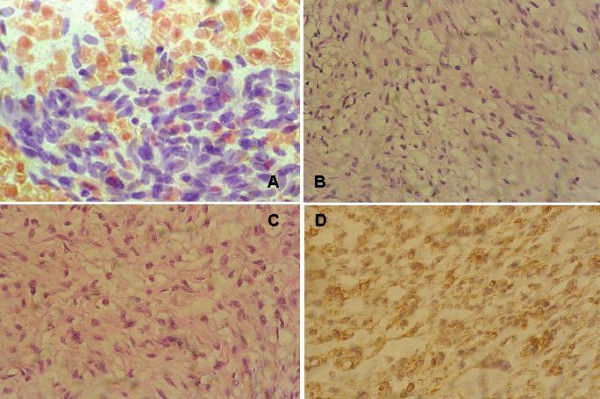
spindle cell lipoma. (a) Benign cytology BSCT, moderately cellular smear with benign looking spindle cells, Papanicolaou × 400. (b) Bland spindle cells intermingled with mature adipocytes and thick collagen bundles, H&E × 400. (c) Another field of the same case where the adipocytes are less prominent compared to "b", H&E × 400. (d) Adipocytes and spindle cells positive for CD34 IHC, DAB, Hx, × 400. See additional files 1, 2, 3, 4 for higher resolution images.
Figure 4.
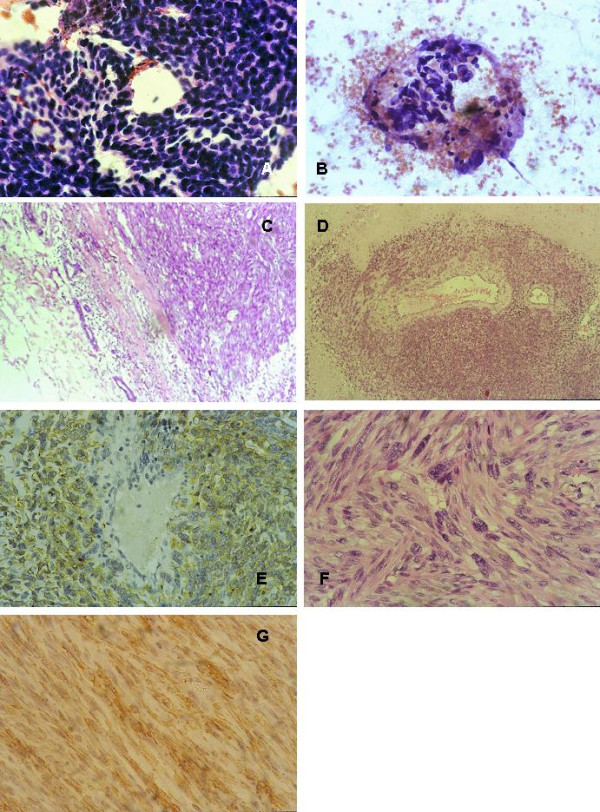
leiomyosarcoma. (a) Malignant BSCTs cytology, cellular smear with sheets of spindle cells, Papanicolaou staining × 400. (b) Another field showing neoplastic ovoid cells, Papanicolaou staining × 400. (c) Well circumscribed tumor pushing the normal ductal cells at the periphery, H&E × 10. (d) Spindle cells are merging from blood vessels, H&E, × 10. (e) Desmin IHC highlighting cells merging from the blood vessels, DAB Hx × 400. (f) Intersecting fascicles of pleomorphic malignant spindle cells having cigar shaped blunt ended nuclei, H&E, × 400. (g) Desmin IHC, DAB, Hx × 400. See additional files 16, 17, 18, 1920, 21, 22 for higher resolution images.
Table 2.
