Abstract
Caterpillars of the herbivore Pieris rapae stimulate the production of jasmonic acid (JA) and ethylene (ET) in Arabidopsis (Arabidopsis thaliana) and trigger a defense response that affects insect performance on systemic tissues. To investigate the spectrum of effectiveness of P. rapae-induced resistance, we examined the level of resistance against different pathogens. Although the necrotrophic fungus Alternaria brassicicola is sensitive to JA-dependent defenses, herbivore-induced resistance was not effective against this pathogen. By contrast, caterpillar feeding significantly reduced disease caused by the bacterial pathogens Pseudomonas syringae pv tomato and Xanthomonas campestris pv armoraciae. However, this effect was apparent only locally in caterpillar-damaged tissue. Arabidopsis mutants jar1, coi1, ein2, sid2, eds5, and npr1 showed wild-type levels of P. rapae-induced protection against P. syringae pv tomato, suggesting that this local, herbivore-induced defense response does not depend exclusively on either JA, ET, or salicylic acid (SA). Resistance against the biotroph Turnip crinkle virus (TCV) requires SA, but not JA and ET. Nevertheless, herbivore feeding strongly affected TCV multiplication and TCV lesion formation, also in systemic tissues. Wounding alone was not effective, but application of P. rapae regurgitate onto the wounds induced a similar level of protection. Analysis of SA-induced PATHOGENESIS RELATED-1 (PR-1) expression revealed that P. rapae grazing primed Arabidopsis leaves for augmented expression of SA-dependent defenses. Pharmacological experiments showed that ET acts synergistically on SA-induced PR-1, suggesting that the increased production of ET upon herbivore feeding sensitizes the tissue to respond faster to SA, thereby contributing to an enhanced defensive capacity toward pathogens, such as TCV, that trigger SA-dependent defenses upon infection.
Plants possess a broad range of defense mechanisms to effectively combat attack by microbial pathogens and herbivorous insects. These mechanisms include preexisting physical and chemical barriers, as well as inducible defense responses that become activated upon attack (Van Loon, 2000). An important question in plant defense signaling research is: How are plants capable of integrating signals induced by pathogenic microorganisms or herbivorous insects into defenses that are specifically active against the invader encountered? The plant hormones salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) are the main players in the regulation of signaling networks involved in induced defense (Reymond and Farmer, 1998; Pieterse and Van Loon, 1999; Feys and Parker, 2000; Glazebrook, 2001; Thomma et al., 2001; Kessler and Baldwin, 2002). SA-, JA-, and ET-dependent pathways regulate defense responses that are differentially effective against specific types of attackers. Although there are exceptions (Thaler et al., 2004), in general it can be stated that pathogens with a biotrophic lifestyle are more sensitive to SA-dependent responses, whereas necrotrophic pathogens and herbivorous insects are resisted by JA/ET-dependent defenses (Thomma et al., 2001; Glazebrook, 2005). For instance, in Arabidopsis (Arabidopsis thaliana), induction of SA-dependent systemic acquired resistance (SAR) by avirulent Pseudomonas syringae pv tomato provides a significant level of protection against the biotrophic pathogen Turnip crinkle virus (TCV), whereas activation of JA/ET-dependent induced systemic resistance (ISR) by nonpathogenic Pseudomonas fluorescens rhizobacteria is ineffective against this virus. Conversely, rhizobacteria-mediated ISR provides enhanced resistance against the necrotrophic fungus Alternaria brassicicola, whereas pathogen-induced SAR is ineffective against this pathogen (Ton et al., 2002).
The production of SA, JA, and ET varies greatly, depending on the type of pathogen or attacking insect. The quantity, composition, and timing of the signal signature results in the activation of a specific set of genes that eventually determines the nature of the defense response that is triggered by the attacker encountered (De Vos et al., 2005; Mur et al., 2006). There is ample evidence that SA-, JA-, and ET-dependent defense pathways interact, either positively or negatively (Felton and Korth, 2000; Feys and Parker, 2000; Pieterse et al., 2001; Kunkel and Brooks, 2002; Bostock, 2005). Global expression profiling of pathogen-infected wild-type Arabidopsis plants and a large number of SA-, JA-, or ET-signaling mutants revealed substantial cross talk between the SA-, JA-, and ET-dependent signaling pathways (Glazebrook et al., 2003). In some cases, the signaling compounds act additively on the level of resistance (Van Wees et al., 2000). In other cases, simultaneous activation of multiple defense-signaling pathways results in antagonistic effects on pathogen and insect resistance (Thaler et al., 2002b; Bostock, 2005). Several key elements involved in pathway cross talk have been identified. For instance, the SAR regulatory protein NONEXPRESSER OF PATHOGENESIS-RELATED GENES1 (NPR1) has been shown to play an important role in the antagonistic effect of SA on JA-responsive gene expression (Spoel et al., 2003). Furthermore, the Arabidopsis transcription factor WRKY70 was shown to act as both an activator of SA-responsive genes and a repressor of JA-inducible genes, thereby integrating signals from these two pathways (Li et al., 2004). In addition, the transcription factors ETHYLENE RESPONSE FACTOR1 (ERF1) and MYC2 were found to integrate signals from the JA and ET pathways in activating defense-related genes that are responsive to both JA and ET (Lorenzo et al., 2003, 2004). Cross communication between defense pathways can provide a regulatory potential for activating multiple resistance mechanisms in varying combinations and may help the plant to prioritize the activation of a particular defense pathway over another, thereby activating an appropriate defense response against the invader encountered.
