Abstract
Deficiency of ornithine-δ-aminotransferase (OAT) in humans results in gyrate atrophy of the choroid and retina (GA), an autosomal recessive disorder characterized by ornithine accumulation and a progressive chorioretinal degeneration of unknown pathogenesis. To determine whether chronic, systemic reduction of ornithine can prevent this form of retinal degeneration, we used an arginine-restricted diet to maintain long term reduction of ornithine in a mouse model of OAT-deficiency (Oat−/−) produced by gene targeting. We evaluated the mice over a 12-month period by measurement of plasma amino acids, electroretinograms, and retinal histologic and ultrastructural studies. We found that an arginine-restricted diet substantially reduces plasma ornithine levels and completely prevents retinal degeneration in Oat−/−. This result indicates that ornithine accumulation is a necessary factor in the pathophysiology of the retinal degeneration in GA and that restoration of OAT activity in retina is not required for effective treatment of GA.
In humans, deficiency of ornithine-δ-aminotransferase (OAT) results in gyrate atrophy of the choroid and retina (GA), a progressive blinding chorioretinal degeneration inherited as an autosomal recessive trait (1, 2). GA patients present in childhood with myopia followed by the development of night blindness, cataracts, and progressive constriction of vision fields, leading to blindness in the fourth to fifth decade (3–5). These visual symptoms are associated with the development of sharply demarcated, nummular areas of chorioretinal atrophy initially occurring in the midperiphery of the ocular fundus. The atrophic lesions gradually increase in number, enlarge, coalesce, and eventually occupy the entire posterior fundus. Biochemically, GA patients accumulate ornithine to levels 10- to 15-fold above normal in all body fluids and develop concomitant overflow ornithinuria. Additionally, they have modest but statistically significant reduction in plasma lysine, glutamate, glutamine, and creatine (2). The human OAT gene has been cloned and mapped (10q26), and more than 60 mutations causing GA have been identified (2, 6–9).
Despite this extensive clinical, biochemical, and molecular characterization of GA, the pathophysiologic mechanism of the progressive retinal degeneration is unknown (2). OAT is a pyridoxal phosphate-dependent mitochondrial matrix enzyme expressed in most tissues, including kidney, small intestine, liver, and retina (10–12). High OAT activity has been documented in the retinal pigment epithelium (RPE) (13–15), the cell layer external to the retinal photoreceptors. The metabolic function(s) of OAT in the RPE and elsewhere in the retina is poorly understood. Several pathophysiological models have been proposed, including ornithine toxicity and deficiency of proline and/or creatine (2). The lack of access to retinas from GA patients for direct evaluation has made pathophysiologic studies difficult (2). Indeed, retinal histopathology has been described in only one GA patient, a 98-year-old woman with the vitamin B6-responsive form of the disease (16).
Development of effective therapy for GA has been difficult, in part because of our limited understanding of the pathogenesis of chorioretinal degeneration. Furthermore, the long time course and extensive allelic heterogeneity make evaluation of any therapeutic intervention difficult (17). A small fraction of patients (<5%) exhibits reduction in plasma ornithine in response to pharmacologic doses of pyridoxine hydrochloride (vitamin B6). These individuals have OAT alleles with mutations affecting the vitamin B6 binding site of the enzyme (18–20). Unfortunately, the long term consequences of pyridoxine treatment in these patients have not been described. Several other experimental treatments, including supplementation with creatine or proline, failed to show unequivocal efficacy (21–24). Attempts at correcting ornithine accumulation by dietary reduction of its precursor, arginine, have been described in several studies (Fig. 1). Only a fraction of patients (≈20%) are able to follow the highly restrictive diet. In some patients who have maintained a chronic reduction of ornithine to near normal levels, there was evidence of modest early improvement and subsequent stabilization in both subjective and objective tests of visual function (25–27). In other patients, however, there was funduscopic evidence of enlargement of the atrophic areas despite moderate biochemical control of plasma ornithine (28–31). Even the most promising studies noted some progression of the chorioretinal atrophy in patients on this diet (27). Whether this results from damage already presented at the time of institution of the diet, less than perfect control of the ornithine accumulation, or a pathophysiologic mechanism unrelated to ornithine is not clear.