Patients' pathological summaries
| N° | Age | Cytology | Gross pathology | Histopathology | Provisional diagnosis |
| 1 | 54 | Benign | Well defined, 2 cm | Spindle cells, adipocytes, thick collagen bundles in variable proportions | SCL? |
| 2 | 55 | Suspicious | Well defined, 3 cm | Spindle and oval cells haphazardly arranged in short intersecting fascicles, ribbons of collagen | MFB? |
| 3 | 46 | Benign | Irregular infiltrative, 3.7 cm | Spindle cells forming interlacing bundles | Benign BSCTs? |
| 4 | 37 | Benign | Well defined, 2.5 cm | Cellular neoplasm formed of spindle and oval cells, arranged in storiform pattern or in parallel fascicles | Benign BSCTs? |
| 5 | 51 | Suspicious | Well defined with irregular margins, 3 cm | Highly cellular neoplasm formed of spindle cells surrounding epithelial lined spaces, high mitotic activity 20/10 hpfs | Malignant PT? |
| 6 | 39 | Benign | Well defined, 4 cm | Interlacing bundles of spindle-shaped cells with cigar shaped-nuclei, eosinophilic cytoplasm, rare mitotic figures | leiomyoma |
| 7 | 52 | Malignant | Well defined, 5 cm | Eosinophilic spindle cells arranged in interlacing bundles, marked cellular and nuclear atypia, mitosis 7/10 hpfs, wide areas of necrosis | leiomyosarcoma |
| 8 | 69 | Malignant | Well defined, 4 cm | Highly pleomorphic spindle cells, high mitotic activity 23/10hpfs | Malignant BSCTs? |
Hpfs: high power fields.
Table 3.
IHC profile of the studied cases
| Provisional diagnosis | Vimentin | CK | S100 | Desmin | SMA | CD34 | CD10 | Final diagnosis | |
| 1 | SCL? | + | - | - | - | - | + | - | SCL |
| 2 | MFB? | + | - | - | + | +/- | + | - | MFB |
| 3 | Benign BSCTs? | + | - | - | - | + | - | Fibromatosis | |
| 4 | Benign BSCTs? | - | + | + | - | +/- | - | -/+ | ME |
| 5 | Malignant PT? | - | + | + | - | -/+ | +/- | MME | |
| 6 | leiomyoma | + | - | - | + | + | - | - | Leiomyoma |
| 7 | leiomyosarcoma | + | - | - | + | + | - | - | Leiomyosarcoma |
| 8 | Malignant BSCTs? | -/+ | + | + | - | + | - | + | SCC |
- negative.
-/+ rare cells not exceeding 10% of the total population.
+/-between 10 and 50%.
+ more than 50%.
Figure 5.
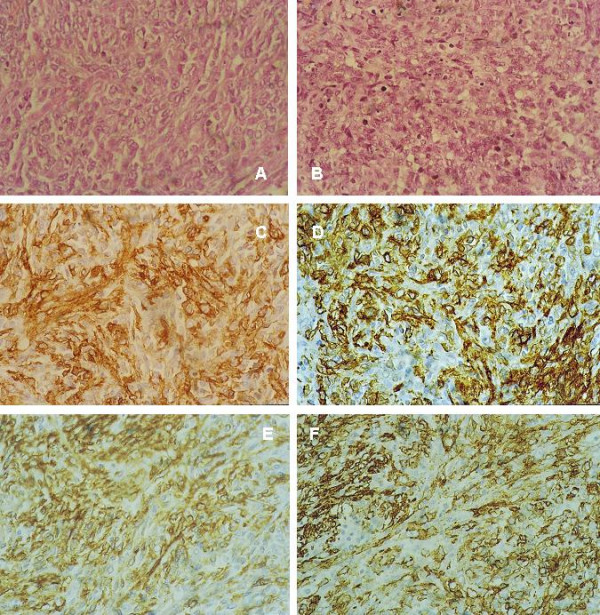
spindle cell carcinoma. (a) Area with bland morphology compared to "fig 4b", H&E ×400. (b) Area with pleomorphic cells, and high mitotic activity, H&E × 400. (c) CK IHC, DAB, Hx, × 400. (d) CD10 IHC, DAB, Hx, × 400. (e) s100p, DAB, Hx, × 400 f- SMA IHC, DAB, Hx, × 400. See additional files 23, 24, 25, 2627, 28 for higher resolution images.
Discussion
The diagnosis of the first case (fig 1), SCL was raised on histopathological basis and confirmed after IHC. Histologically, the lesion consisted essentially of spindle cells intermingled with adipocytes and collagen bundles, features described for soft tissue and breast SCL [1,2,21]. However, contrary to the infiltrative nature of the lesion described by Mulvany et al [2], no mammary tissue was seen entrapped in SCL in the present work. In addition, mast cells usually identified in SCL of soft tissue [21] were not found here. IHC confirmed the histological diagnosis and showed reactivity of the spindle cells for vimentin and CD34, finding previously described [2,21].
