Abstract
CD1-restricted presentation of lipid or glycolipid antigens derived from Mycobacterium tuberculosis has been demonstrated by in vitro experiments using cultured T-cell lines. In the present work, the frequency of T-cell responses to natural mycobacterial lipids was analyzed in ex vivo studies of peripheral blood lymphocytes from human patients with pulmonary tuberculosis, from asymptomatic individuals with known contact with M. tuberculosis documented by conversion of their tuberculin skin tests, and from healthy tuberculin skin test-negative individuals or individuals vaccinated with Mycobacterium bovis BCG. Proliferation and gamma interferon enzyme-linked immunospot assays using peripheral blood lymphocytes and autologous CD1+ immature dendritic cells revealed that T cells from asymptomatic M. tuberculosis-infected donors responded with significantly greater magnitude and frequency to mycobacterial lipid antigen preparations than lymphocytes from uninfected healthy donors. By use of these methods, lipid-antigen-specific proliferative responses were minimally detectable or absent in blood samples from patients with active tuberculosis prior to chemotherapy but became detectable in blood samples drawn 2 weeks after the start of treatment. Lipid antigen-reactive T cells were detected predominantly in the CD4-enriched T-cell fractions of circulating lymphocytes, and anti-CD1 antibody blocking experiments confirmed the CD1 restriction of these T-cell responses. Our results provide further support for the hypothesis that lipid antigens serve as targets of the recall response to M. tuberculosis, and they indicate that CD1-restricted T cells responding to these antigens comprise a significant portion of the circulating pool of M. tuberculosis-reactive T cells in healthy individuals with previous exposure to M. tuberculosis.
Mycobacterium tuberculosis is a human pathogen of enormous importance to global public health. According to the most recent data published by the World Health Organization, approximately 2.2 million deaths per year are attributable to M. tuberculosis, an additional 8 million people develop symptoms of tuberculosis (TB) every year, and every third human being on earth is infected with the bacterium (36). In spite of this devastating impact on human populations, it is also clear that the human immune system is capable of providing effective protection against disease due to M. tuberculosis infection, since the majority of immunocompetent people infected by this bacterium do not develop signs of serious illness. A major goal for future efforts to control tuberculosis is thus to understand how the immune system successfully recognizes and suppresses the growth of M. tuberculosis. Understanding the underlying mechanisms of the natural adaptive immune response to M. tuberculosis may allow development of novel vaccination strategies to control disease caused by this pathogen.
A substantial body of clinical and experimental data indicate that antigen-specific T cells play a major role in maintaining solid and long-lived immunity to M. tuberculosis (reviewed in reference 4). It also has been shown for animal models and for humans that both CD4+ and CD8+ T cells are involved in the adaptive immune response to the pathogen (4, 24, 25). Thus, both classical pathways of antigen presentation, which depend on the peptide binding and presenting functions of the major histocompatibility complex (MHC) class I and class II molecules, have been shown to be involved in the protective immune response to M. tuberculosis. However, it has also become clear in recent years that CD1 molecules, a family of antigen-presenting molecules that bind lipids and present these to T cells, are also involved in the generation of cell-mediated immune responses to mycobacterial pathogens (6, 21, 33). The precise role and relative importance of this novel pathway for antigen recognition in generating protective immunity to M. tuberculosis remains poorly understood.
Studies of the human CD1 system have identified this as a family of antigen-presenting molecules related in structure and evolution to MHC class I and class II molecules (21). CD1 is conserved in most or all mammals, although the size and number of CD1 genes and the variety of different CD1 isoforms vary widely among species. In humans, the CD1 family consists of five isoforms (CD1a, -b, -c, -d, and -e) encoded by a cluster of minimally polymorphic genes that map outside of the MHC. The current system of classification divides the human CD1 proteins into at least two distinct groups (group 1 and group 2) based on differences in structure, expression, and function. Group 1 CD1 proteins, which include CD1a, -b, and -c, are expressed predominantly on professional antigen-presenting cells (APCs) such as myeloid lineage dendritic cells (DC). CD1 group 2, which consists only of CD1d, is more widely expressed on hematopoietic lineage cells and on certain epithelia. Isolation of T cells specific for antigens presented by CD1 molecules has led to the demonstration that the foreign antigens presented by CD1 molecules to T cells include an array of mycobacterial lipids and glycolipids (1, 20, 21). Investigation of T-cell lines derived from healthy individuals has revealed that T cells recognizing CD1-restricted mycobacterial antigens have a broad range of functional activities, suggesting that they may contribute to the generation or maintenance of immunity against mycobacteria (30).
In support of the hypothesis that lipid antigen recognition occurs in vivo during infection, it was previously demonstrated that fresh lymphocytes from humans with prior infection by M. tuberculosis recognize a synthetic analogue of a CD1c-presented mycobacterial isoprenoid glycolipid antigen (16). In the present study, we sought to determine whether T cells against natural mycobacterial lipid antigens are expanded in humans as a result of previous infection with M. tuberculosis and also to analyze the frequency and phenotypic properties of such T cells. By measuring T-cell proliferation and gamma interferon (IFN-γ) production by enzyme-linked immunospot (ELISPOT) assay, we show that CD1-restricted T-cell responses against natural mycobacterial lipid antigens in the peripheral blood correlate strongly with prior M. tuberculosis infection. Such responses could be detected in both CD4+ and CD8+ fractions of circulating lymphocytes and constituted a substantial fraction of the total IFN-γ-producing cells responding to M. tuberculosis in some individuals. In addition, CD1-restricted T-cell responses were absent or significantly reduced during active pulmonary tuberculosis but appeared soon after the institution of successful antimicrobial chemotherapy, thus indicating a significant effect of active M. tuberculosis infection on the modulation of this component of the immune response.
MATERIALS AND METHODS
Human subjects and clinical samples.
. The following populations of human subjects were recruited as donors.
(i) M. tuberculosis-infected healthy donors.
Blood samples from 48 individuals (25 females and 23 males; mean age, 31.25 years) were obtained on a volunteer basis after informed consent from the ambulatory population of the TB clinic of St. Elizabeth's Hospital in Boston, Mass., and from the outpatient clinic of the Lungenklinik Heckeshorn in Berlin, Germany. All donors had previous evidence of subclinical M. tuberculosis infection, as documented by conversion of the tuberculin skin test. A positive tuberculin skin test was defined as >10 mm of induration at the injection site 48 h after intradermal injection of 1 U of purified protein derivative (PPD). All individuals in the M. tuberculosis-infected healthy donor group showed >15 mm of induration on their most recent tuberculin skin test and had documentation of a previous negative test. These individuals were free of active disease and had normal chest X-rays at the time of recruitment.
(ii) Active TB patients.
Blood samples from 24 TB patients (8 females and 16 males; mean age, 43.4 years) were obtained from the Lungenklinik Heckeshorn in Berlin, Germany, after informed consent. TB patients presented to the hospital with symptoms of pulmonary TB and in some cases with extrapulmonary symptoms, and the diagnosis of TB was confirmed by culture of M. tuberculosis. Blood samples were obtained prior to and at several time points after initiation of anti-TB chemotherapy using standard multidrug regimens.
(iii) Healthy donors without M. tuberculosis infection.