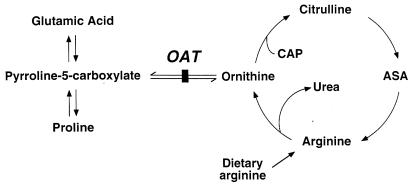
Figure 1.
Ornithine metabolic pathways. The vertical bar indicates the site of the block caused by OAT deficiency. ASA, argininosuccinic acid; CAP, carbamyl phosphate.
To develop a model for studies of pathophysiology and treatment, we produced an OAT-deficient mouse by gene targeting (32). Like human GA patients, adult OAT-deficient mice exhibit hyperornithinemia with plasma ornithine levels 10- to 15-fold above normal (Oat−/− animals 1,172 ± 251 μM; Oat+/+ 95 ± 21 μM). These animals exhibit a gradual reduction in ERG amplitude beginning around 4 months of age. The histology of their retinas is normal at 2 months, but by 7 months there is irregular swelling of the RPE and shortening and disorganization of the photoreceptor outer segments. There is also a gradual loss of photoreceptors so that by 12 months the number of photoreceptor nuclei is reduced to 30% of normal (33). Here, we use an arginine-restricted diet to evaluate the biochemical and retinal effects of chronic reduction of ornithine in this model. We show that this therapy results in normalization of plasma ornithine and completely prevents retinal degeneration.
Materials and Methods
Animal Husbandry.
Mouse husbandry and manipulations were carried out according to National Institutes of Health guidelines at the Johns Hopkins University School of Medicine. OAT−/− mice require arginine supplementation during their first 2 weeks of life to prevent lethal hyperammonemia as the result of deficiency of urea cycle intermediates (32). In brief, mice born from heterozygote matings were given intraperitoneal (i.p.) arginine injections at 12-h intervals starting within hours after birth and continuing for the first 2 weeks of life (32). The initial dose was 10 mmol/kg of body weight/dose. The absolute amount given remained constant, so as the animals grew, the dose tapered to ≈2 mmol/kg of body weight/dose at the end of the 2 weeks. The arginine stock solution consisted of 1 mol/liter arginine hydrochloride and 1 mol/liter arginine free base (Sigma) at a ratio of 2:1 (vol:vol) with a final concentration of arginine of 1 mol/liter and pH of ≈9. After 2 weeks of age, the mice no longer require arginine supplementation. Postweaning, animals were maintained on an amino acid synthetic pellet diet containing standard supplementation of amino acids including 1.12% (wt/wt) arginine (ICN) until they were 6 weeks of age.
Arginine-Restricted Diet.
At the age of 6 weeks, male mice were divided into two groups, each containing six Oat−/− mice and six aged-matched Oat+/+ littermates. One group was maintained on complete mouse chow diet as above. The other was maintained on the same synthetic amino acid diet except that arginine was deleted and sucrose added to maintain an isocaloric content. The minimal arginine requirement for normal growth and development was satisfied with arginine-supplemented drinking water (620 mg/1,000 ml) with ad libitum intake. All mice were kept in a 12-h light/12-h dark environment at an incident luminance of <25 cd with six animals/cage at room temperature of 25°C. The body weight of each mouse was recorded at 2- to 4-week intervals for the first 6 months on diet and every 2–3 months thereafter.
Plasma Amino Acid Determination.
Tail vein blood was collected from each mouse at 6 weeks, 9 weeks, and 4, 8, and 12 months of age. Using ammonium-free heparin (Sigma) as anticoagulant, we obtained 30–50 μl of plasma from each animal. Plasma amino acid determinations were made with a Beckman Coulter 6300 amino acid analyzer as described (32).
Electroretinography.
The ERGs of each mouse were recorded at 1.5, 4, 6, and 12 months of age by using a gold foil recording electrode on an EPIC-2000 system in a dark environment (LKC) with minor modifications of a previously described method (34). Mice were anesthetized with a mixture of 0.1% ketamine, 0.1% xylazine, and 4% urethane in Ringer solution (0.3 ml/20–25 g animals). All mice were subjected to at least 30 min of dark adaptation before recording. Pupil dilation was achieved by using 1/4 drop of 0.25% tropicamide. A scotopic single flash light without filter (0 dB) was used as the source of light stimulation to record the maximal response from retina. Sixteen responses were automatically averaged, with 5 sec between stimuli. The ERG amplitude was calculated as the difference between the peak of the A wave and the peak of the B wave (33).