MFB is a tumor closely related to SCL [22,23] (case 2, fig 2). However, contrary to the begnin cytological nature of SCL, MFB was unobvious based on the presence of nuclear pleomorphism in some spindle cells. This finding is consistent with other studies, cytologically misinterpreting this tumor as malignant soft tissue tumor or PT [24,25]. Histologically, MFB was formed of spindle cells, hyalinized collagen bundles and adipocytes, criteria previously described by in the original study by Wargotz and colleagues[3]. The diagnosis of this case was based on IHC positivity for vimentin, CD34, desmin and focal positivity for SMA, a finding described in the literature [26]. While all the literature agrees on the positivity of MFB for vimentin and CD34, controversies regarding the positivity for desmin and SMA are reported [3,27].
Figure 2.
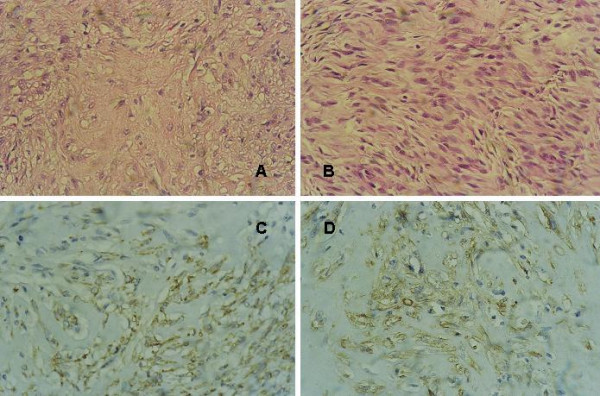
myofibroblastoma. (a) Most of the tumor is formed of hyalinized collagen bundles surrounded by spindle cells, H&E × 400. (b) Cellular area formed of palissading spindle cells, H&E × 400. (c) Staining of spindle and oval cells is more pronounced in the lower right part, CD34 IHC, DAB, Hx × 400. (d) Focal area of spindle and oval cells positive for SMA, IHC, DAB, Hx ×400. See additional files 5, 6, 7, 8 for higher resolution images.
This tumor, of myofibroblastic origin, is capable of diverse lines of differentiation [28]; and based on the proportion of the spindle cells, collagenous stroma and cellular pleomorphism, is subclassified as classic, collagenized, epithelioid, cellular, lipomatous and variants resembling SFT [22,27,29]. Therefore this case was signed out as collagenized MFB. It is crucial to recognize this tumor and to distinguish it from its malignant counterpart, myofibrosarcoma. The latter, characterized by marked cellular pleomorphism, infiltrating margins and high mitotic rate, necessitate more aggressive extensive and radical surgery [30].
At a molecular level, MFB shares cytogenetic abnormalities with SCL [23], leading to the hypothesis of a dual, myofibroblastic and lipomatous, differentiation from a common pluripotential mesenchymal precursor cell, represented by the vimentin+/CD34+ fibroblast of the mammary stroma as suggested by Magro and co-workers [28]. In latter studies, the same investigators postulated that the "vimentin+/CD34+ cell" is the precursor cell of all benign spindle cell neoplasm [31,32]. This hypothesis provides explanation for the phenotypic heterogeneity of these neoplasm and their variable IHC profiles taking into consideration the well-known inherent plasticity of the "vimentin+/CD34+ cell" to differentiate toward several mesenchymal lines.
Breast fibromatosis (case 3) is a lesion that clinically and radiologically suggests breast cancer [33,34]. Fibromatosis is an infiltrative fibroblastic and myofibroblastic proliferation with significant risk for local recurrence, without metastatic potential. Specific histological features, such as size, cellularity, atypia, and mitotic figures, are not helpful in predicting recurrence [5]. The diagnosis of fibromatosis in this work was based on positivity of the spindle cells for vimentin and SMA, a finding described in other studies [35,36].
ME tumors are defined as lesions arising from or composed of a dominant to pure population of myoepithelial cells (ME cells) in the WHO classification 2003 [7]. ME cells are immunoreactive for SMA, CD10, s100, CK and high molecular weight CK [7,37,38]. ME tumors are divided into myoepitheliosis, adenomyoepithelial adenosis, ME and MME. A case of spindle cell ME (case 4) has been diagnosed by IHC. Cells were strongly reactive for CK, s100, SMA and focally positive for CD10. Variable levels of expression for CD10 have been reported depending on the type of ME tumors [39]. It has been postulated that CD10 expression in combination with SMA is more informative on ME cells' nature [40]. A second tumor encountered here of ME origin is MEC (case 5, fig 3). Histologically, it mimicked malignant PT, as it was composed of malignant spindle cells surrounding luminal epithelia. However, based on the negativity of the spindle cells for CD34 and their positivity for CK, s100, SMA and CD10 [37,38], the diagnosis of malignant PT have been excluded. This diagnosis is important in view of the aggressive behaviour of MME compared to malignant PT [41,42].