Thirty-two healthy donors (17 females and 15 males; mean age, 30.6 years) with no history of M. tuberculosis exposure were recruited from laboratory and hospital staff personnel, and blood samples were collected after informed consent was obtained. None of these individuals had any known history of contact with TB, and all had negative PPD skin tests at the time of recruitment. Tuberculin skin tests were considered negative if an initial injection of 1 U of PPD and a follow-up injection of 10 U of PPD 2 weeks later were both negative (defined as ≤10 mm of induration). The individuals in this group who had not received Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccination showed 0 to 3 mm of induration. Nine of the subjects in this group had a remote history of vaccination with BCG. Most of these BCG-vaccinated subjects had weakly reactive PPD skin tests (between 3 and 10 mm of induration) which were classified as negative based on our criteria. Other inclusion criteria that applied for all three study groups were absence of a positive human immunodeficiency virus test, age between 18 and 65 years, and the absence of any other known concurrent infection. Because of limitations in the sample size and the numbers of mononuclear cells obtained, detailed analyses including ELISPOT assays and antibody blocking studies were carried out only on representative subsets of subjects from the various groups. These subsets were in most cases selected as consecutively recruited subjects. They were not selected on the basis of any criteria other than those described above, except that in some cases it was necessary to exclude samples from analysis because of insufficient mononuclear cell yields. All procedures involving human subjects were fully reviewed and approved by the appropriate institutional review boards.
Preparation of cells.
Peripheral blood mononuclear cells (PBMC) from blood samples from all three study groups were isolated on the same day as sample collection (generally within 2 h) by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density centrifugation. Isolated PBMC were suspended in complete medium (RPMI 1640, 10 mM HEPES buffer, 200 mM l-glutamine, 5 U of streptomycin-penicillin/ml [all from Gibco-BRL, Rockville, Md.]) supplemented with 10% human male AB serum (Sigma, St. Louis, Mo.) and were cultured in plastic tissue culture flasks for 1 h to allow firm adherence of monocytes. After adherence, nonadherent cells were removed, suspended in freezing medium (85% complete medium-15% dimethyl sulfoxide), and cryopreserved in liquid nitrogen until further use. Adherent cells were washed and then incubated with complete medium supplemented with 10% fetal calf serum (FCS), 200 U of granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex, Seattle, Wash.)/ml, and 200 U of interleukin-4 (IL-4; PeproTech, Rocky Hill, N.J.)/ml for 4 days to obtain immature monocyte-derived DC. DC were detached and collected by use of 5 mM EDTA in phosphate-buffered saline (PBS) and a cell scraper, irradiated with 5,000 rads, suspended in freezing medium (85% FCS-15% dimethyl sulfoxide), and cryopreserved in liquid nitrogen until use. Expression of CD1a, CD1b, and CD1c by DC was determined by flow cytometry (see below).
MACS cell sorting.
For depletion of CD3+ cells, nonadherent PBMC were incubated with anti-CD3 conjugated magnetic microbeads (Miltenyi, Bergisch-Gladbach, Germany), washed with PBS containing 0.05% EDTA, and passed over a magnetic microbead-associated cell sorting (MACS) magnetic separation column (Miltenyi). Cells in the flowthrough were collected and passed over the column a second time, after which they were found to have <1% residual anti-CD3 staining by fluorescence-activated cell sorter (FACS) analysis. For enrichment of CD4+ and CD8+ populations, nonadherent cells were incubated with magnetic microbeads conjugated with anti-CD4 or anti-CD8 antibodies (Miltenyi). After a wash with PBS-0.05% EDTA, cells were applied to MACS magnetic separation columns, and retained cells were collected to obtain enriched CD4+ or CD8+ fractions. Passage over MACS columns was repeated once to obtain populations that were >95% CD4 positive or CD8 positive by FACS analysis.
Flow cytometry. (i) CD1 expression.
DC were resuspended in PBS plus 5% FCS and aliquoted in V-bottom plates. After a wash, cells were stained with unlabeled antibodies to CD1a, -b, -c, or -d or to MHC class I or class II molecules (see below) for 15 min at room temperature. The nonspecific mouse myeloma protein P3 (produced by myeloma line P3X63Ag8; American Type Culture Collection, Manassas, Va.) was used as an immunoglobulin G1 (IgG1) isotype control. Cells were washed with PBS and then stained with a 1/100 dilution of fluorescein isothiocyanate-conjugated goat anti-mouse Ig F(ab′)2 fragments (Becton Dickinson, Mountain View, Calif.). Propidium iodide was used to identify and exclude dead cells. Between 5,000 and 10,000 viable cells were analyzed for each sample with a FACScalibur cytometer (Becton Dickinson) and Cellquest software.
(ii) MACS-sorted cells.
Nonadherent cells were analyzed similarly except that they were stained with Cy5-conjugated anti-CD4, phycoerythrin-conjugated anti-CD8 (Becton Dickinson), and fluorescein isothiocyanate-conjugated anti-CD3 (Pharmingen) antibodies.
Mycobacterial antigen preparations.
Mycobacterial antigens were derived from M. tuberculosis strain H37Rv grown in Middlebrook 7H9 medium (Difco) containing albumin-dextrose-catalase supplement and 0.1% Tween 80. Bacterial pellets were resuspended in ethanol and allowed to stand for 24 h. Ethanol was removed by evaporation, and bacteria were lyophilized until completely dessiccated. A total sonicate of M. tuberculosis was prepared by suspending 10 mg of dried bacilli per ml in ice-cold PBS and sonicating with a probe sonicator (Sonifier 450; Branson Ultrasonics Corp., Danbury, Conn.) at 40% maximum power output for 5 min. This M. tuberculosis sonicate represents a suspension of all of the antigens of the bacillus. The total extractable lipids of M. tuberculosis strain H37Rv (M. tuberculosis total-lipid extract) were obtained by a modification of the Folch extraction method using 4 volumes of chloroform-methanol (2:1) for each volume of M. tuberculosis total sonicate as previously described (5, 8). Evaluation of the M. tuberculosis lipid extracts by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining revealed no detectable protein bands, indicating a level of contamination of <0.01% by weight for any single protein constituent (8; unpublished data). Glucose monomycolate (GMM) was purified from the lipid extract of Mycobacterium phlei grown in Middlebrook 7H9 medium supplemented with 1 mg of glucose/ml by silica column chromatography as previously described (16). The purity of isolated GMM was assessed by chromatography on silica plates followed by treatment with 3% (wt/vol) cupric acetate and 8% (wt/vol) phosphoric acid and charring at 140°C for 20 min. Computer-assisted quantitative analysis of charred lipid species was carried out by using Scion Image software (Scion Corporation, Frederick, Md.) and demonstrated the purity of GMM to be >98%.
Proliferation assays.
Irradiated (5,000 rads) immature DC were plated in U-bottom microtiter plate wells at 25,000 per well in 0.2 ml of complete medium containing 10% human male AB serum (Sigma). Lipid antigens were added at a final concentration of 20 μg/ml. In antibody blocking experiments, monoclonal antibodies (MAbs) used were purified IgG obtained from hybridoma culture supernatants and were added to a final concentration of 20 μg/ml. The following antibodies were used: 10H3.9.3 (anti-CD1a) (18), BCD1b5 (anti-CD1b) (13), F10/21A3 (anti-CD1c) (12), CD1d42 (anti-CD1d) (27), and W6/32 (anti-HLA-A, -B, and -C) (12). Responding autologous nonadherent lymphocytes were added to a density of 50,000 unseparated or MACS-sorted cells per well. Wells were pulsed with 1 μCi of [3H]thymidine per well during the last 6 h of a 96-h incubation period. Cells were harvested onto glass fiber filters, and tritium incorporation was measured using a Microbeta Trilux scintillation counter (Wallac, Turku, Finland).