Retinal Histology and Electron Microscopy.
Eyes were removed from deeply anesthetized OAT-deficient mice and controls and were immersion-fixed in a mixture of 4% paraformaldehyde and 0.1% glutaraldehyde in 0.13 M phosphate buffer. The eyes were bisected along the vertical meridian, postfixed in 1% OsO4 in phosphate buffer, and embedded in Medcast resin. One-micrometer-thick sections were stained with a mixture of toluidine blue, methylene blue, and basic fuchsin for light microscopy, and ninety-nanometer sections were contrasted with uranyl acetate and lead citrate for transmission electron microscopy.
Results
Effect of an Arginine-Restricted Diet on Ornithine Accumulation.
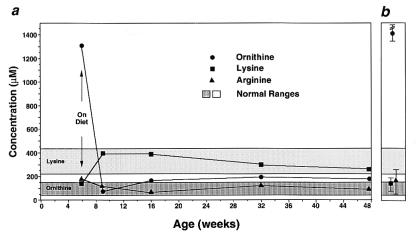
The Oat−/− mice used in this study were weaned at 3 weeks of age to a diet of standard rodent chow (22% protein). By 6 weeks of age, when first studied, they exhibited the plasma amino acid abnormalities typical for Oat−/− mice (Fig. 2). Plasma ornithine averaged 13-fold over Oat+/+ littermates on the same diet, and the mean plasma lysine was ≈50% of control values. We then placed the mice on the arginine-restricted diet consisting of an arginine-free mouse chow with 22% protein provided as essential amino acids. To meet minimal requirements for growth, we supplemented the drinking water with arginine. In preliminary experiments, we found that a supplement of ≈0.6 mmol arginine/kg/day was required for normal growth. Oat−/− mice on this diet had a rapid decline in plasma ornithine to normal levels while maintaining a normal growth rate (Figs. 2 and 3). Plasma lysine levels rose to normal, and plasma arginine levels were in the low normal range (Fig. 2). This normalization of plasma amino acid concentrations was maintained for the remaining 42 weeks of the study until the mice were killed at ≈1 year of age.
Figure 2.
Selected plasma amino acid levels of Oat−/− mice on an arginine-restricted diet over 12 months (a) or Oat−/− mice on a standard diet (b). Ranges of plasma ornithine and lysine in control animals are indicated with dark or light gray rectangles, respectively. In a, the Oat−/− mice were placed on an arginine-restricted diet at the age of 6 weeks, as indicated by the arrow. Each point represents the mean plasma concentration for the indicated amino acid from 5–8 Oat−/− mice. In b, each point represents the mean of measurements for the indicated amino acid in 5–8 adult Oat−/− animals on a standard diet.
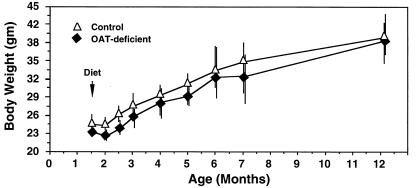
Figure 3.
Growth of Oat−/− and control mice on an arginine-restricted diet over 12 months. The animals were placed on an arginine-restricted diet at the age of 6 weeks, as indicated by an arrow. Each point and vertical line indicates the mean and range of weight of the 5–8 animals in each group, respectively.
Ornithine Reduction Preserves Retinal Physiologic Function.
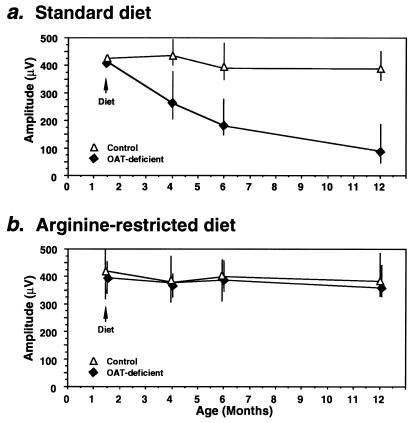
To determine the consequence of correction of ornithine accumulation on the electrical activity of the retina, we compared the ERG amplitudes of Oat−/− and Oat+/+ mice on arginine-restricted and standard diets (Fig. 4 a and b). The Oat−/− mice on a standard diet with hyperornithinemia exhibited a gradual reduction of ERG amplitude indistinguishable from our previously published results (Fig. 4a) (33). By contrast, ERG amplitudes of Oat−/− mice on the arginine-restricted diet with near normal plasma ornithine values remained constant over the 12-month period (Fig. 4b) and were identical to the amplitudes measured in Oat+/+ controls. These results indicate that long term reduction of ornithine preserves the electrophysiologic function of the retinas of Oat−/− mice.