Many studies have indicated that JA and its derivatives are the most important regulators of induced resistance against herbivore attack. A classic example is the observation that following attack by larvae of Manduca sexta, tomato (Solanum lycopersicum) leaves accumulate JA, resulting in the activation of genes encoding proteinase inhibitor proteins that inhibit digestive Ser proteinases of herbivorous insects and reduce further insect feeding (Farmer and Ryan, 1992; Howe, 2005). In agreement with this, JA-deficient tomato mutants that are affected in the DEFENSELESS1 (DEF1) gene are more susceptible to attack by herbivores such as M. sexta, Spodoptera exigua, Frankliniella occidentalis, and Tetranychus urticae (Howe et al., 1996; Li et al., 2002; Thaler et al., 2002a). Also, in Arabidopsis, genetic evidence demonstrates that JA plays an important role in induced defense against different types of herbivores (McConn et al., 1997; Stintzi et al., 2001; Ellis et al., 2002; Stotz et al., 2002; Reymond et al., 2004; Van Poecke and Dicke, 2004). Besides being more vulnerable to herbivore attack, various Arabidopsis mutants affected in JA biosynthesis or signaling are altered in their resistance against pathogens, such as the fungi A. brassicicola, Botrytis cinerea, Erysiphe cichoracearum, Erysiphe orontii, Fusarium oxysporum, and Oidium lycopersicum, the oomycetous pathogens Pythium irregulare and Pythium mastophorum, the bacterial pathogens Erwinia carotovora, P. syringae, and Xanthomonas campestris, and the viral pathogen Cucumber mosaic virus (Pozo et al., 2005, and refs. therein).
The dual role of JA in herbivore and pathogen resistance prompted us to investigate the effectiveness of herbivore-induced resistance against infection by microbial pathogens. Aiming to understand how plants integrate pathogen- and insect-induced signals into specific defense responses, we recently monitored the dynamics of SA, JA, and ET signaling in Arabidopsis after attack by a set of microbial pathogens and herbivorous insects with different modes of attack (De Vos et al., 2005). Of these, the tissue-chewing caterpillar of the cabbage white butterfly (Pieris rapae) is a specialist on cruciferous plant species (Van Loon et al., 2000). While feeding on Arabidopsis, P. rapae larvae induced significant levels of JA and ET and a large number of predominantly JA-responsive genes. In other studies, P. rapae feeding has been demonstrated to induce the expression of JA-responsive genes as well (Reymond et al., 2000, 2004). Because of the nature of the response of Arabidopsis to feeding by P. rapae, we hypothesized that caterpillar-induced resistance would be effective against pathogens that are sensitive to JA/ET-dependent defense responses, but not against pathogens that are sensitive exclusively to SA-dependent defenses.
In Arabidopsis, the dependence of induced resistance against specific pathogens on SA and/or JA and ET reflects the involvement of these signaling compounds in basal resistance that is expressed upon primary infection (Ton et al., 2002). Basal resistance against the fungus A. brassicicola is reduced only in JA-insensitive mutants and not in genotypes that are nonresponsive to SA (Thomma et al., 1998). Conversely, basal resistance against TCV is controlled exclusively by a SA-dependent pathway (Kachroo et al., 2000). Only SA-nonaccumulating NahG plants exhibited enhanced disease susceptibility to this pathogen, whereas mutants affected in JA or ET signaling did not. Basal resistance against the bacterial pathogens P. syringae pv tomato and X. campestris pv armoraciae was found to be affected in SA-, JA-, and ET-responsive mutants (Pieterse et al., 1998; Ellis et al., 2002; Ton et al., 2002), indicating that basal resistance against these pathogens depends on the combined action of these signals. Here, we studied whether P. rapae-induced resistance is differentially effective against the microbial pathogens A. brassicicola, P. syringae pv tomato, X. campestris pv armoraciae, and TCV.
RESULTS
P. rapae-Induced Defense against Herbivore Feeding
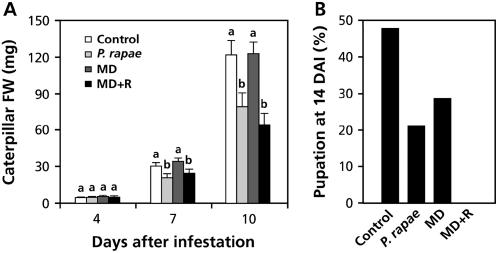
Feeding of P. rapae larvae on Arabidopsis stimulates the production of JA and ET and induces changes in the expression of a large number of defense-related genes (Reymond et al., 2004; De Vos et al., 2005). To verify that this induced defense response is associated with enhanced insect resistance, we monitored the fresh weight of P. rapae larvae on untreated and preinfested Arabidopsis Columbia-0 (Col-0) plants. For induction of resistance, five first-instar larvae of P. rapae were allowed to feed for 24 h on 5-week-old Col-0 plants. Subsequently, the caterpillars were removed and replaced by a fresh first-instar larva of which the fresh weight was monitored over a 10-d period. Figure 1A shows that the increase in weight of the P. rapae larvae was significantly reduced on preinfested plants. To investigate whether herbivore-induced resistance could be mimicked by wounding, Arabidopsis leaves were mechanically damaged with a needle and tested for enhanced resistance against P. rapae feeding. Moreover, mechanically damaged leaves were supplemented with regurgitate that was collected from other P. rapae larvae that had fed on Col-0 plants. Whereas wounding alone did not reduce larval weight gain, application of regurgitate onto the wounds induced similar levels of herbivore resistance as P. rapae feeding did (Fig. 1A).
Figure 1.
Effect of herbivore-induced resistance on P. rapae performance. A, Growth of P. rapae larvae on herbivore-induced (P. rapae), mechanical damage (MD)-induced, or MD- and regurgitate-induced (MD + R) Arabidopsis Col-0 plants. Freshly hatched P. rapae larvae were transferred onto uninduced (control) and induced plants 24 h after the start of the induction treatment. Caterpillar fresh weight (FW) was measured after 4, 7, and 10 d of feeding. The values presented are means (±se) of 20 larvae that received the same treatment. Different letters indicate statistically significant differences between treatments (Fisher's lsd test; α = 0.05). B, Percentage of P. rapae larvae (n = 20) that developed into pupae within 14 d after infestation (DAI). The experiment was repeated with similar results.