IFN-γ ELISPOT assay.
ELISPOT membrane plates (Millipore, Bedford, Mass.) were coated overnight with the IFN-γ-specific antibody AB285 (R&D Systems, Minneapolis, Minn.). After being blocked with 1% bovine serum albumin in PBS for 2 h and washed three times with PBS, immature DC (25,000 per well) were plated into the wells and incubated together with autologous nonadherent cells (50,000 per well) in 0.2 ml of complete medium. Mycobacterial antigens were added to wells in triplicate, and some wells contained medium without antigen for determination of background levels of IFN-γ spot-forming cells. After 48 h, cells were removed and wells were washed 10 times with washing buffer (0.05% Tween 20 in PBS). The biotinylated anti-IFN-γ detection antibody BAF285 (R&D Systems) was added according to standardized protocols (31). Spots were stained by using alkaline phosphatase chromogen detection and were counted both by microscopy and by using a computerized ELISPOT reader (Biosys, Karben, Germany).
RESULTS
Proliferative responses of M. tuberculosis-infected donors to M. tuberculosis lipid antigens.
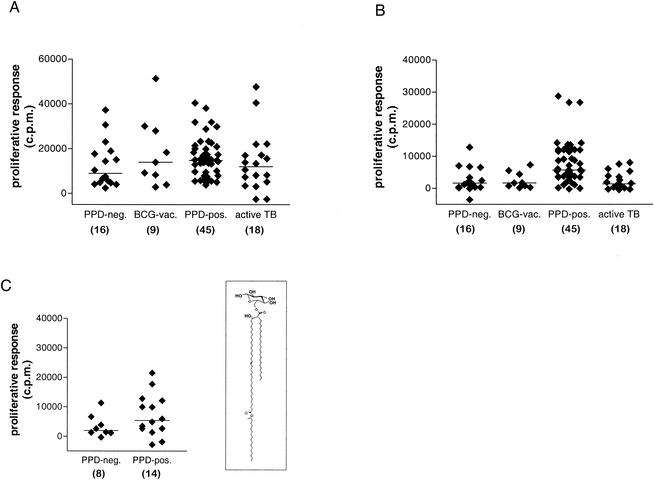
In order to assess the effect of previous infection with M. tuberculosis on the development of T-cell responses against mycobacterial lipids, we first studied the ex vivo responses of PBMC isolated from humans with or without documented M. tuberculosis infection. Proliferative responses of the whole PBMC to mycobacterial antigens revealed that the vast majority of all individuals studied, including PPD-negative healthy donors, responded strongly to the M. tuberculosis total sonicate containing most or all of the antigens of the bacillus (Fig. 1A). In marked contrast, significant responses to the M. tuberculosis total-lipid extract were seen in most cases for PPD-positive healthy donors known to have had contact with TB but only rarely and at low levels for PPD-negative subjects, BCG-immunized subjects without known contact with M. tuberculosis, and patients with active TB (Fig. 1B) (P = 0.0006 for responses of PPD-positive healthy donors to the M. tuberculosis lipid extract compared to medium alone, and P > 0.05 for other groups, by the Mann-Whitney U test). A similar result was obtained by calculation of the stimulation indices (SI) for the responses of each group to the M. tuberculosis lipid extract [SI = (counts per minute of [3H]thymidine incorporation with M. tuberculosis lipid stimulation)/(counts per minute of [3H]thymidine incorporation with medium alone)]. This revealed significant elevations (i.e., >3 standard deviations above the background level determined in the absence of added antigen) of the SI in response to the M. tuberculosis lipid extract for the PPD-positive healthy donor group (median SI, 11.5) but not for the PPD-negative control group (median SI, 1.5).
FIG. 1.
Proliferative responses of the four study groups to M. tuberculosis whole-antigen extract and M. tuberculosis lipid antigens. Proliferation assays were performed by culturing nonadherent PBMC from each individual with irradiated autologous monocyte-derived iDC in complete medium containing M. tuberculosis antigens added to a final concentration of 20 μg/ml. (A) Responses to M. tuberculosis total sonicate; (B) responses to M. tuberculosis total-lipid extract; (C) responses to purified GMM (inset shows structure of M. phlei C80 GMM). Proliferation was measured as counts per minute of [3H]thymidine incorporation, and results are expressed as counts per minute with backgrounds subtracted (see below). Each symbol represents the mean of triplicate values for a single individual, and horizontal bars drawn through each group of data points show median proliferation values. Subjects are grouped into the following clinical categories: tuberculin skin test negative with no history of M. tuberculosis exposure or BCG vaccination (PPD-neg), tuberculin skin test negative with a history of previous BCG vaccination (BCG-vac), tuberculin skin test positive without active disease (PPD-pos), and active TB prior to initiation of treatment (active TB). BCG-vac, PPD-pos, and active TB study groups were compared statistically to the PPD-neg control group (by the Mann-Whitney U test). Significant increases were observed only for the proliferative responses of the PPD-pos group relative to those of the PPD-neg group for M. tuberculosis whole-lipid antigen (B) (P = 0.0006) and for purified GMM (C) (P = 0.0223). The number of individuals in each group is given in parentheses below the x-axis labels. The median background value (i.e., proliferation in medium alone without M. tuberculosis antigens) for each group was as follows: 1,930 cpm (range, 445 to 1,629 cpm) for the PPD-neg control group, 630 cpm (range, 401 to 1,667 cpm) for BCG-vac, 641 cpm (range, 134 to 2,823) for PPD-pos, and 1,554 cpm (range, 394 to 2,780 cpm) for active TB.
We also assessed the proliferative responses of some individuals to GMM, a purified and structurally defined mycobacterial glycolipid antigen (Fig. 1C). Previous studies have identified GMM as a CD1b-presented antigen, and T-cell cross-reactivity between GMMs isolated from different mycobacterial species has been well documented (14, 15). Here, we used GMM purified to near-homogeneity from extracts of the rapidly growing saprophytic mycobacterium M. phlei. Most of the PPD-negative subjects tested (n = 8) failed to respond to GMM (median proliferative response, 1,373 cpm; P > 0.05 for comparison to medium-only controls). In contrast, the proliferative responses of a subset of PPD-positive healthy donors (n = 12) to GMM revealed clear responses in the majority of cases and a significant increase relative to the background level for the group (median proliferative response, 5,416 cpm; P = 0.0003 for comparison to medium-only controls). Calculation of SI also revealed proliferative responses to GMM to be significantly increased (>3 standard deviations above background) for the PPD-positive group (mean SI, 9.15) but not the PPD-negative control group (mean SI, 3.45).
Frequencies of IFN-γ-producing cells responding to M. tuberculosis lipid antigens.