Figure 4.
ERG amplitudes of Oat−/− and control mice on either a standard (a) or an arginine-restricted (b) diet over 12 months of life. Oat−/− and control mice were divided into groups of 5–8 animals. In a, a group of control and a group of Oat−/− mice were placed on standard diet for 12 months whereas in b, groups of control and Oat−/− mice were placed on an arginine-restricted diet at age 6 weeks. The symbols represent the mean, and the vertical bars the range, of ERG amplitudes recorded from control mice (open triangles) or Oat−/− mice (filled triangles) at indicated ages. The ERG amplitude was calculated as the difference between the peak of the A wave and the peak of the B wave (33).
Correction of Ornithine Accumulation Prevents Retinal Degeneration.
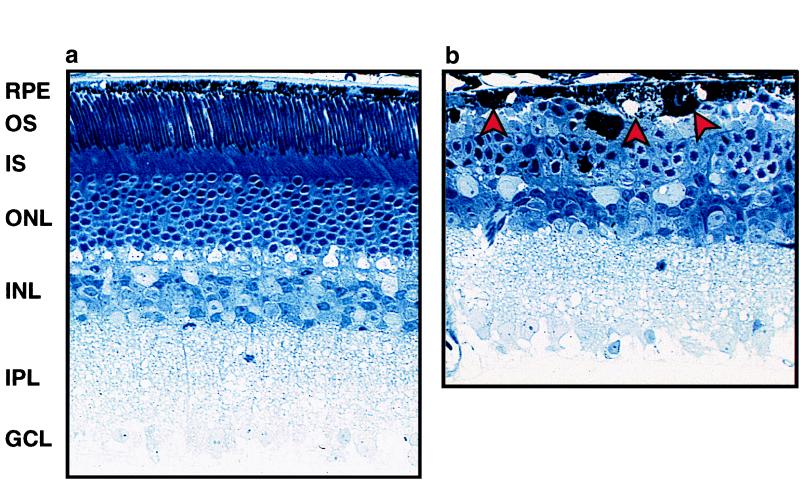
At the end of the study, we compared the retinal histology of Oat−/− mice on either standard or arginine-restricted diets. The Oat−/− mice on a standard diet had severe retinal degeneration (Fig. 5b). Some RPE cells were markedly swollen, and others were flat, irregularly shaped, and engorged with abnormal inclusions. The photoreceptor outer segments were virtually absent, and the inner segments were shortened and highly disorganized. The outer nuclear layer, comprised of the photoreceptor nuclei, was markedly reduced to 2–3 layers of nuclei (normal is 9–10 layers). These results are consistent with our previously published descriptions of retinal degeneration in the Oat−/− mice. By contrast, Oat−/− mice on the arginine-restricted diet had histologically normal retinas at 12 months (Fig. 5a). The RPE cells were cuboidal with normal thickness. The photoreceptor inner and outer segments had normal organization and length and the number of layers of photoreceptor nuclei was normal (9–10; see Fig. 5a).
Figure 5.
Retinal histopathology of Oat−/− mice on an arginine-restricted diet (a) or a standard diet (b) at age 12 months. Note the reduced number of ONL cells and loss of IS and OS in the right panel. The arrows indicate abnormal RPE cells. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; IS and OS, inner and outer segments, respectively, of photoreceptors.