Figure 3.
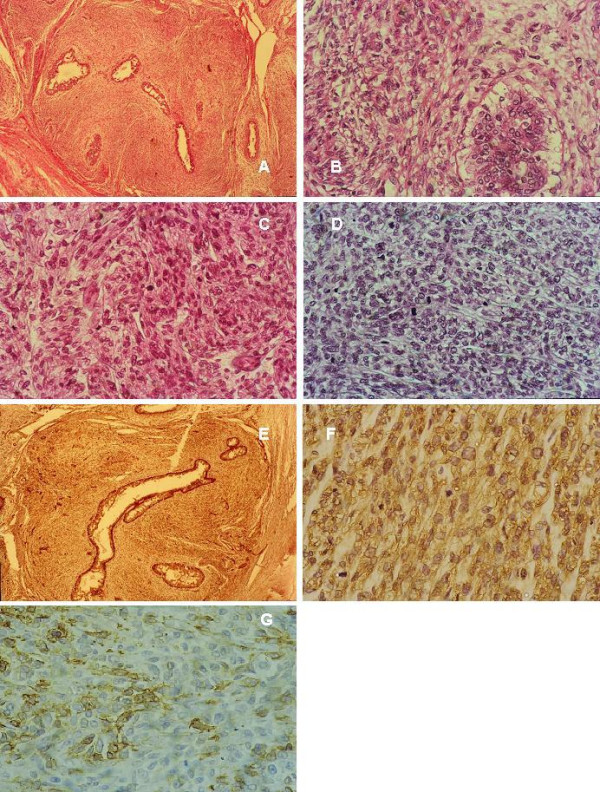
myoepithelial carcinoma. (a) Spindle cell proliferation surrounding epithelial lumina, H&E × 400. (b) Higher power view showing pleomorphic spindle cells with eosinoplilic and clear cytoplasm surrounding an epithelial lined space, H&E × 400. (c) Another area showing pleomorphic spindle and ovoid cells with eosinophilic cytoplasm arranged in fascicles; cells have high mitotic activity, H&E × 400. (d) Pleomorphic spindle and ovoid cells with clear cytoplasm arranged in wavy fascicles, H&E × 400. (e) s100 p IHC staining of the myoepithelial cells around the epithelial lumina and the neoplastic spindle cells × DAB, Hx, ×10. (f) Higher magnification of "fig 4e" showing staining of almost all the cells for s100 p IHC, DAB, Hx, × 400. (g) CD10 IHC, DAB, Hx, × 400. see additional files 9, 10, 11, 1213, 14, 15 for higher resolution images.
Two smooth muscle tumors had been encountered in this study, leiomyoma and leiomyosarcoma. These tumors, since their initial description by Strong [8], are rare breast neoplasm. Two types of breast leiomyoma are identified, superficial and vascular leiomyoma. Superficial leiomyomas, are located in the skin and subcutaneous tissues and involve the nipple or areolar region, while vascular leiomyomas are located deep within the breast parenchyma and are less common than the superficial counterpart. The true histogenesis of breast smooth muscle tumors remains unknown. Cells may be considered as hamartomatous proliferation of smooth muscle surrounding blood vessels, embryonal migration of smooth-muscle cells from the nipple, differentiation from multipotent mesenchymal cells in breast tissue or derivation from myoepithelial cells of breast ducts with frank differentiation to smooth muscle [9,43]. The leiomyoma diagnosed in the present work (case 6) was a deep one arising from smooth muscles around blood vessels. Histologically, it demonstrated interlacing bundles of spindle cells with blunt-ended cigar shaped nuclei, and eosinophilic cytoplasm. There was no nuclear atypia, no hypercellularity. The mitotic rate was low and necrosis was absent. However, the importance resides in differentiating leiomyoma from leiomyosarcoma. The latter diagnosis has been made for case 7 (fig 4). On cytological basis, it was difficult to nominate the lesion which was designed as malignant BSCT. The same difficulty has been reported in other studies, as the tumor was misdiagnosed as poorly differentiated ductal carcinoma or as malignant PT [16,17]. By the contrary, histologically, it fulfilled the criteria for diagnosing leiomyosarcoma. In addition, cells were centred on blood vessels, a feature identified for the leiomyoma case. On IHC bases, both leiomyoma and leiomyosarcoma were positive for both SMA and desmin (fig 5).