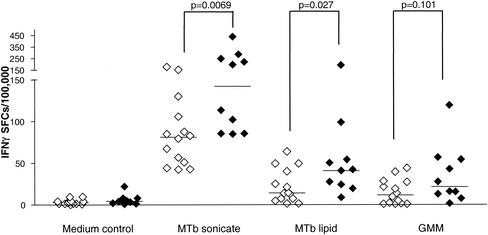
By use of IFN-γ ELISPOT assays with cells from PPD-positive healthy donors with known TB exposure and from PPD-negative healthy donors, the differential responses to mycobacterial lipid antigens were confirmed and quantitative estimates of the responding cell populations were obtained (Fig. 2). Frequencies of IFN-γ-producing cells were determined after stimulation with either the M. tuberculosis total-lipid extract or highly purified GMM. Control cultures were either exposed to medium alone or stimulated with a suspension of total sonicated M. tuberculosis. The median frequencies of IFN-γ-producing cells after lipid antigen stimulation in the PPD-positive group were 46 per 100,000 for the M. tuberculosis total-lipid extract and 43 per 100,000 for purified GMM. Both of these values represented statistically significant increases in levels of IFN-γ-producing cells relative to those in the medium-only control wells (P = 0.0003 for total-lipid antigen, and P = 0.0012 for GMM, by the Mann-Whitney U test). The frequency of IFN-γ-producing cells in response to both lipid antigen preparations represented a significant fraction of the overall response to the complete M. tuberculosis sonicate, which produced a median value of 113 spot-forming cells per 100,000 PBMC. The PPD-negative donor group also showed evidence of IFN-γ responses to both lipid antigen preparations, although these were generally lower than the responses seen in the PPD-positive group (median values, 23 and 24 IFN-γ-producing cells per 100,000 PBMC for total-lipid antigen and purified GMM, respectively; P = 0.0002 for total-lipid antigen, and P = 0.0003 for GMM, compared to the medium-only control). Significant differences between the PPD-positive and PPD-negative groups could be observed in the frequencies of IFN-γ-producing cells in response to the whole M. tuberculosis sonicate (P = 0.0069 by the Mann-Whitney U test) and to the total-lipid antigen (P = 0.027 by the Mann-Whitney U test).
FIG. 2.
Frequency of IFN-γ-producing cells responding to M. tuberculosis whole-antigen extract and to M. tuberculosis lipid antigens. IFN-γ-producing cells in peripheral blood leukocyte samples were quantitated by ELISPOT assay as described in Materials and Methods. M. tuberculosis antigens were used at a final concentration of 20 μg/ml. Results are presented as numbers of spot-forming cells (SFCs) per 100,000 cells. Open symbols, PPD-negative healthy donors (n = 14); filled symbols, PPD-positive healthy donors (n = 10). Horizontal bars show median values for each group. P values for the comparisons indicated were determined by using the Mann-Whitney U test.
Mycobacterial lipid-antigen-specific T cells in active TB.
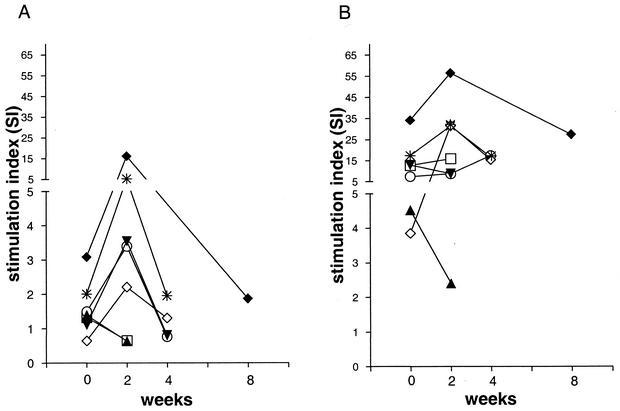
In order to investigate the response to mycobacterial lipid antigens in the setting of clinically active infection, additional blood samples were obtained from patients with active pulmonary TB prior to the start of anti-TB chemotherapy and at several points thereafter (i.e., 2 and 4 weeks after initiation of treatment and at the time of discharge from the hospital). Proliferation assays comparing the reactivities of the PBMC collected at these time points showed that lipid-antigen-specific T-cell responses were generally detected weakly or not at all prior to initiation of therapy and became detectable in peripheral blood of TB patients approximately 2 weeks after initiation of anti-TB chemotherapy (Fig. 3A). The maximum proliferative response to mycobacterial lipid antigen was observed at 2 weeks following the start of therapy, and this tended to decline over the next 2 to 4 weeks. The proliferative responses of these samples to the total M. tuberculosis sonicate showed less variation over time than the responses to the lipid antigen preparation and tended to remain elevated at a similar level throughout the time course of the analysis (Fig. 3B). Similarly, proliferative responses to the mitogen phytohemagglutinin or to the purified recombinant M. tuberculosis protein antigen Ag85B (as previously described by Hess et al. [7]) were elevated in several individuals and showed relatively less variation over time than lipid-antigen-specific responses (data not shown).
FIG. 3.
T-cell responses to M. tuberculosis antigens before and after initiation of anti-TB chemotherapy. Peripheral blood leukocytes collected from seven patients with active TB before and at different time points after initiation of anti-TB chemotherapy were tested for proliferative responses to M. tuberculosis antigens. (A) Responses to M. tuberculosis whole-lipid antigen fraction (20 μg/ml); (B) responses to M. tuberculosis total sonicate (10 μg/ml). Each symbol represents a different TB patient. The median background value for cultures containing medium without M. tuberculosis antigens was 1,053 cpm (range, 204 to 5,533 cpm).
Characterization of M. tuberculosis lipid antigen-reactive T cells.
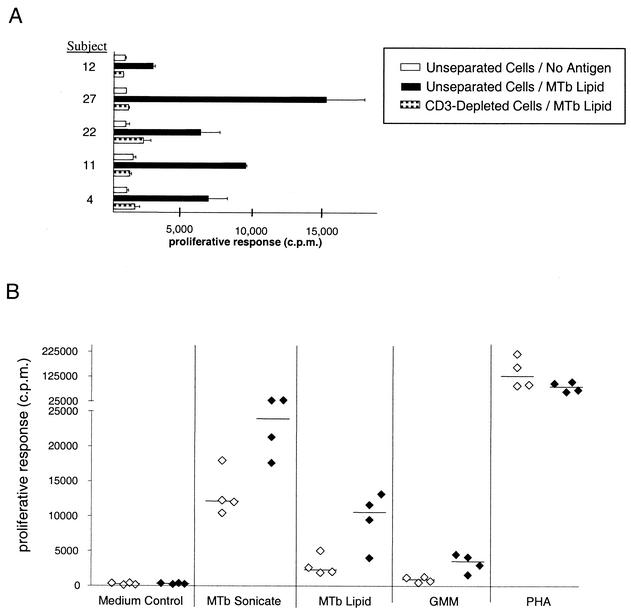
In initial studies to demonstrate the T-cell dependence of the responses observed, proliferation and IFN-γ ELISPOT assays using responding nonadherent PBMC that were depleted of CD3+ cells were performed. These studies showed that depletion of CD3+ cells completely eliminated responses to the M. tuberculosis total-lipid fraction (Fig. 4A). The observed proliferative responses to the M. tuberculosis total-lipid fraction were also dependent on the presence of APCs, as no responses were observed when nonadherent lymphocytes were cultured with antigens in the absence of autologous adherent cells (data not shown). In addition, proliferation assays using either unstimulated APCs (monocytes) or immature DC (iDC) obtained by pretreatment of monocytes with IL-4 and GM-CSF revealed that while both APC preparations supported responses to the M. tuberculosis total sonicate, significant responses to lipid antigens were obtained only when iDC were used as the APCs (Fig. 4B).
FIG. 4.