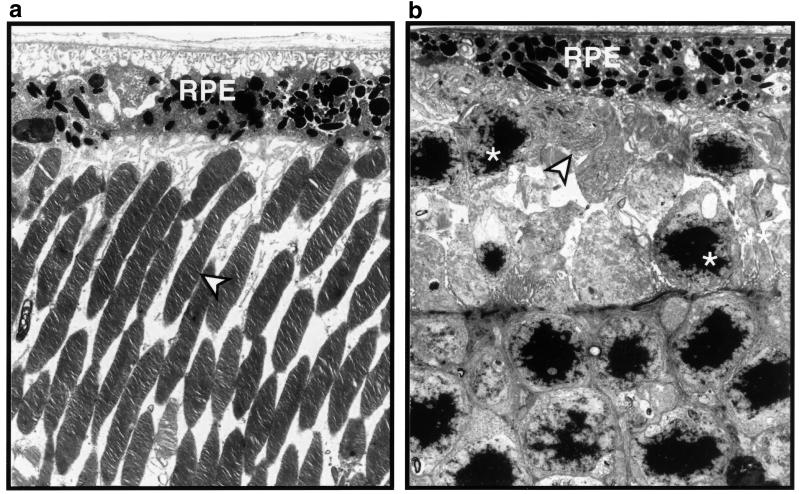
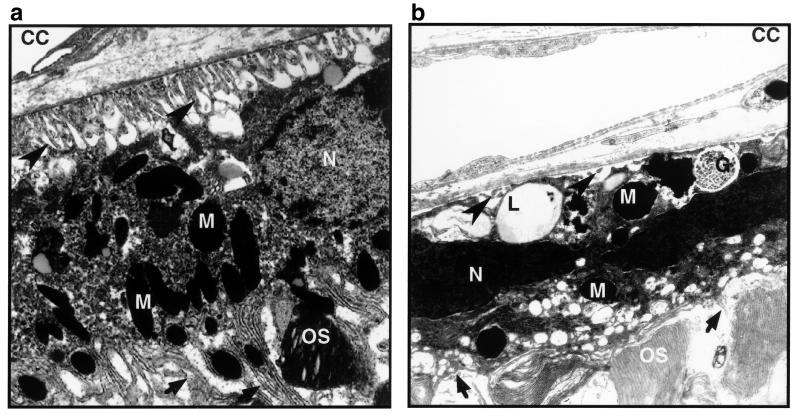
This striking difference in retinal histology was confirmed by ultrastructural analysis (Figs. 6 and 7). In Oat−/− mice on the standard diet, most RPE cells had lost their basal infoldings and had greatly reduced apical microvilli (Figs. 6b and 7b). Some were swollen and engorged with lipid inclusions (Fig. 7b); others contained clusters of small electron dense granules with the fine structure of glycogen. The central retinas in these mice were devoid of photoreceptor inner and outer segments or had only remnants of disorganized inner and outer segment-like membranes (Fig. 6b). By contrast, the retinas of Oat−/− mice on the arginine-restricted diet at 12 months of age were entirely normal in appearance (Fig. 6a). The RPE cells formed a continuous epithelium of uniform cells with normal basal infoldings, apical microvilli, and organelles (Fig. 7a). Photoreceptor inner and outer segments retained their characteristic fine structure as found in normal retinas (Fig. 6a).
Figure 6.
Electron micrographs of retinas from Oat−/− mice on an arginine-restricted diet (a) or a standard diet (b) at age 12 months. The arrow heads indicate normal appearing photoreceptor outer segments in a and a near complete loss of the photoreceptor outer segments in b. The asterisks in b indicate photoreceptor nuclei.
Figure 7.
Electron micrograph of the outer retinas of the Oat−/− mice on an arginine-restricted diet (a) or a standard diet (b) at age 12 months. The large arrow heads indicate the basal infoldings of retinal pigment epithelium, healthy-appearing in a and virtually gone in b. The small arrows indicate the apical processes of the retinal pigment epithelium, long and delicate in a and short and disorganized in b. CC, choriocapillaris; N, nuclei of the retinal pigment epithelium; M, melanosome; OS, photoreceptor outer segment; L, lipid droplets; G, glycogen accumulation.
Discussion
More than 90 disorders have a slowly progressive, inherited retinal degeneration as a prominent aspect of their phenotype [RetNet, the Retinal Information Network (www.sph.uth.tmc.edu/Retnet/home.htm); ref. 35]. Development and evaluation of treatments for these disorders is a daunting task. Central to this challenge is our lack of understanding the pathophysiologic mechanisms for most of these diseases. Evaluation of potential therapies is hampered by the small number of affected individuals, the slow and variable rate of progression of retinal degeneration in untreated patients, allelic heterogeneity of the disease genes, other genetic and environmental variables in the patient populations, and lack of direct access to the affected tissue for study.