SCC of the breast, account for less than 1% of invasive carcinoma [44]. It represents a variant of metaplastic carcinoma (MSC), and includes a wide spectrum of lesions with histomorphologic and nuclear features ranging from overtly malignant to mildly atypical. The case presented in this study (case 8, fig 5) was suspicious for malignancy on FNAC, based on the presence of some atypical nuclei in the spindle cells and scattered mitotic activity. This corresponded histologically to bland areas with mildly pleomorphic cells and low mitotic activity, while most of the tumor was formed of frankly malignant cells with a mitotic activity up to 23 mitosis/10 hpfs. Vimentin, CK, SMA s100 and CD10 were positive on the spindle cells while desmin and CD34 were negative. The expression of SMA, s100 and CD10 on SCC favour the current concept of its myoepithelial origin [15,19,45,46]. Controversies in the literature exist concerning the prognosis being better or worse compared to ordinary breast duct carcinoma [19,20,47-49].
Conclusion
In conclusion, although BSCTs are infrequent, awareness of this category is essential for patient categorization and optimal therapy. The combination of conventional H&E and IHC using a small panel of antibodies is fundamental. The role of cytology is less obvious in this category.
Abbreviations
BSCTs: breast spindle cell tumors, SCL: spindle cell lipoma, MFB: myofibroblastoma, ME: myoepithelioma, MME: malignant myoepithelioma, SCC: spindle cell carcinoma, SFT: solitary fibrous tumor, PT: phyllode tumor, CK: cytokeratin, SMA: smooth muscle actin, hpfs: high power fields, Hx: hematoxylin
Competing interests
The author(s) declare that they have no competing interests.
Supplementary Material
High resolution image of figure 1a
High resolution image of figure 1b
High resolution image of figure 1c
High resolution image of figure 1d
High resolution image of figure 2a
High resolution image of figure 2b
High resolution image of figure 2c
High resolution image of figure 2d
High resolution image of figure 3a
High resolution image of figure 3b
High resolution image of figure 3c
High resolution image of figure 3d
High resolution image of figure 3e
High resolution image of figure 3f
High resolution image of figure 3g
High resolution image of figure 4a
High resolution image of figure 4b
High resolution image of figure 4c
High resolution image of figure 4d
High resolution image of figure 4e
High resolution image of figure 4f
High resolution image of figure 4g
High resolution image of figure 5a
High resolution image of figure 5b
High resolution image of figure 5c
High resolution image of figure 5d
High resolution image of figure 5e
High resolution image of figure 5f
Acknowledgments
Acknowledgements
I gratefully thank the skilful technical assessment of Mr. Mohammed El Laboudy and Mr. Reda Shaheen.
References
- Smith DN, Denison CM, Lester SC. Spindle cell lipoma of the breast. A case report. Acta Radiol. 1996;37:893–895. doi: 10.1177/02841851960373P290. [DOI] [PubMed] [Google Scholar]
- Mulvany NJ, Silvester AC, Collins JP. Spindle cell lipoma of the breast. Pathology. 1999;31:288–291. doi: 10.1080/003130299105188. [DOI] [PubMed] [Google Scholar]
- Wargotz ES, Weiss SW, Norris HJ. Myofibroblastoma of the breast. Sixteen cases of a distinctive benign mesenchymal tumor. Am J Surg Pathol. 1987;11:493–502. doi: 10.1097/00000478-198707000-00001. [DOI] [PubMed] [Google Scholar]
- Torngren S, Frisell J, Nilsson R, Wiege M. Nodular fasciitis and fibromatosis of the female breast simulating breast cancer. Case reports. Eur J Surg. 1991;157:155–158. [PubMed] [Google Scholar]