Dependence of responses to mycobacterial lipid antigen on CD3+ cells and CD1+ APCs. (A) Proliferative response of unseparated and CD3-depleted nonadherent cells. Nonadherent PBMC from PPD-positive healthy donors were incubated with anti-CD3-conjugated MACS beads. After separation on MACS columns, cells were harvested and washed. A total of 50,000 unseparated or CD3-depleted cells and 25,000 iDC were incubated either with the whole-lipid antigen (MTb lipid) or with medium alone (No antigen). Results obtained from five representative PPD-positive healthy donors are shown. Error bars represent standard errors of the means for triplicate samples. (B) Proliferative responses of unstimulated adherent cells or monocyte-derived DC. Nonadherent PBMC from four PPD-positive healthy donors were incubated with M. tuberculosis antigens (20 μg/ml) or medium alone together with either untreated adherent cells (open symbols) or monocyte-derived DC (filled symbols). Each symbol represents the mean of triplicate values for one donor. Horizontal bars show median values for each group. PHA, phytohemagglutinin.
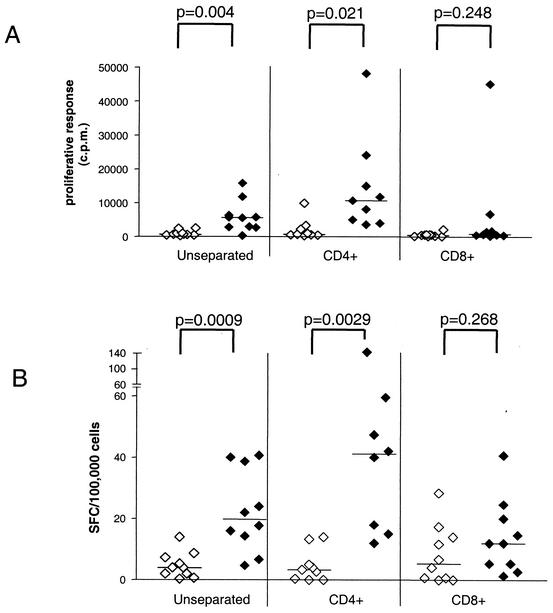
In order to determine if the M. tuberculosis lipid antigen reactivity was predominantly a feature of either CD4+ or CD8+ T cells, we studied the responses of these populations separately after their purification by immunomagnetic sorting. These studies showed that responses to the M. tuberculosis whole-lipid antigen preparation were much more pronounced in isolated CD4+ T cells and were only weakly detected in most samples of CD8+ T cells by proliferation assay (Fig. 5A). This suggested that for the proliferative responses observed, the T cells responding to M. tuberculosis lipid antigens were predominantly CD4 positive. Very similar findings were obtained by IFN-γ ELISPOT assay (Fig. 5B).
FIG. 5.
Involvement of CD4+ and CD8+ T cells in the responses to M. tuberculosis lipid antigen. CD4+ and CD8+ nonadherent PBMC of PPD-positive healthy donors were purified from a group of 10 representative PPD-positive healthy donors. Either 50,000 of the selected CD4+ or CD8+ cells or 50,000 unseparated nonadherent PBMC were cultured with 25,000 autologous DC in complete medium with or without M. tuberculosis whole-lipid antigen (20 μg/ml). Responses were measured by a proliferation assay (A) or by an IFN-γ ELISPOT assay (B). Open symbols, medium control; filled symbols, M. tuberculosis lipid antigen stimulation. Results are expressed as counts per minute of [3H]thymidine incorporation for proliferative responses and as numbers of spot-forming cells (SFC) per 100,000 cells for the ELISPOT assay. P values were calculated by an unpaired t test. Horizontal bars show median values for each group. Each symbol represents the mean of triplicate values for one individual. Results for all 10 donors are shown for unseparated and CD8+ cells. Results for only nine (proliferation) or eight (ELISPOT) donors are shown for CD4+ cells because of insufficient cell recovery from two samples.
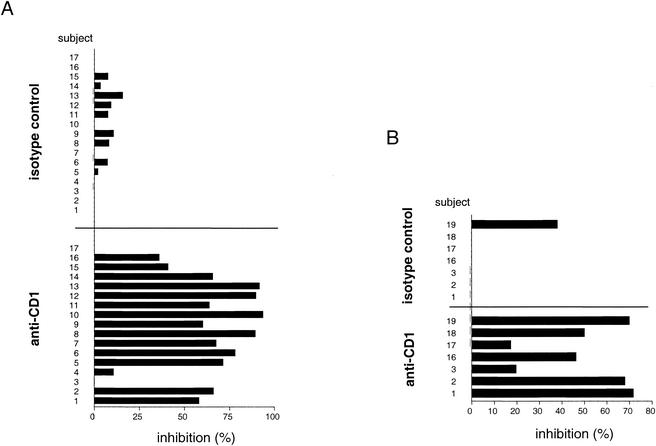
The requirement for GM-CSF and IL-4 pretreatment of adherent mononuclear cells to generate effective APCs for responses to M. tuberculosis lipid antigens suggested a direct involvement of group 1 CD1 molecules, since these are absent on normal monocytes but strongly upregulated by treatment with these cytokines (20, 21). To more directly assess the involvement of CD1 molecules in triggering the lipid-antigen-specific responses, we determined the effects of adding a mixture of MAbs specific for all of the group 1 CD1 isoforms (i.e., anti-CD1a, -b, and -c) in the proliferation and IFN-γ ELISPOT assays. In proliferation assays using a series of representative PPD-positive donors, we observed significant blocking of the proliferative responses against the M. tuberculosis total-lipid-antigen preparation for 15 of 17 individuals, with the level of inhibition ranging from 10.7 to 93.5% (mean, 57.8%) (Fig. 6A). Blocking with an isotype-matched control antibody (P3) was consistently less than 16% (mean, 4.5%). Similar results were obtained in ELISPOT assays using a sample of seven PPD-positive individuals (Fig. 6B). This revealed a 19.6 to 77.6% (mean, 50.9%) inhibition of IFN-γ spot-forming cells by addition of the anti-CD1 antibody cocktail, whereas the isotype control antibody revealed virtually no inhibition (mean, 9.5%, maximum, 38.1%).
FIG. 6.
Blocking of the immune response to mycobacterial lipid antigen by CD1-specific antibodies. Nonadherent cells and autologous DC of PPD-positive healthy donors were incubated with or without MAbs in the presence or absence of the M. tuberculosis total-lipid extract (20 μg/ml). Responses were measured by a proliferation assay (A) or an IFN-γ ELISPOT assay (B) using lipid antigen alone or together with a blocking MAb cocktail against CD1 (i.e., a mixture of anti-CD1a, -b, and -c antibodies). The nonbinding myeloma protein P3 was used as an isotype control. Results are expressed as percent inhibition of proliferation relative to proliferation in replicate cultures that contained no anti-CD1 or isotype control MAbs. The individuals used for these antibody blocking studies were a representative series of consecutively recruited PPD-positive individuals who showed responses significantly above background to the M. tuberculosis lipid-antigen preparation (>1,000 cpm in the proliferation assay, or >10 spot-forming cells/100,000 cells in the ELISPOT assay, above background levels with medium alone).