All of these problems have contributed to the difficulty in developing and evaluating possible treatments for GA. Reduction of ornithine with an arginine-restricted diet has been the most extensively studied treatment for this disorder. Some investigators have concluded that this approach was either ineffective or unworkable (29, 30) whereas others have obtained promising but inconclusive results (26, 36). The strongest data in favor of a beneficial effect came from a study of affected sib pairs (27). Preliminary evaluation of six sib pairs of various ages indicated that there is a strong familial similarity in severity and rate of progression. This observation validated the use of an older sib as a control for a younger sib. In two young affected sib pairs, the sibs were begun on diet at the same chronologic time and were followed for several years. By the time the younger sibs reached the age of the older at the time of diet institution, they had been on diet for most of their lives. In both sib pairs, the retinas of the younger treated sibs were much less affected than those of the older untreated sibs at a comparable age. Despite this promising result, there continues to be uncertainty regarding the effectiveness of reduction of ornithine because of the small sample size in the study and because there has been clear evidence of progression of retinal lesions in patients even after achieving optimal dietary control of their plasma ornithine (27, 30).
The availability of the Oat−/− mice provided us with an opportunity to evaluate the effect of reduction of ornithine under highly controlled conditions from an early age in animals with an identical genotype at the OAT locus. These animals develop hyperornithinemia soon after weaning (age 3 weeks) (32, 33). We placed them on the arginine-restricted diet at age 6 weeks, before there is electrophysiologic, histologic, or ultrastructural evidence of retinal damage (32, 33). The biochemical effect of the diet was a sustained reduction in plasma ornithine. The electrophysiologic, histologic, and ultrastructural consequences of this biochemical correction were striking. The Oat−/− animals on diet maintained completely normal retinas whereas their counterparts with high ornithine levels had the expected severe retinal degeneration. Based on these results and those obtained from clinical trials on human GA patients, we conclude that systemic ornithine reduction to near normal levels is a highly effective treatment for GA.
The Mechanism of Retinal Degeneration in GA in Humans Is Unknown.
The only available human retinal histopathology of GA to date was obtained from a 98-year-old GA patient with vitamin B6-responsive OAT alleles who had a small, isolated central area of functional retina surrounded by large areas totally devoid of choroid and retinal layers (37). There was focal photoreceptor atrophy and RPE hyperplasia in the region of demarcation between atrophic and relatively normal retina. Unfortunately, this observation on a single patient provided limited insight into the pathophysiology of GA. For example, it is not certain if ornithine accumulation in retinal tissues is similar to that in plasma and which of the many types of specialized retinal cells are involved in the early stages of the degeneration. In earlier studies, we found that ultrastructural changes in the RPE cells are the earliest morphologic sign of retinal damage in hyperornithinemic Oat−/− mice (33). We also found that the magnitude of ornithine accumulation and lysine depletion in retinas of the Oat−/− mice is comparable to the changes in plasma (T.W. and D.V., unpublished work). The present study provides conclusive evidence that a systemic reduction of ornithine prevents retinal degeneration in Oat−/− mice. Taken together, these results favor the hypothesis that direct ornithine toxicity to retinal cells, particularly to the RPE, is involved in the earliest stages of the pathophysiology of GA. The focal degeneration and malfunction of the RPE cells likely results in the photoreceptor abnormalities and in secondary atrophy of the choriocapillaries (38). It is also possible that photoreceptor damage in GA may result from a combination of insults, including ornithine toxicity, damage from toxic debris resulting from degenerating RPE cells, failure of the RPE cells to provide their normal nutritive functions for the photoreceptors, reduced nutrition as the result of choriocapillaris atrophy, and failure to maintain the protected microenvironment required for optimal photoreceptor function secondary to the breakdown of the blood–retina barrier.
Our results demonstrate that early correction of ornithine accumulation is critical for prevention of irreversible retinal degeneration. For optimal outcome, therefore, it is important to institute this therapy at the earliest age possible. This observation emphasizes the value of early and accurate diagnosis of GA. Furthermore, our results indicate that it is not necessary to restore OAT activity in retina to prevent retinal degeneration in GA. Rather, any mechanism that achieves a reduction in ornithine to a near normal range should be beneficial for human GA patients. This conclusion also has significant implications for development of strategies for somatic gene therapy of GA. Restoration of sufficient OAT activity to reduce plasma ornithine levels to near normal should be sufficient to prevent retinal degeneration. The choice of tissue localization for expression of OAT activity should be determined by consideration of accessibility, maximization of introduced activity, and access to the total body ornithine pool. If necessary, somatic gene therapy could also be coupled with a modest to moderate degree of dietary arginine restriction. This combined approach would reduce OAT activity required and would still allow the patient to ingest a more tolerable diet.