To investigate whether the reduced larval performance on induced plants affected the development of the larvae into pupae, the percentage of the larvae that reached pupation was assessed 14 d after transfer of the first-instar larvae onto the Arabidopsis plants. Figure 1B shows that the number of caterpillars that developed into pupae was clearly lower in herbivore-induced plants. Mechanically damaged plants also slowed down pupation, but to a lesser extent than mechanically damaged plants that were treated with P. rapae regurgitate. Wounding alone resulted in moderate reduction in the speed of pupation, whereas this treatment had no effect on caterpillar weight gain (Fig. 1A). Evidently, wounding and insect-derived elicitors induce plant defenses that differentially affect the various stages of larval development. Together, these results indicate that P. rapae feeding induces a defense response that inhibits growth and development of other larvae that subsequently feed on the leaves. This herbivore-induced resistance can be mimicked by applying regurgitate of P. rapae onto the wound sites of mechanically damaged Arabidopsis leaves.
Herbivore-Induced Resistance Is Not Effective against A. brassicicola
Because feeding by P. rapae increased production of both JA and ET (De Vos et al., 2005), we hypothesized that the resulting resistance would also be effective against the necrotrophic fungal pathogen A. brassicicola. Wild-type Arabidopsis Col-0 plants are highly resistant to A. brassicicola infection. However, the phytoalexin-deficient mutant pad3-1 is substantially more susceptible (Thomma et al., 1999) and has been used successfully to study induced resistance against this pathogen (Ton et al., 2002). To trigger herbivore-induced resistance, three P. rapae larvae were allowed to feed on pad3-1 plants for 24 h. As a positive control, pad3-1 plants were treated with 0.1 mm methyl jasmonate (MeJA), which has been shown to induce resistance against A. brassicicola (Ton et al., 2002). Both P. rapae feeding and MeJA treatment activated the JA-responsive genes LOX2 and VSP2 to an extent that was equal to that observed in similarly treated wild-type Col-0 plants (data not shown), indicating that JA signaling is not affected in pad3-1, which confirms previous findings (Van Wees et al., 2003).
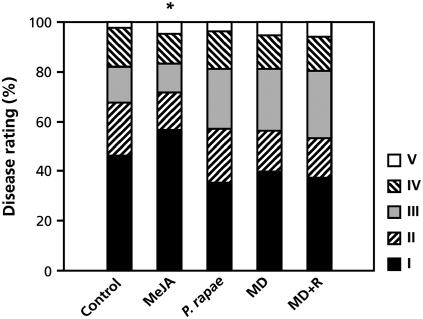
To assess the effectiveness of herbivore-induced resistance against A. brassicicola, noninduced, MeJA-treated, and herbivore-damaged plants were inoculated with this fungus. In noninduced plants, necrotic lesions started to appear within 2 to 3 d after inoculation and progressed into typical spreading lesions that were surrounded by extensive chlorosis. By 6 d after inoculation, the leaves were extensively damaged and sporulation of the pathogen was evident. Exogenous application of MeJA 24 h prior to challenge inoculation resulted in a significant reduction in disease severity. However, although JA levels were increased up to 10-fold in P. rapae-induced plants (De Vos et al., 2005), no enhanced resistance against A. brassicicola infection could be observed in these plants (Fig. 2). Moreover, neither wounding nor application of P. rapae regurgitate onto the wounds resulted in enhanced resistance. It must be concluded, therefore, that P. rapae-induced resistance is not effective against A. brassicicola.
Figure 2.
Effectiveness of herbivore-induced resistance against A. brassicicola. To trigger herbivore-induced resistance, three first-instar larvae of P. rapae were allowed to feed for 24 h on mutant pad3-1 plants, which are a susceptible host for this pathogen. MeJA-induced resistance was elicited by dipping the leaves into a solution containing 0.1 mm MeJA 24 h before challenge. Plants were challenge inoculated with A. brassicicola when 5 weeks old and scored for final disease symptoms 6 d later. Disease severity is expressed on the basis of symptom severity and lesion size (increasing severity from I to V; see “Materials and Methods” for details). Asterisk indicates statistically significant difference distributions within the disease-severity classes compared with the noninduced control treatment (χ2, α = 0.05; n = 15).
P. rapae-Induced Resistance Is Locally Effective against Two Bacterial Leaf Pathogens
Previously, Arabidopsis mutants affected in SA, JA, or ET signaling were demonstrated to be affected in the level of resistance to the bacterial pathogens X. campestris pv armoraciae and P. syringae pv tomato (Pieterse et al., 1998; Ellis et al., 2002; Ton et al., 2002), implying a role for all three signals in the defense against these pathogens. To investigate the effectiveness of herbivore-induced resistance against both of these bacterial pathogens, Col-0 plants were exposed to P. rapae feeding for 24 h and subsequently challenge inoculated with X. campestris pv armoraciae or P. syringae pv tomato. Disease symptoms on P. rapae-damaged leaves were less severe than on nondamaged leaves on the same plants. Therefore, damaged (local) and nondamaged (systemic) leaves were assessed separately. Figure 3, A and B, shows that P. rapae feeding induced a significant level of resistance against both X. campestris pv armoraciae and P. syringae pv tomato in the P. rapae-damaged local leaves, but not in the undamaged systemic leaves.
Figure 3.
Herbivore-induced resistance against X. campestris pv armoraciae and P. syringae pv tomato. Herbivore-induced resistance was triggered in Col-0 plants by allowing P. rapae to feed on the leaves for 24 h. Immediately after removal of the caterpillars or 3 d later (=3 d after infestation [DAI]), plants were challenge inoculated with either X. campestris pv armoraciae or P. syringae pv tomato by dipping the leaves into a bacterial suspension containing 108 or 5 × 106 CFU mL−1, respectively. Three days after challenge inoculation, the percentage of diseased leaves per plant was determined and the disease index was calculated relative to challenged control plants (set at 100%). To discriminate between local (L) and systemic (S) effects, P. rapae-damaged and undamaged leaves on the same plants were scored separately. The values presented are means (±se) of 20 to 25 plants that received the same treatment. Different letters indicate statistically significant differences between treatments (Fisher's lsd test; α = 0.05). A, P. rapae-induced resistance against X. campestris pv armoraciae. B, P. rapae-induced resistance against P. syringae pv tomato. C, Effect of a 3-d interval between induction and challenge inoculation on P. rapae-induced resistance against P. syringae pv tomato. D, Effect of mechanical damage (MD) and MD in combination with P. rapae regurgitate (MD + R) on the level of resistance against P. syringae pv tomato. E, P. rapae-induced resistance against P. syringae pv tomato in Arabidopsis defense-signaling mutants. The absolute proportions of diseased leaves of uninduced control plants were 41% (A), 60% (B), 62% (C), 79% (D), and 72% (Col-0), 85% (sid2-1), 75% (eds5-1), 77% (npr1-1), 44% (coi1-16), 78% (jar1-1), and 78% (ein2-1; E).