- Wargotz ES, Norris HJ, Austin RM, Enzinger FM. Fibromatosis of the breast. A clinical and pathological study of 28 cases. Am J Surg Pathol. 1987;11:38–45. doi: 10.1097/00000478-198701000-00005. [DOI] [PubMed] [Google Scholar]
- Tavassoli FA. Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. 1991;15:554–568. doi: 10.1097/00000478-199106000-00004. [DOI] [PubMed] [Google Scholar]
- Tavassoli FA, Soares J. Myoepithelial lesions. In: Tavassoli FA, Devilee P, editor. pathology and genetics Tumors of the breast and female genital organs. WHO classification of tumors. IARC Press; 2003. [Google Scholar]
- Strong LW. Leiomyoma of the breast. Am J Obstet. 1913;68:53–55. [Google Scholar]
- Diaz-Arias AA, Hurt MA, Loy TS, Seeger RM, Bickel JT. Leiomyoma of the breast. Hum Pathol. 1989;20:396–399. doi: 10.1016/0046-8177(89)90052-X. [DOI] [PubMed] [Google Scholar]
- Damiani S, Miettinen M, Peterse JL, Eusebi V. Solitary fibrous tumour (myofibroblastoma) of the breast. Virchows Arch. 1994;425:89–92. doi: 10.1007/BF00193955. [DOI] [PubMed] [Google Scholar]
- Dahlstrom J, Buckingham J, Bell S, Jain S. Nodular fasciitis of the breast simulating breast cancer on imaging. Australas Radiol. 2001;45:67–70. doi: 10.1046/j.1440-1673.2001.00879.x. [DOI] [PubMed] [Google Scholar]
- Tokita K, Nakano A, Suko S, Arase M. Benign schwannoma of the breast: report of a case. Surg Today. 1996;26:449–452. doi: 10.1007/BF00311936. [DOI] [PubMed] [Google Scholar]
- Uchida N, Yokoo H, Kuwano H. Schwannoma of the breast: report of a case. Surg Today. 2005;35:238–242. doi: 10.1007/s00595-004-2904-4. [DOI] [PubMed] [Google Scholar]
- Bennett IC, Khan A, De Freitas R, Chaudary MA, Millis RR. Phyllodes tumours: a clinicopathological review of 30 cases. Aust N Z J Surg. 1992;62:628–633. doi: 10.1111/j.1445-2197.1992.tb07534.x. [DOI] [PubMed] [Google Scholar]
- Dunne B, Lee AH, Pinder SE, Bell JA, Ellis IO. An immunohistochemical study of metaplastic spindle cell carcinoma, phyllodes tumor and fibromatosis of the breast. Hum Pathol. 2003;34:1009–1015. doi: 10.1053/S0046-8177(03)00414-3. [DOI] [PubMed] [Google Scholar]
- Szekely E, Madaras L, Kulka J, Jaray B, Nagy L. Leiomyosarcoma of the breast. Pathol Oncol Research. 2001;7:151–153. doi: 10.1007/BF03032583. [DOI] [PubMed] [Google Scholar]
- Jun Wei X, Hiotis K, Garcia R, Hummel Levine P. Leiomyosarcoma of the breast: a difficult diagnosis on fine-needle aspiration biopsy. Diagn Cytopathol. 2003;29:172–178. doi: 10.1002/dc.10359. [DOI] [PubMed] [Google Scholar]
- Medina-Franco H, Gamboa-Dominguez A, de La Medina AR. Malignant peripheral nerve sheath tumor of the breast. Breast J. 2003;9:332. doi: 10.1046/j.1524-4741.2003.09420.x. [DOI] [PubMed] [Google Scholar]
- Al-Bozom IA, Abrams J. Spindle cell carcinoma of the breast, a mimicker of benign lesions: case report and review of the literature. Arch Pathol Lab Med. 1996;120:1066–1068. [PubMed] [Google Scholar]
- Carter MR, Hornick JL, Lester S, Fletcher CD. Spindle cell (sarcomatoid) carcinoma of the breast: a clinicopathologic and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 2006;30:300–309. doi: 10.1097/01.pas.0000184809.27735.a1. [DOI] [PubMed] [Google Scholar]
- Miettien MM, Mandhal M. Spindle cell lipoma, pleomorphic lipoma. In: Fletcher CDM, editor. Tumours of soft tissue and bone. WHO classification of tumors. IARC Press; 2002. [Google Scholar]
- Magro G, Fraggetta F, Torrisi A, Emmanuele C, Lanzafame S. Myofibroblastoma of the breast with hemangiopericytoma-like pattern and pleomorphic lipoma-like areas. Report of a case with diagnostic and histogenetic considerations. Pathol Res Pract. 1999;195:257–262. doi: 10.1016/S0344-0338(99)80044-2. [DOI] [PubMed] [Google Scholar]