Acknowledgments
We thank S. Muscelli for help in preparation of this manuscript. This work was supported in part by National Eye Institute Grants EY02948 (D.V.) and EY01311 and EY01730 (A.H.M.); the Foundation Fighting Blindness (Hunt Valley, MD) (D.V. and A.H.M.); the Mackall Foundation Trust (New York) (A.H.M.); the Chatlos Foundation (Longwood, FL) (A.H.M.); Research to Prevent Blindness, Inc. (New York) (A.H.M.); and National Institute Research Center Grant HD24605 (D.V.). D.Valle is an Investigator and T. Wang was a Research Associate with the Howard Hughes Medical Institute.
Abbreviations
- OAT
ornithine-δ-aminotransferase
- GA
gyrate atrophy of the choroid and retina
- RPE
retinal pigment epithelium
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Jacobsohn E. Klin Monatsbl Augenheilkd. 1888;26:202–206. [Google Scholar]
- 2.Valle D, Simell O. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C, Beaudet A, Sly W, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1147–1185. [Google Scholar]
- 3.Takki K, Milton R C. Ophthalmology. 1981;88:292–301. doi: 10.1016/s0161-6420(81)35031-3. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser-Kupfer M I, Ludwig I H, DeMonasterio F M, Valle D, Krieger I. Ophthalmology. 1985;92:394–401. doi: 10.1016/s0161-6420(85)34022-8. [DOI] [PubMed] [Google Scholar]
- 5.Francois J. Ophthalmologica (Basel) 1979;178:311–320. doi: 10.1159/000308842. [DOI] [PubMed] [Google Scholar]
- 6.Brody L C, Mitchell G A, Obie C, Michaud J, Steel G, Fontaine G, Robert M-F, Kaiser-Kupfer M I, Valle D. J Biol Chem. 1992;267:3302–3307. [PubMed] [Google Scholar]
- 7.Mitchell G A, Looney J E, Brody L C, Steel G, Suchanek M, Engelhardt J F, Willard H F, Valle D. J Biol Chem. 1998;263:14288–14295. [PubMed] [Google Scholar]
- 8.Inana G, Totsuks S, Redmond M, Dougherty T, Nagle J, Shiono R, Ohura T, Kominami E, Katunuma N. Proc Natl Acad Sci USA. 1986;83:1203–1207. doi: 10.1073/pnas.83.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramesh V, Shaffer M M, Allaire J M, Shih V E, Gusella J F. DNA. 1986;5:493–501. doi: 10.1089/dna.1.1986.5.493. [DOI] [PubMed] [Google Scholar]
- 10.Herzfeld A, Raper S. Biochim Biophys Acta. 1976;428:600–610. doi: 10.1016/0304-4165(76)90188-4. [DOI] [PubMed] [Google Scholar]
- 11.Riby J E, Hurwitz R E, Kretchmer N. Pediatr Res. 1990;28:261–265. doi: 10.1203/00006450-199009000-00022. [DOI] [PubMed] [Google Scholar]
- 12.De Jonge W J, Dingemanse M A, de Boer P A, Lamers W H, Moorman A F. Pediatr Res. 1998;43:442–451. doi: 10.1203/00006450-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Hayakasa S, Shiono T, Takaku Y, Mizuno K. Invest Ophthalmol. 1980;19:1457–1460. [PubMed] [Google Scholar]
- 14.Rao G N, Cotler E. Neurochem Res. 1984;9:555–562. doi: 10.1007/BF00964382. [DOI] [PubMed] [Google Scholar]
- 15.Ratzlaff K, Batch A. Comp Biochem Physiol B Biochem Mol Biol. 1987;88:35–37. doi: 10.1016/0305-0491(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 16.Weleber R G, Kennaway N G. Ophthalmology. 1981;88:316–324. doi: 10.1016/s0161-6420(81)35035-0. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser-Kupfer M I, De Monasterio F, Valle D, Walser M, Brusilow S W. Ophthalmology. 1981;88:307–310. doi: 10.1016/s0161-6420(81)35033-7. [DOI] [PubMed] [Google Scholar]