Because P. rapae-induced plants were challenge inoculated with the bacterial pathogens immediately after removal of the caterpillars, the time between induction and expression of resistance may have been too short to mount an effective systemic effect. To clarify this point, P. rapae-induced plants were challenge inoculated with P. syringae pv tomato 3 d after removal of the caterpillars. P. rapae-damaged leaves mounted a significant level of local resistance against P. syringae pv tomato infection (Fig. 3C) that was also expressed as a reduction in bacterial growth in the leaves (data not shown). However, again resistance was not expressed systemically, even though the leaves had been allowed more time to mount a defense response. It can thus be concluded that P. rapae feeding enhances the level of resistance against both bacterial pathogens, but that this resistance is localized to the herbivore-damaged tissues and is not expressed systemically.
To investigate whether elicitors of herbivore-induced local resistance against P. syringae pv tomato are present in the regurgitate of P. rapae, we applied regurgitate onto the wounded sites of mechanically damaged leaves and assessed the level of induced protection against P. syringae pv tomato. Figure 3D shows that neither mechanical damage nor a combination treatment of mechanical damage and P. rapae regurgitate mimicked the resistance reaction that was induced upon caterpillar feeding.
To study the role of SA, JA, and ET in P. rapae-induced local resistance against P. syringae pv tomato, we tested different Arabidopsis genotypes that are affected in either SA (sid2-1, eds5-1, npr1-1), JA (coi1-16, jar1-1), or ET (ein2-1) signaling. Figure 3E shows that all genotypes tested were fully capable of expressing caterpillar-induced resistance against P. syringae pv tomato, resulting in a significant decrease in the proportion of leaves with disease symptoms in herbivore-induced leaves. To investigate whether this reduction in disease symptoms correlated with a reduction in bacterial proliferation, we monitored growth of the pathogen in control and P. rapae-induced leaves of Col-0, and one mutant of each of the three signaling pathways (eds5-1, jar1-1, and ein2-1). Herbivore-induced leaves showed a statistically significant reduction in bacterial titers in comparison to uninfested leaves in Col-0 (Student's t test; P = 0.008), eds5-1 (Student's t test; P = 0.007), jar1-1 (Student's t test; P = 0.008), and ein2-1 (Student's t test; P < 0.001). Together, these data indicate that the observed local resistance does not depend exclusively on SA, JA, or ET signaling.
Local and Systemic Effects of Herbivore-Induced Resistance against TCV
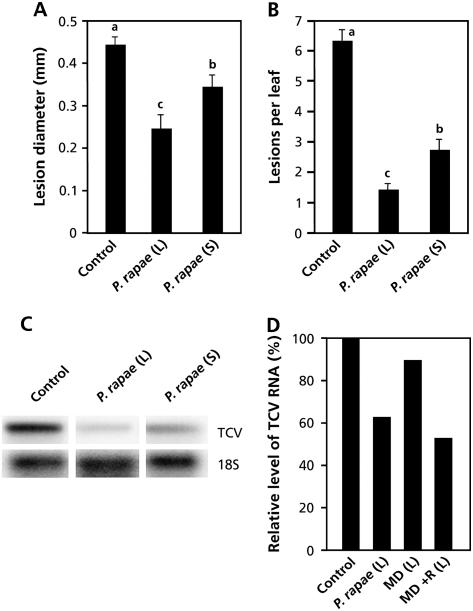
TCV is virulent on most Arabidopsis accessions, including Col-0 (Simon et al., 1992), but avirulent on accession Dijon (Di-0), which develops a hypersensitive response and does not allow systemic spreading of the pathogen (Simon et al., 1992; Dempsey et al., 1997). To investigate the effectiveness of P. rapae-induced resistance against TCV, Di-0 plants were exposed to P. rapae feeding for 24 h and subsequently challenge inoculated with TCV. Five days later, the level of induced protection was examined by determining lesion size and TCV RNA levels in control and P. rapae-induced plants. Caterpillar feeding resulted in a significant reduction in lesion size (Fig. 4A) and a strong reduction in the number of lesions per leaf (Fig. 4B). Moreover, TCV RNA accumulated to much lower levels in the P. rapae-induced plants than in controls (Fig. 4C). The effects on lesion development and TCV multiplication were apparent in herbivore-damaged and nondamaged leaves of herbivore-induced plants, indicating that P. rapae-induced resistance against TCV is effective both locally and systemically.
Figure 4.
Herbivore-induced resistance against TCV. Herbivore-induced resistance was triggered in Di-0 plants by allowing P. rapae to feed on the leaves for 24 h. Immediately after removal of the caterpillars, plants were challenge inoculated with TCV by rubbing 3-μL droplets of TCV RNA (0.1 μg μL−1) in bentonite buffer onto three P. rapae-damaged local (L) leaves, and three undamaged, systemic (S) leaves. Five days after challenge, average lesion size, average number of lesions per leaf, and TCV RNA levels were determined. Different letters indicate statistically significant differences between treatments (Fisher's lsd test; α = 0.05). A, Local and systemic effects of P. rapae-induced resistance on TCV lesion size. The values presented are means (±se) of all lesions measured on 15 plants that received the same treatment. B, Local and systemic effects of P. rapae-induced resistance on the number of lesions per leaf. The values presented are means (±se) from 15 plants that received the same treatment. C, Accumulation of TCV RNA 5 d after challenge inoculation of control and P. rapae-induced Di-0 plants. Blots were hybridized with a TCV-specific probe. Equal loading of RNA samples was checked using a probe for 18S rRNA. D, Effect of mechanical damage (MD) and MD in combination with P. rapae regurgitate (MD + R) on TCV RNA accumulation. Signal intensities of TCV RNA on the RNA blots were quantified using a phosphor imager, normalized for equal levels of 18S rRNA, and compared to the normalized TCV RNA levels in the uninduced control plants (set at 100%).