- Pauwels P, Sciot R, Croiset F, Rutten H, Van den Berghe H, Dal Cin P. Myofibroblastoma of the breast: genetic link with spindle cell lipoma. J Pathol. 2000;191:282–285. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH635>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Powari M, Srinivasan R, Radotra BD. Myofibroblastoma of the male breast: a diagnostic problem on fine-needle aspiration cytology. Diagn Cytopathol. 2002;26:290–293. doi: 10.1002/dc.10046. [DOI] [PubMed] [Google Scholar]
- Simsir A, Cangiarella J, Boppana S, Waisman J. Aspiration cytology of the collagenized variant of mammary myofibroblastoma: a case report with review of the literature. Diagn Cytopathol. 2001;24:399–402. doi: 10.1002/dc.1088. [DOI] [PubMed] [Google Scholar]
- Schmitt-Graff A, Desmouliere A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch. 1994;425:3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- Lazaro-Santander R, Garcia-Prats MD, Nieto S, Andres-Gozalvo C, Cortes-Vizcaino V, Vargas-Holguin S, Vera-Roman JM. Myofibroblastoma of the breast with diverse histological features. Virchows Arch. 1999;434:547–550. doi: 10.1007/s004280050382. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Ushigome S. Myofibroblastoma of the breast with diverse differentiations. Arch Pathol Lab Med. 1997;121:599–603. [PubMed] [Google Scholar]
- Magro G, Michal M, Vasquez E, Bisceglia M. Lipomatous myofibroblastoma: a potential diagnostic pitfall in the spectrum of the spindle cell lesions of the breast. Virchows Arch. 2000;437:540–544. doi: 10.1007/s004280000297. [DOI] [PubMed] [Google Scholar]
- Gocht A, Bosmuller HC, Bassler R, Tavassoli FA, Moinfar F, Katenkamp D, Schirrmacher K, Luders P, Saeger W. Breast tumors with myofibroblastic differentiation: clinico-pathological observations in myofibroblastoma and myofibrosarcoma. Pathol Res Pract. 1999;195:1–10. doi: 10.1016/S0344-0338(99)80087-9. [DOI] [PubMed] [Google Scholar]
- Magro G, Michal M, Bisceglia M. Benign spindle cell tumors of the mammary stroma: diagnostic criteria, classification, and histogenesis. Pathol Res Pract. 2001;197:453–466. doi: 10.1078/0344-0338-00112. [DOI] [PubMed] [Google Scholar]
- Magro G, Bisceglia M, Michal M, Eusebi V. Spindle cell lipoma-like tumor, solitary fibrous tumor and myofibroblastoma of the breast: a clinico-pathological analysis of 13 cases in favor of a unifying histogenetic concept. Virchows Arch. 2002;440:249–260. doi: 10.1007/s00428-001-0572-y. [DOI] [PubMed] [Google Scholar]
- Greenberg D, McIntyre H, Ramsaroop R, Arthur J, Harman J. Aggressive fibromatosis of the breast: a case report and literature review. Breast J. 2002;8:55–57. doi: 10.1046/j.1524-4741.2002.08013.x. [DOI] [PubMed] [Google Scholar]
- Nakano S, Ohtsuka M, Hasegawa T, Kudoh T, Ikebata K, Sakata H, Yamamoto M, Satake T. Fibromatosis of the breast: a case report. Breast Cancer. 2002;9:179–183. doi: 10.1007/BF02967585. [DOI] [PubMed] [Google Scholar]
- Pettinato G, Manivel JC, Petrella G, Jassim AD. Fine needle aspiration cytology, immunocytochemistry and electron microscopy of fibromatosis of the breast. Acta Cytol. 1991;35:403–408. [PubMed] [Google Scholar]
- Yamaguchi H, Sakakibara T, Hino M, Ryu M, Senuma K, Nakai K, Tomiki Y, Sakamoto K, Kamano T, Tsurumaru M, Matsumoto T. A case of fibromatosis of the breast. Breast Cancer. 2002;9:175–178. doi: 10.1007/BF02967584. [DOI] [PubMed] [Google Scholar]
- Weidner N, Levine JD. Spindle-cell adenomyoepithelioma of the breast. A microscopic, ultrastructural, and immunocytochemical study. Cancer. 1988;62:1561–1567. doi: 10.1002/1097-0142(19881015)62:8<1561::AID-CNCR2820620817>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Felipo F, Del Agua C, Eguizabal C, Vaquero M. Benign adenomyoepithelioma of the breast: a case with gross mimicking of malignancy. Breast J. 2002;8:383–384. doi: 10.1046/j.1524-4741.2002.08610.x. [DOI] [PubMed] [Google Scholar]