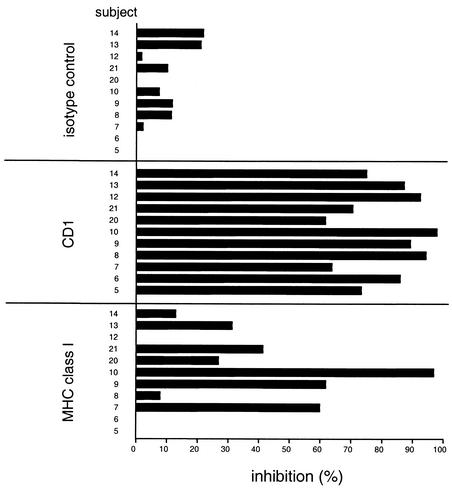
The CD1 restriction of the lipid-antigen response was also studied for a single isolated mycobacterial glycolipid antigen, GMM (Fig. 7). A series of 11 PPD-positive subjects who were responsive to purified GMM in proliferation assays were analyzed. GMM responses by cells from all of these donors were markedly inhibited by the anti-CD1 antibody cocktail (61.9 to 98.2% inhibition; mean, 81.2%). In contrast, the nonbinding isotype control antibody showed little or no inhibition (mean, 8%). An antibody to MHC class I molecules (W6/32), which bound to the surfaces of the APCs at levels comparable to the binding of the anti-CD1 antibodies, showed variable levels of inhibition (0 to 87%; mean, 30.9%) that were in most cases much lower than the inhibitory effects of anti-CD1 treatment.
FIG. 7.
Blocking of proliferative responses to GMM by anti-CD1 MAbs. Nonadherent cells and autologous DC of PPD-positive healthy donors were incubated with GMM (20 μg/ml) in a proliferation assay as described in the legend to Fig. 1, by using GMM either alone or together with MAbs specific for CD1 (a mixture of anti-CD1a, -b, and -c), a MAb specific for MHC class I molecules (W6/32), or a nonbinding isotype control MAb (P3). Blocking of the response is expressed as percent inhibition of proliferation relative to proliferation in replica cultures containing no anti-CD1 or isotype control MAbs. The individuals used for these antibody blocking studies were a representative series of consecutively recruited PPD-positive subjects who showed responses significantly above background to purified GMM (proliferation, >1,000 cpm above background level with medium alone).
DISCUSSION
The recognition of bacterial lipid antigens by T cells through CD1 presentation is now established as a pathway that can potentially lead to the development of specific immunity to microbial pathogens. In the present study, we have examined ex vivo T-cell responses to natural lipid antigens of M. tuberculosis in a cohort of humans to assess the contribution of this form of cell-mediated recognition to the anti-mycobacterial immune response. In agreement with a previous study that used a synthetic analogue of a mycobacterial glycolipid antigen presented by CD1c, our results show for the first time that responses to natural M. tuberculosis lipid antigens are routinely found in healthy humans who have previously been infected with M. tuberculosis (i.e., PPD-positive, healthy M. tuberculosis-exposed subjects). Such responses could be demonstrated by significant increases both in T-cell proliferation and in the frequency of IFN-γ-producing cells in response to M. tuberculosis lipid antigens. These M. tuberculosis lipid-antigen-specific responses were most prominent in individuals who proved to have had previous exposure to M. tuberculosis, establishing an extremely strong correlation between the presence of M. tuberculosis lipid-specific T cells in the circulation and previous infection with this pathogen. Taken together, our findings indicated that the observed M. tuberculosis lipid-antigen responses represent a component of the normal human adaptive immune response to M. tuberculosis.
The comparison among the four study populations revealed that all groups recognized whole mycobacterial sonicate regardless of previous contact with mycobacteria either by vaccination or by infection. The reason for such responses in PPD-negative subjects with no known TB exposure is not clearly known. They may reflect cross-reactivity between antigens of ubiquitous environmental mycobacteria and M. tuberculosis, or they could be due to γδ T-cell responses to phosphoantigens that are contained in most crude mycobacterial antigen preparations (33). In contrast, the lipid fraction was in most cases recognized only by PBMC from previously infected healthy individuals, while most PPD-negative healthy individuals without known contact with TB showed either no detectable response or only weak responses (Fig. 1 and 2). At present, we do not know whether the weak responses observed in some of the PPD-negative individuals represent specific responses to M. tuberculosis lipids that may have been primed by exposure to other bacteria with cross-reacting lipid antigens or simply weak nonspecific mitogenic activities that may be inherent in the M. tuberculosis lipid extract. Nevertheless, it is noteworthy that both proliferation and ELISPOT assays demonstrated that these responses to M. tuberculosis lipid antigens were significantly augmented in healthy PPD-positive subjects. Interestingly, even BCG-vaccinated individuals, who were generally weakly tuberculin skin test reactive (between 3 and 10 mm of induration), did not respond or responded only weakly to the lipid fraction. This could reflect the need for a continuous interaction between persistent M. tuberculosis and an active immune response in latently M. tuberculosis infected healthy individuals in order to produce and maintain measurable recall responses to lipid antigens. Alternatively, the relatively low level of antigen exposure that is likely to result from BCG vaccination, or the administration of BCG early in the first year of life, might explain why the BCG-vaccinated individuals in the present study did not show T-cell responses that cross-reacted with M. tuberculosis lipids.
Patients with active TB did not respond to mycobacterial lipid antigens prior to anti-TB chemotherapy, but lipid-antigen-specific responses increased about 2 weeks after initiation of chemotherapy (Fig. 3). In contrast, considerable frequencies of lipid-antigen-specific T cells could be detected in the blood of infected individuals who did not develop active disease, regardless of the time point of blood drawing after infection (ranging from months to more than 10 years after TB exposure or conversion of the tuberculin skin test). It is possible that in TB patients, the lipid-antigen-specific T cells were sequestered at the site of mycobacterial infection in the lung and thus were not detectable in the peripheral blood. After initiation of anti-TB chemotherapy, which results in cessation of bacterial growth and a decline in bacterial burden, lipid-antigen-specific T cells may have been redistributed so that they were again detectable in the blood. Alternatively, individuals who develop active TB may have intrinsic defects that lead to delayed or reduced reactivity to M. tuberculosis lipid antigens, and these responses may be further suppressed by the pathogen once it has entered an actively growing phase. This possibility is suggested by previous studies showing cytokine-dependent suppression of T-cell reactivity in patients with active TB disease, such as the effects attributed to transforming growth factor β in humans with active TB and to prostaglandin E2 in the mouse model (9, 19, 23). In addition, an active role for certain mycobacterial products, such as the 19-kDa lipoprotein, has also been suggested in the apparent suppression of T-cell responses that accompanies active TB (22). T-cell responses to M. tuberculosis lipid antigens were observed to decrease again in these patients later during chemotherapy and in several cases were found to have returned to very low levels at the completion of anti-TB treatment (Fig. 3; additional data not shown). One could speculate that this represented a short-term expansion and subsequent contraction of the T-cell population in response to changes in the levels of CD1-presented lipid antigens.