To investigate whether elicitors of P. rapae are involved in herbivore-induced resistance against TCV, we examined whether mechanical damage or a combination of mechanical damage and regurgitate treatment affects TCV RNA multiplication. Figure 4D shows that mechanical damage alone did not result in a reduction of TCV RNA levels. However, application of P. rapae regurgitate onto the wounded sites resulted in a reduction in TCV RNA levels similar to what was observed upon caterpillar feeding, indicating that factors in the regurgitate of P. rapae are responsible for herbivore-induced resistance against TCV.
P. rapae-Induced Resistance Is Associated with Priming for SA-Dependent Defense Responses
Previously, Kachroo et al. (2000) demonstrated that TCV resistance in Di-0 is dependent on SA, but not on JA and ET. Hence, TCV is sensitive to SA-dependent defenses, whereas JA/ET-dependent defense responses do not contribute to an enhanced level of resistance. Although feeding by P. rapae larvae does not trigger increased SA levels (De Vos et al., 2005), it did induce local and systemic resistance against TCV. This prompted us to investigate whether feeding by P. rapae primes the plant tissue for enhanced expression of SA-responsive genes following TCV infection. TCV infection induces PATHOGENESIS RELATED-1 (PR-1) gene expression in a SA-dependent manner (Kachroo et al., 2000). Therefore, we analyzed the expression of the SA-responsive PR-1 gene in control and SA-treated leaves of uninfested and P. rapae-infested Di-0 and Col-0 plants. Confirming previous findings in Col-0 (De Vos et al., 2005), P. rapae-infested Di-0 and Col-0 plants that were not treated with SA showed no increase in PR-1 transcript levels, indicating that herbivory by itself did not induce the SA-signaling pathway. In uninfested Di-0 and Col-0 plants, PR-1 transcripts accumulated within 24 h after SA treatment (Fig. 5). However, in P. rapae-infested plants of both accessions, increased levels of PR-1 mRNA were already detectable at 6 h after SA treatment and PR-1 transcript levels had accumulated further by 24 h. These results indicate that herbivore feeding primed the plant tissue for augmented expression of the SA-responsive PR-1 gene.
Figure 5.
P. rapae-induced priming of SA-induced PR-1 gene expression. P. rapae was allowed to feed on Di-0 and Col-0 plants for 24 h. After removal of the caterpillars, uninfested and P. rapae-infested plants were either treated with 1 mm SA or not treated. Six and 24 h later, the leaf tissue was harvested for RNA-blot analysis of PR-1 mRNA. Equal loading of RNA samples was checked by staining rRNA bands with ethidium bromide.
P. rapae feeding is associated with production of both ET and JA (De Vos et al., 2005). Both hormones have been demonstrated to modulate SA-dependent defense responses (Pieterse et al., 2001). To investigate whether ET or JA plays a role in priming P. rapae-induced tissue for augmented SA-dependent defense responses, we analyzed the effect of 1-aminocyclopropane-1-carboxylic acid (ACC) and MeJA on SA-induced expression of PR-1. Figure 6A shows the changes in PR-1 gene expression in Col-0 plants upon treatment with ACC, SA, or a combination of SA and increasing concentrations of ACC. Exogenous application of SA resulted in an 11-fold increase in PR-1 transcript levels, whereas ACC treatment had no effect. In the combination treatments, ACC enhanced the level of SA-induced PR-1 expression in a dose-dependent manner. This additive effect of ACC on SA-induced PR-1 expression was not apparent in the ET-insensitive mutant ein2-1 (Fig. 6A), indicating that ET primed the leaf tissue for enhanced expression of PR-1 by SA. Figure 6B shows a similar analysis of PR-1 gene expression upon treatment with MeJA, SA, or a combination of SA and MeJA. Alone, MeJA did not induce PR-1 gene expression. In the combination treatments, increasing concentrations of MeJA did not significantly affect SA-induced PR-1 mRNA levels, indicating that MeJA has neither an additive nor an antagonistic effect on this SA-induced defense response.
Figure 6.
Effect of ACC and MeJA on SA-induced expression of PR-1. Analysis of the SA-responsive PR-1 gene in wild-type Col-0 and mutant ein2-1 plants. Five-week-old plants were treated with 100 μm ACC, 100 μm MeJA, 1 mm SA, or a combination of 1 mm SA and increasing concentrations of either ACC or MeJA. Twenty-four hours after chemical treatment, the leaf tissue was harvested for RNA-blot analysis of PR-1 mRNA. To check for equal loading, RNA blots were stripped and hybridized with a probe for 18S rRNA. Signal intensities of PR-1 mRNA on the RNA blots were quantified using a phosphor imager, normalized for equal levels of 18S rRNA, and compared to the normalized PR-1 mRNA levels in the untreated control plants (control; set at 1). A, Effect of ACC on SA-induced PR-1 expression in Col-0 and ET-insensitive ein2-1 plants. B, Effect of MeJA on SA-induced PR-1 expression in wild-type Col-0 plants.
DISCUSSION
Little is known about how plants coordinate attacker-induced signals into specific defense responses. Previously, we studied the signal signature and the whole-genome expression profile of Arabidopsis upon attack by pathogens and insects with very different modes of action (De Vos et al., 2005). In four of the five Arabidopsis-attacker combinations tested, JA played an important role in the differential regulation of a large proportion of the attacker-activated/repressed genes (i.e. in the interactions of Arabidopsis with P. syringae pv tomato, A. brassicicola, P. rapae, and the western flower thrips F. occidentalis). Nevertheless, the vast majority of the JA-responsive changes were specific for each plant-attacker combination. Evidently, signal molecules such as JA play an important role in the primary response of the plant to pathogen and insect attack, but the final outcome of the resistance reaction is shaped by so far unidentified additional factors.