- 18.Weleber R G, Kennaway N G, Buist N R. Lancet. 1978;2:1213. doi: 10.1016/s0140-6736(78)92211-0. [DOI] [PubMed] [Google Scholar]
- 19.Ramesh V, Gusella J, Shih V. Mol Biol Med. 1991;8:81–93. [PubMed] [Google Scholar]
- 20.Michaud J, Mitchell G A, Thompson G N, Brody L C, Steel G, Fontaine G, Schappert K, Keith C G, Valle D. Am J Hum Genet. 1995;56:616–622. [PMC free article] [PubMed] [Google Scholar]
- 21.Giordano D, DeSanto N G, Pluvio M, Santinelli R, Stoppoloni G. Nephron. 1978;22:97–106. doi: 10.1159/000181428. [DOI] [PubMed] [Google Scholar]
- 22.Yatziv S, Statter M, Merin S. J Lab Clin Med. 1979;93:749–757. [PubMed] [Google Scholar]
- 23.Sipila I, Rapola J, Simell O, Vannas A. N Engl J Med. 1981;304:867–870. doi: 10.1056/NEJM198104093041503. [DOI] [PubMed] [Google Scholar]
- 24.Hayasaka S, Saito T, Nakajima H, Takahashi O, Mizuno K, Tada K. Br J Ophthalmol. 1985;69:283–290. doi: 10.1136/bjo.69.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valle D, Walser M, Brusilow S, Kaiser-Kupfer M. J Clin Invest. 1980;65:371–378. doi: 10.1172/JCI109680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser-Kupfer M I, De Monasterio F M, Valle D, Walser M, Brusilow S. Science. 1980;210:1128–1131. doi: 10.1126/science.7444439. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser-Kupfer M I, Caruso R C, Valle D. Arch Ophthalmol. 1991;109:1539–1548. doi: 10.1001/archopht.1991.01080110075039. [DOI] [PubMed] [Google Scholar]
- 28.Berson E L, Shih V E, Sullivan P L. Ophthalmology. 1981;88:311–315. doi: 10.1016/s0161-6420(81)35029-5. [DOI] [PubMed] [Google Scholar]
- 29.Berson E L, Hanson A H, Rosner B, Shih V E. Birth Defects Orig Artic Ser. 1982;18:209–218. [PubMed] [Google Scholar]
- 30.Vannas Sulonen K, Simell O, Sipila I. Ophthalmology. 1987;94:1428–1433. [PubMed] [Google Scholar]
- 31.Vannas Sulonen K. Acta Ophthalmol. 1987;65:101–109. doi: 10.1111/j.1755-3768.1987.tb08499.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Lawler A M, Steel G, Sipila I, Milam A H, Valle D. Nat Genet. 1995;11:185–190. doi: 10.1038/ng1095-185. [DOI] [PubMed] [Google Scholar]
- 33.Wang T, Milam A H, Steel G, Valle D. J Clin Invest. 1996;97:2753–2762. doi: 10.1172/JCI118730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory-Evans K, Bhattacharya S S. Trends Genet. 1998;14:103–108. doi: 10.1016/s0168-9525(98)01402-4. [DOI] [PubMed] [Google Scholar]
- 35.McInnes R R, Arshinoff S A, Bell L, Marliss E B, McCulloch J C. Lancet. 1981;1:513–516. doi: 10.1016/s0140-6736(81)92858-0. [DOI] [PubMed] [Google Scholar]
- 36.Wilson D J, Weleber R G, Green W R. Am J Ophthalmol. 1991;111:24–33. doi: 10.1016/s0002-9394(14)76892-8. [DOI] [PubMed] [Google Scholar]
- 37.Hamasaki D I, Dix R D, Atherton S S. Invest Ophthal Visual Sci. 1988;29:1242–1254. [PubMed] [Google Scholar]
- 38.Korte G E, Reppucci V, Henkind P. Invest Ophthalmol Visual Sci. 1984;25:1135–1145. [PubMed] [Google Scholar]