- Popnikolov NK, Ayala AG, Graves K, Gatalica Z. Benign myoepithelial tumors of the breast have immunophenotypic characteristics similar to metaplastic matrix-producing and spindle cell carcinomas. Am J Clin Pathol. 2003;120:161–167. doi: 10.1309/G6CT-R8MD-TFUW-19XV. [DOI] [PubMed] [Google Scholar]
- Moritani S, Kushima R, Sugihara H, Bamba M, Kobayashi TK, Hattori T. Availability of CD10 immunohistochemistry as a marker of breast myoepithelial cells on paraffin sections. Mod Pathol. 2002;15:397–405. doi: 10.1038/modpathol.3880536. [DOI] [PubMed] [Google Scholar]
- Foschini MP, Eusebi V. Carcinomas of the breast showing myoepithelial cell differentiation. A review of the literature. Virkows Arch. 1988;432:303–310. doi: 10.1007/s004280050170. [DOI] [PubMed] [Google Scholar]
- Lakhani SR, Michael J, O'Hare MJ. The mammary myoepithelial cell – Cinderella or ugly sister? Breast Cancer Res. 2001;3:1–4. doi: 10.1186/bcr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir G, Yampolsky I, Sandbank J. Parenchymal leiomyoma of the breast. Report of a case and clinicopathological review. Eur J Surg Oncol. 1995;21:88–89. doi: 10.1016/S0748-7983(05)80077-0. [DOI] [PubMed] [Google Scholar]
- Huvos AG, Lucas JC, Jr, Foote FW., Jr Metaplastic breast carcinoma. Rare form of mammary cancer. NY State J Med. 1973;73:1078–1082. [PubMed] [Google Scholar]
- Nakayama Y, Iwasaki H, Iwanaga S, Nakamura H, Shiroshita T, Kikuchi M, Jozaki H, Hashimoto M, Ikeda S. Spindle cell carcinoma of the breast: a case report and an immunohistochemical study including p53 and Ki-67 expression. Pathol Int. 1997;47:404–411. doi: 10.1111/j.1440-1827.1997.tb04515.x. [DOI] [PubMed] [Google Scholar]
- Gobbi H, Simpson JF, Jensen RA, Olson SJ, Page DL. Metaplastic spindle cell breast tumors arising within papillomas, complex sclerosing lesions, and nipple adenomas. Mod Pathol. 2003;16:893–901. doi: 10.1097/01.MP.0000085027.75201.B5. [DOI] [PubMed] [Google Scholar]
- Maemura M, Iino Y, Oyama T, Hikino T, Yokoe T, Takei H, Horiguchi J, Ohwada S, Nakajima T, Morishita Y. Spindle cell carcinoma of the breast. Jpn J Clin Oncol. 1997;27:46–50. doi: 10.1093/jjco/27.1.46. [DOI] [PubMed] [Google Scholar]
- Sneige N, Yaziji H, Mandavilli SR, Perez ER, Ordonez NG, Gown AM, Ayala A. Low-grade (fibromatosis-like) spindle cell carcinoma of the breast. Am J Surg Pathol. 2001;25:1009–1016. doi: 10.1097/00000478-200108000-00004. [DOI] [PubMed] [Google Scholar]
- Khan HN, Wyld L, Dunne B, Lee AH, Pinder SE, Evans AJ, Robertson JF. Spindle cell carcinoma of the breast: a case series of a rare histological subtype. Eur J Surg Oncol. 2003;29:600–603. doi: 10.1016/S0748-7983(03)00107-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High resolution image of figure 1a
High resolution image of figure 1b
High resolution image of figure 1c
High resolution image of figure 1d
High resolution image of figure 2a
High resolution image of figure 2b
High resolution image of figure 2c
High resolution image of figure 2d
High resolution image of figure 3a
High resolution image of figure 3b
High resolution image of figure 3c
High resolution image of figure 3d
High resolution image of figure 3e
High resolution image of figure 3f
High resolution image of figure 3g
High resolution image of figure 4a
High resolution image of figure 4b
High resolution image of figure 4c
High resolution image of figure 4d
High resolution image of figure 4e
High resolution image of figure 4f
High resolution image of figure 4g
High resolution image of figure 5a
High resolution image of figure 5b
High resolution image of figure 5c
High resolution image of figure 5d
High resolution image of figure 5e
High resolution image of figure 5f