Herbivore-Induced Resistance against Microbial Pathogens
Feeding of P. rapae caterpillars on Arabidopsis is associated with enhanced production of both JA and ET, whereas the levels of SA remain unaltered (De Vos et al., 2005). Upon P. rapae feeding, Arabidopsis plants mount a defense response that is effective against subsequent infestation by the same herbivore (Fig. 1, A and B), confirming previous findings in other plant species (Kessler and Baldwin, 2002; Agrawal and Kurashige, 2003; Howe, 2005). Because of the dual role of JA in both pathogen and insect resistance, we investigated whether P. rapae feeding triggers cross-resistance against microbial pathogens. Our data show that herbivore-induced resistance in Arabidopsis is ineffective against the necrotrophic pathogen A. brassicicola (Fig. 2), locally effective against the bacterial pathogens X. campestris pv armoraciae and P. syringae pv tomato (Fig. 3), and locally and systemically effective against TCV (Fig. 4). Mechanical damage alone was ineffective, but in combination with P. rapae regurgitate the effectiveness of P. rapae-induced resistance could be mimicked in most cases.
A. brassicicola
Because A. brassicicola has been demonstrated to be sensitive to JA-dependent defense responses (Thomma et al., 1998; Ton et al., 2002), the lack of cross-resistance against this necrotrophic fungal pathogen was unexpected. Whole-genome expression profiling revealed that, although about 50% of all the P. rapae- or A. brassicicola-induced genes are regulated by JA, less than 10% of these JA-responsive gene sets overlap (De Vos et al., 2005). Hence, whereas JA may be an important primary signal in the defense response that is activated upon attack by either P. rapae or A. brassicicola, the final outcome of the resistance reaction is highly divergent and, in the case of P. rapae, feeding is only effective against the herbivore and not against the necrotrophic fungus. So how can this difference in effectiveness be explained? First, JA-mediated resistance against P. rapae and A. brassicicola may be conferred via different JA intermediates. Previously, different members of the oxylipin and jasmonate family have been shown to have different biological activities (Stintzi et al., 2001; Feussner and Wasternack, 2002; Farmer et al., 2003). Hence, the oxylipin signature of herbivore-induced plants may act positively on the level of resistance against further insect feeding, but for enhanced resistance against fungi, a different oxylipin signature may be required. Second, specific regulatory factors may be involved in shaping the JA-dependent defense response. Previously, Lorenzo et al. (2004) demonstrated that the transcription factors AtMYC2 and ERF1 antagonistically regulate differential sets of JA-responsive genes that are activated in response to herbivore and pathogen attack. They showed that AtMYC2 represses JA-responsive genes that are involved in defense against pathogens (e.g. PDF1.2), whereas ERF1 acts as a positive regulator in this respect. Expression-profiling studies indeed revealed that AtMYC2 is up-regulated upon feeding by P. rapae, whereas ERF1 is not (Reymond et al., 2004; De Vos et al., 2005). This supports the notion that AtMYC2 serves as an important regulator in discriminating between different JA-regulated defense responses.
X. campestris pv armoraciae and P. syringae pv tomato
P. rapae-induced resistance was effective against the bacterial pathogens X. campestris pv armoraciae and P. syringae pv tomato. However, enhanced resistance could only be observed in caterpillar-damaged tissue and not systemically in undamaged leaves of P. rapae-infested plants. Whereas application of P. rapae regurgitate onto mechanically damaged sites mimicked the herbivore-induced effect on P. rapae performance (Fig. 1), it had no effect on the level of resistance against P. syringae pv tomato (Fig. 3D). Hence, herbivore-induced defense responses seem to branch into at least two distinct types of resistance: one that affects P. rapae performance and another that is effective against the bacterial pathogens. This is supported by the fact that P. rapae performance is affected in the JA-insensitive coi1 mutant (Reymond et al., 2004), whereas the P. rapae-induced resistance against P. syringae pv tomato is still functional in this mutant (Fig. 3E). Another JA response mutant, jar1-1, as well as the SA- and ET-signaling mutants sid2-1, eds5-1, npr1-1, and ein2-1, mounted wild-type levels of resistance against P. syringae pv tomato in herbivore-induced leaves, suggesting that this type of induced resistance does not depend exclusively on one of these three regulators. SA, JA, and ET have all been implicated in the regulation of induced resistance against P. syringae pv tomato (Pieterse et al., 1998; Ellis et al., 2002; Ton et al., 2002; Glazebrook et al., 2003). Hence, the effectiveness of P. rapae-induced resistance against this pathogen in the signaling mutants is not unexpected. Previously, Stout et al. (1999) showed that damage caused by the corn earworm Helicoverpa zea induced resistance in tomato against P. syringae pv tomato, suggesting that herbivore-induced resistance against this bacterial pathogen is effective in different plant species. Interestingly, in the same study, P. syringae infection was shown to induce resistance against feeding by the corn earworm. In Arabidopsis, it was shown that P. syringae infection triggers resistance against the herbivore cabbage looper (Trichoplusia ni; Cui et al., 2002, 2005), indicating that induced resistance triggered by P. syringae and certain herbivorous insects is reciprocally effective.