Using the IFN-γ ELISPOT assay, we found a surprisingly high frequency of lipid-antigen-specific T cells in individuals immune to M. tuberculosis. As shown by the results in Fig. 2, the contribution of lipid-antigen-reactive T cells to the overall response to the M. tuberculosis total sonicate was considerable. Furthermore, frequencies of IFN-γ-producing cells after stimulation with a single purified lipid antigen (GMM) were comparable to frequencies observed in published studies following stimulation with protein antigens such as ESAT-6 and Ag85, which have been shown to stimulate 50 to 100 IFN-γ-producing T cells per 100,000 circulating lymphocytes (17, 32). Characterization of the cells responding to M. tuberculosis lipids in human PBMC indicated that these were CD3 positive, thus identifying them as T cells. Previous studies using in vitro-expanded T-cell lines have established that CD1 restriction can be observed for both αβ and γδ T-cell receptor (TCR) T cells (28). However, in preliminary experiments we found that reactivity to M. tuberculosis lipids was not significantly affected by depletion of cells expressing γδ TCRs, indicating that the majority of the M. tuberculosis lipid-responsive cells detected in our studies express αβ TCRs (data not shown). Among peripheral T cells, CD4+ T cells were generally responsible for the majority of proliferative responses and IFN-γ production that we observed upon stimulation with mycobacterial lipid antigens (Fig. 4A and 5). Although many of the previously described in vitro-cultivated T-cell lines responding to mycobacterial lipid antigens have been phenotypically either CD4− CD8− or CD4− CD8+, our findings suggest that this may reflect a bias inherent in the methods used to isolate and cultivate such cell lines rather than a true representation of the phenotypic distribution of M. tuberculosis lipid-reactive T cells. In fact, in agreement with the findings reported here, it has been reported that CD4+ T-cell lines recognizing lipid antigens presented by CD1 can be isolated both from human PBMC and from mice (26, 35). Although proliferation assays may be biased because they preferentially detect strongly proliferating cells (i.e., CD4+ cells), the results suggest that CD4+ T cells could represent the major subset of T cells responsive to mycobacterial lipid antigens in humans.
Several lines of evidence in the present study indicate that the observed T-cell responses against M. tuberculosis lipids are mediated mainly by presentation via CD1 molecules. Thus, only DC-expressing CD1 could present lipid antigen to M. tuberculosis-specific T cells, whereas monocytes that lacked expression of CD1 failed to activate T cells after pulsing with mycobacterial lipid antigen (Fig. 4B). Although this is consistent with a requirement for CD1 presentation, it could also reflect other differences between monocytes and DC that enable the latter to more efficiently present antigens and activate resting T cells. Stronger evidence for the CD1 restriction of the observed T-cell responses to the whole mycobacterial lipid (Fig. 6) and a single purified lipid antigen (GMM) (Fig. 7) was provided by our finding that these could be strongly inhibited in most cases by a MAb cocktail recognizing all three group 1 CD1 proteins, and not by isotype control antibodies that did not bind to the surfaces of the cells. Moderate variability of the blocking effects of anti-CD1 MAbs on the responses to the M. tuberculosis total-lipid extract was observed in this study, with a few individuals actually showing little or no inhibition of their responses by anti-CD1 (Fig. 6). This variability in the level of inhibition showed no correlation with the strength of the proliferative or IFN-γ ELISPOT responses of the subjects (data not shown). Instead, it may be related to the heterogeneity of the M. tuberculosis total-lipid-antigen preparation, which could potentially evoke qualitatively different responses from different donors. In support of this possibility was our finding that the responses to a homogeneous purified M. tuberculosis glycolipid antigen (GMM) were consistently and strongly inhibited by anti-CD1 MAbs (Fig. 7). Since our present data have evaluated CD1 restriction of M. tuberculosis lipid-specific responses by using only a mixture of MAbs against all three group 1 CD1 isoforms, further studies will be required to determine whether one or more of these CD1 molecules play a dominant role in these ex vivo responses.
As an additional control for these experiments, we assessed the effects of MAbs against MHC class I molecules, which are expressed on both the responding T cells and the APCs. Although we did observe partial inhibition of T-cell responses to M. tuberculosis lipid antigens in some cases by using anti-MHC class I MAbs, these effects were generally much weaker and less consistent than the blocking effects observed with anti-CD1 MAbs (Fig. 7). The mechanism for the partial blocking effects of anti-MHC class I MAbs in these experiments has not been investigated by us. However, reports in the literature have shown that MHC class I molecules may associate on the T-cell surface with a variety of molecules involved in T-cell activation, including the CD3-TCR complex and CD2 (11, 34). Thus, anti-MHC class I MAbs might inhibit T-cell activation in a non-antigen-specific manner by interfering with the function of associated receptor complexes. Alternatively, it is interesting to speculate that this finding might reflect a relevant accessory interaction between CD1-restricted T cells and APCs that is dependent on MHC class I molecules, or the presence of a population of MHC-restricted accessory cells that is required in some subjects for the effective stimulation of CD1-restricted responses to lipid antigens.
The results provided by the present study show that lipid-antigen-specific T cells are present in the circulation of humans following natural infection with M. tuberculosis and thus may represent one component of the protective immune response to this organism. Indeed, the presence of these T-cell responses in PPD-positive subjects who developed no clinical signs of disease, and the suppression of such responses in active TB patients, suggests a correlation between circulating lipid-antigen-reactive T cells and control of the infection. Our data indicate that exposure to M. tuberculosis by natural infection can elicit recall responses to M. tuberculosis lipids, which most likely represent part of the adaptive immune response to this pathogen. This function of the group 1 component of the CD1 system appears to contrast with the dominant role of the group 2 CD1 molecules, which have been linked to the selection and activation of natural killer T cells that may function more as effectors of innate immunity and in immunoregulation (2, 3, 10, 29).
Understanding the basis of the overall acquired immunity to M. tuberculosis infection in humans is crucial for developing new approaches to therapy and prevention through vaccination. Recent data from studies using guinea pigs as an in vivo model for the function of group 1 CD1 molecules have shown that long-term memory against mycobacterial lipid antigens can be stimulated by specific immunization, further suggesting that lipids might be useful targets to evaluate further in the ongoing search for novel vaccines against M. tuberculosis (8). In humans, further investigation of the role of unconventional T cells such as CD1-restricted and nonclassical HLA-restricted T cells is required for better understanding of the basis for optimal and appropriate acquired immunity to M. tuberculosis.
Acknowledgments
This work was supported by grants from the NIH (AI48933 and AI45889) and by a grant from the Irene Diamond Foundation to S.A.P. D.B.M. was supported by grants from the NIH (AR48632 and AI49313) and by the Research and Education Foundation of the American College of Rheumatology. T.U. received a fellowship from the Deutsche Forschungsgemeinschaft (DFG, UL 176/1-1).
We thank Daphney Frederique and Tan-Yun Cheng for excellent technical assistance in the preparation of lipid antigens, and we thank Sadamu Ishikawa for assistance in identifying human subjects for this study.