TCV
Prior infestation with P. rapae inhibited multiplication of TCV and significantly reduced the size and number of TCV lesions (Fig. 4). The effect of P. rapae-induced resistance against TCV was apparent not only locally in herbivore-damaged tissue, but also systemically in undamaged leaves of infested plants. The inhibition of TCV multiplication could be mimicked by application of P. rapae regurgitate, suggesting that elicitors in the regurgitate of P. rapae are responsible for the activation of this systemic defense response. Resistance against this biotrophic pathogen is regulated predominantly by SA (Kachroo et al., 2000; Ton et al., 2002). P. rapae feeding is not accompanied by changes in SA levels either in Col-0 (De Vos et al., 2005) or in Di-0 (data not shown). In this study, we demonstrated that P. rapae feeding primes the plant tissue for augmented, SA-inducible gene expression in both Col-0 and Di-0 (Fig. 5). Moreover, we show that ET acts synergistically on the level of SA-induced PR-1 gene expression, confirming previous findings (Lawton et al., 1994), whereas MeJA does not (Fig. 6). Hence, the increased production of ET that occurs in both Col-0 (De Vos et al., 2005) and Di-0 (data not shown) upon herbivore feeding can sensitize the tissue to respond faster or stronger to SA and may thereby contribute to the enhanced resistance against TCV. In this scenario, herbivore-induced ET primes the leaf tissue for augmented SA-dependent defenses, thereby providing an enhanced defensive capacity toward pathogens, such as TCV, that trigger SA-dependent defense responses upon infection. Priming for augmented expression of pathogen-induced defense responses is implicated in different types of chemically and microbially induced resistance (Zimmerli et al., 2000; Conrath et al., 2002; Newman et al., 2002; Pozo et al., 2002; Verhagen et al., 2004; Ton et al., 2005; Van Hulten et al., 2006). Here, we show that herbivore feeding induces a similar alarmed state leading to cross-resistance against a viral pathogen. Future research will be focused on elucidating the role of ET in this herbivore-induced priming phenomenon.
Mechanical Damage and P. rapae Regurgitate
In this study, we demonstrated that herbivore-induced resistance against P. rapae feeding and TCV could not be mimicked by mechanical damage alone. However, application of regurgitate of P. rapae to the wounded sites resulted in similar levels of resistance as did prior infestation with P. rapae (Figs. 1 and 4D). Puncturing leaves or scratching the leaf surface is a common way to imitate feeding by herbivores. However, mechanically damaging leaf tissue only partially mimics the response of plants to herbivore feeding. For instance, artificially wounded leaf tissues do not produce the same blends of volatiles as do leaf tissues that have been injured by grazing herbivores (Mattiacci et al., 1995; Van Poecke and Dicke, 2002). Using a mechanical caterpillar named MecWorm, Mithöfer et al. (2005) demonstrated that computerized continuous damage resembles the insect's feeding process much better, leading to the production of a volatile blend that is more similar than wounding at a single time point. Evidently, the dynamics of wounding inflicted by grazing herbivores influence the nature of the induced plant defense response to a large extent.
Other factors that influence the wound response upon insect feeding are elicitors that are released by the herbivore during feeding. Application of gut regurgitate from feeding herbivores to mechanically damaged sites has been demonstrated to mimic specific herbivore-induced defense responses. For instance, cabbage (Brassica capitata) leaves that are artificially damaged and subsequently treated with gut regurgitate of P. brassicae caterpillars release a volatile blend similar to that of herbivore-damaged plants, leading to the attraction of parasitic wasps that attack the herbivores (Mattiacci et al., 1995). Insect-derived compounds, such as the enzymes Glc oxidase and β-glucosidase, and fatty acid-amino acid conjugates such as volicitin, have been identified as potent elicitors of volatile production in different plant-herbivore interactions (Mattiacci et al., 1995; Turlings et al., 2000; Halitschke et al., 2001; Musser et al., 2002). Besides insect-derived elicitors, caterpillar regurgitate also contains high levels of plant-derived molecules, including JA, the jasmonate precursor 12-oxo-phytodienoic acid, and dinor oxo-phytodienoic acid (Reymond et al., 2004), which have been shown to play a critical role in herbivore resistance in Arabidopsis (Stintzi et al., 2001).
Our study of the spectrum of effectiveness of P. rapae-induced resistance demonstrates that components of the caterpillar regurgitate play an important role in the activation of resistance against the insect itself and against TCV. However, the nature of the elicitors involved remains to be elucidated. Neither application of P. rapae regurgitate onto artificially damaged leaves nor wounding alone induced resistance against the bacterial pathogens X. campestris pv armoraciae and P. syringae pv tomato (Fig. 3D). Hence, elicitors in the regurgitate of P. rapae are not involved in the defense response against these pathogens.
Spectrum of Effectiveness of Induced Resistance
Previously, we demonstrated that pathogen-induced SAR is effective against pathogens that in noninduced plants are resisted through SA-dependent defenses, whereas rhizobacteria-mediated ISR is effective against pathogens that in noninduced plants are resisted through JA/ET-dependent defenses (Ton et al., 2002; H. Van Pelt and C.M.J. Pieterse, unpublished data). This suggests that SAR and ISR constitute a reinforcement of extant SA- or JA/ET-dependent basal defense responses, respectively. Here, we showed that herbivore feeding induces cross-resistance against several microbial pathogens. However, the observed spectrum of effectiveness was clearly different from that predicted on the basis of the known effectiveness of JA and ET that are produced upon feeding by P. rapae. We expected enhanced resistance against the necrotrophic fungus A. brassicicola because this pathogen has been shown to be sensitive to JA-dependent defenses. On the other hand, we expected no effect on the level of resistance against the biotrophic pathogen TCV because resistance against this pathogen has been demonstrated to be regulated by SA, which is not produced during feeding by P. rapae. Both expectations appeared to be false because other regulating factors influenced the outcome of the defense response. We confirmed that ET acts synergistically on SA-inducible defenses, suggesting that herbivore-induced ET production may be involved in the observed enhanced resistance against TCV.
Evolution provided plants with sophisticated defensive strategies to perceive attack by microbial pathogens and herbivorous insects and to translate that perception into an appropriate defense response. Our study demonstrates that the defense response that is triggered upon insect feeding is surprisingly complex. Synergistic and antagonistic effects of cross talk between and within SA-, JA-, and ET-dependent signaling pathways play a role in determining the final outcome of the resistance reaction (Bostock, 1999; Spoel et al., 2003; Mur et al., 2006). Understanding the complexity of the coordinated cellular responses involved in this process is a major challenge for future research.