Editor: J. D. Clements
REFERENCES
- 1.Beckman, E. M., S. A. Porcelli, C. T. Morita, S. M. Behar, S. T. Furlong, and M. B. Brenner. 1994. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372:691-694. [DOI] [PubMed] [Google Scholar]
- 2.Behar, S. M., C. C. Dascher, M. J. Grusby, C. R. Wang, and M. B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brossay, L., M. Chioda, N. Burdin, Y. Koezuka, G. Casorati, P. Dellabona, and M. Kronenberg. 1998. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn, J. L., and J. D. Ernst. 2000. Immune responses in tuberculosis. Curr. Opin. Immunol. 12:432-436. [DOI] [PubMed] [Google Scholar]
- 5.Folch, J., M. Lees, and G. H. Sloane Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 6.Gumperz, J. E., and M. B. Brenner. 2001. CD1-specific T cells in microbial immunity. Curr. Opin. Immunol. 13:471-478. [DOI] [PubMed] [Google Scholar]
- 7.Hess, J., L. Grode, J. Hellwig, P. Conradt, I. Gentschev, W. Goebel, C. Ladel, and S. H. Kaufmann. 2000. Protection against murine tuberculosis by an attenuated recombinant Salmonella typhimurium vaccine strain that secretes the 30-kDa antigen of Mycobacterium bovis BCG. FEMS Immunol. Med. Microbiol. 27:283-289. [DOI] [PubMed] [Google Scholar]
- 8.Hiromatsu, K., C. C. Dascher, K. P. LeClair, M. Sugita, S. T. Furlong, M. B. Brenner, and S. A. Porcelli. 2002. Induction of CD1-restricted immune responses in guinea pigs by immunization with mycobacterial lipid antigens. J. Immunol. 169:330-339. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch, C. S., R. Hussain, Z. Toossi, G. Dawood, F. Shahid, and J. J. Ellner. 1996. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc. Natl. Acad. Sci. USA 93:3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawano, T., Y. Tanaka, E. Shimizu, Y. Kaneko, N. Kamata, H. Sato, H. Osada, S. Sekiya, T. Nakayama, and M. Taniguchi. 1999. A novel recognition motif of human NKT antigen receptor for a glycolipid ligand. Int. Immunol. 11:881-887. [DOI] [PubMed] [Google Scholar]
- 11.Khan, A. A., C. Bose, L. S. Yam, M. J. Soloski, and F. Rupp. 2001. Physiological regulation of the immunological synapse by agrin. Science 292:1681-1686. [DOI] [PubMed] [Google Scholar]
- 12.Melián, A., Y. J. Geng, G. K. Sukhova, P. Libby, and S. A. Porcelli. 1999. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am. J. Pathol. 155:775-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melián, A., G. F. Watts, A. Shamshiev, G. De Libero, A. Clatworthy, M. Vincent, M. B. Brenner, S. Behar, K. Niazi, R. L. Modlin, S. Almo, D. Ostrov, S. G. Nathenson, and S. A. Porcelli. 2000. Molecular recognition of human CD1b antigen complexes: evidence for a common pattern of interaction with αβ TCRs. J. Immunol. 165:4494-4504. [DOI] [PubMed] [Google Scholar]
- 14.Moody, D. B., V. Briken, T. Y. Cheng, C. Roura-Mir, M. R. Guy, D. H. Geho, M. L. Tykocinski, G. S. Besra, and S. A. Porcelli. 2002. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat. Immunol. 3:435-442. [DOI] [PubMed] [Google Scholar]
- 15.Moody, D. B., M. R. Guy, E. Grant, T. Y. Cheng, M. B. Brenner, G. S. Besra, and S. A. Porcelli. 2000. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J. Exp. Med. 192:965-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody, D. B., T. Ulrichs, W. Muhlecker, D.C. Young, S. S. Gurcha, E. Grant, J. P. Rosat, M. B. Brenner, C. E. Costello, G. S. Besra, and S. A. Porcelli. 2000. CD1c-mediated T cell recognition of isoprenoid glycolipids in M. tuberculosis infection. Nature 404:884-888. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa, A. S., F. A. Shaban, A. T. Abal, R. Al Attiyah, H. G. Wiker, K. E. Lundin, F. Oftung, and K. Huygen. 2000. Identification and HLA restriction of naturally derived Th1-cell epitopes from the secreted Mycobacterium tuberculosis antigen 85B recognized by antigen-specific human CD4+ T-cell lines. Infect. Immun. 68:3933-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olive, D., P. Dubreuil, and C. Mawas. 1984. Two distinct TL-like molecular subsets defined by monoclonal antibodies on the surface of human thymocytes with different expression on leukemia lines. Immunogenetics 20:253-264. [DOI] [PubMed] [Google Scholar]
- 19.Pathan, A. A., K. A. Wilkinson, P. Klenerman, H. McShane, R. N. Davidson, G. Pasvol, A. V. Hill, and A. Lalvani. 2001. Direct ex vivo analysis of antigen-specific IFN-γ-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J. Immunol. 167:5217-5225. [DOI] [PubMed] [Google Scholar]
- 20.Porcelli, S., C. T. Morita, and M. B. Brenner. 1992. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature 360:593-597. [DOI] [PubMed] [Google Scholar]
- 21.Porcelli, S. A., and R. L. Modlin. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297-329. [DOI] [PubMed] [Google Scholar]
- 22.Post, F. A., C. Manca, O. Neyrolles, B. Ryffel, D. B. Young, and G. Kaplan. 2001. Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits Mycobacterium smegmatis-induced cytokine production by human macrophages in vitro. Infect. Immun. 69:1433-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangel, M. J., G. Estrada, I. De La Luz Garcia Hernandez, L. D. Aguilar, R. Marquez, and P. R. Hernandez. 2002. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology 106:257-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serbina, N. V., C. C. Liu, C. A. Scanga, and J. L. Flynn. 2000. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J. Immunol. 165:353-363. [DOI] [PubMed] [Google Scholar]
- 26.Sieling, P. A., M. T. Ochoa, D. Jullien, D. S. Leslie, S. Sabet, J. P. Rosat, A. E. Burdick, T. H. Rea, M. B. Brenner, S. A. Porcelli, and R. L. Modlin. 2000. Evidence for human CD4+ T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions. J. Immunol. 164:4790-4796. [DOI] [PubMed] [Google Scholar]
- 27.Spada, F. M., F. Borriello, M. Sugita, G. F. Watts, Y. Koezuka, and S. A. Porcelli. 2000. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur. J. Immunol. 30:3468-3477. [DOI] [PubMed] [Google Scholar]
- 28.Spada, F. M., E. P. Grant, P. J. Peters, M. Sugita, A. Melian, D. S. Leslie, H. K. Lee, E. van Donselaar, D. A. Hanson, A. M. Krensky, O. Majdic, S. A. Porcelli, C. T. Morita, and M. B. Brenner. 2000. Self-recognition of CD1 by γδ T cells. Implications for innate immunity. J. Exp. Med. 191:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spada, F. M., Y. Koezuka, and S. A. Porcelli. 1998. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 188:1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 31.Ulrichs, T., P. Anding, S. H. Kaufmann, and M. E. Munk. 2000. Numbers of IFN-γ-producing cells against ESAT-6 increase in tuberculosis patients during chemotherapy. Int. J. Tuberc. Lung Dis. 4:1181-1183. [PubMed] [Google Scholar]
- 32.Ulrichs, T., P. Anding, S. Porcelli, S. H. Kaufmann, and M. E. Munk. 2000. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect. Immun. 68:6073-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulrichs, T., and S. A. Porcelli. 2000. CD1 proteins: targets of T cell recognition in innate and adaptive immunity. Rev. Immunogenet. 2:416-432. [PubMed] [Google Scholar]
- 34.Verhagen, A. M., B. Schraven, M. Wild, R. Wallich, and S. C. Meuer. 1996. Differential interaction of the CD2 extracellular and intracellular domains with the tyrosine phosphatase CD45 and the zeta chain of the TCR/CD3/zeta complex. Eur. J. Immunol. 26:2841-2849. [DOI] [PubMed] [Google Scholar]
- 35.Wang, B., T. Chun, and C. R. Wang. 2000. Comparative contribution of CD1 on the development of CD4+ and CD8+ T cell compartments. J. Immunol. 164:739-745. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. 2003. WHO Report 2003. Global tuberculosis control. [Online.] www.who.int/gtb/publications/globrep/index.html.