MATERIALS AND METHODS
Cultivation of Plants
Seeds of Arabidopsis (Arabidopsis thaliana) accessions Col-0, Di-0, and the Col-0 mutants pad3-1 (Glazebrook and Ausubel, 1994), jar1-1 (Staswick et al., 1992), coi1-16 (Ellis et al., 2002), ein2-1 (Guzmán and Ecker, 1990), sid2-1 (Nawrath and Métraux, 1999; Wildermuth et al., 2001), eds5-1 (Rogers and Ausubel, 1997; Nawrath et al., 2002), and npr1-1 (Cao et al., 1994) were sown in quartz sand. Two-week-old seedlings were transferred to 60-mL pots containing a sand/potting soil mixture that was autoclaved twice for 20 min. Plants were cultivated in a growth chamber with an 8-h d (200 μE m−2 s−1 at 24°C) and 16-h night (20°C) cycle at 70% relative humidity for another 3 weeks. Plants were watered every other day and received one-half-strength Hoagland nutrient solution (Hoagland and Arnon, 1938) containing 10 μm Sequestreen (CIBA-Geigy) once a week.
Herbivore Induction, Wounding, and Regurgitate Treatment
Tissue-chewing larvae of the small cabbage white butterfly Pieris rapae were reared on brussels sprout plants (Brassica oleracea gemmifera cv Cyrus) in a growth chamber with a 16-h d/8-h night cycle (21°C; 50%–70% relative humidity) as described previously (Van Poecke et al., 2001; De Vos et al., 2005). To trigger herbivore-induced resistance, 5-week-old Arabidopsis plants were infested by transferring three to five freshly hatched first-instar larvae (L1) onto each plant. The larvae were allowed to feed for 24 h, after which they were removed. During the feeding period, most of the larvae remained on the leaf to which they had been transferred.
The effect of wounding was assessed by mechanically damaging the leaf tissue. Three small holes (1-mm diameter) were punctured in each of five leaves per plant using a sterile needle. To study the effect of P. rapae regurgitate, 1 μL of freshly collected regurgitate of P. rapae was divided over the three punctured holes of each mechanically damaged leaf. Regurgitate was collected from L4 to L5 larvae that were allowed to feed on uninduced Col-0 plants as described (Mattiacci et al., 1995). Twenty-four hours after the start of the induction treatments, noninduced and induced plants were challenged with P. rapae or one of the microbial pathogens.
P. rapae Assays
To study the effect of herbivore feeding and wounding on P. rapae performance, a single freshly hatched first-instar larva was transferred to each of 20 noninduced or induced Col-0 plants. At 4, 7, and 10 d, the fresh weights of the larvae were determined. After 10 d, the first larvae started to pupate. Therefore, fresh weight was determined only up to 10 d of feeding. To examine effects on caterpillar development, the percentage of caterpillars that had pupated within 14 d after hatching was determined.
Alternaria brassicicola Bioassays
Bioassays with the fungal pathogen Alternaria brassicicola MUCL 20297 were performed essentially as described by Ton et al. (2002). Briefly, A. brassicicola was grown on potato (Solanum tuberosum) dextrose agar plates for 2 weeks at 22°C. Conidia were harvested, as described by Broekaert et al. (1990). Five-week-old pad3-1 mutant plants (n = 15) on which P. rapae had been allowed to feed for 24 h were challenge inoculated with A. brassicicola by applying 3-μL droplets of 10 mm MgSO4, containing 5 × 105 spores mL−1, onto three P. rapae-damaged leaves. As a negative control, leaves from untreated plants were inoculated in a similar manner. As a positive control, pad3-1 plants were pretreated with MeJA by dipping the leaves into a solution containing 0.1 mm MeJA (Serva, Brunschwig Chemie B.V.) 24 h before challenge inoculation. Inoculated plants were kept at 100% relative humidity. At 6 d after challenge, disease severity was determined. Disease ratings were expressed on the basis of intensity of symptoms and lesion size: I, no visible disease symptoms; II, nonspreading lesion; III, spreading lesion without chlorosis; IV, spreading lesion surrounded by chlorotic halo; and V, spreading lesion with extensive tissue maceration and sporulation of the pathogen.
Xanthomonas campestris pv armoraciae and Pseudomonas syringae pv tomato Bioassays
Bioassays with the bacterial pathogens Xanthomonas campestris pv armoraciae and Pseudomonas syringae pv tomato DC3000 were performed as described by Ton et al. (2002) and Pieterse et al. (1998). Briefly, rifampicin-resistant X. campestris pv armoraciae and P. syringae pv tomato DC3000 were cultured at 28°C in liquid 0.8% nutrient broth medium (Difco) and King's B medium (King et al., 1954), respectively. After overnight incubation, bacterial cells were collected by centrifugation and resuspended in 10 mm MgSO4 containing 0.015% (v/v) Silwet L-77 to a final density of 108 and 5 × 106 colony-forming units (CFU) mL−1, respectively. Five-week-old Arabidopsis plants on which P. rapae had been allowed to feed for 24 h were challenge inoculated by dipping the leaves into the bacterial suspension. Challenge inoculations were performed immediately after removal of the caterpillars or 3 d later. Three days after challenge inoculation, the percentage of leaves with symptoms was determined per plant (n = 20–25). Leaves showing necrotic or water-soaked lesions surrounded by chlorosis were scored as diseased. For each plant, caterpillar-damaged leaves (local effects) and undamaged leaves (systemic effects) were scored separately. Mechanically damaged and regurgitate-treated plants were challenged in a similar manner.
Growth of X. campestris pv armoraciae and P. syringae pv tomato was determined by collecting replicate leaf samples from 10 pools of three plants per treatment immediately after challenge inoculation and 3 d later. Leaf samples were weighed, rinsed in water, and homogenized in 10 mm MgSO4. Subsequently, dilutions were plated on selective nutrient broth or King's B medium supplemented with 100 mg L−1 cycloheximide and 50 mg L−1 rifampicin. After incubation at 28°C for 2 d, the number of rifampicin-resistant CFU g−1 of infected leaf tissue was determined, and bacterial growth over the 3-d time interval was calculated.
TCV Bioassays
Bioassays with TCV were performed as described previously (Ton et al., 2002). TCV inoculum was produced by in vitro transcription from plasmid pT7TCV66 (Oh et al., 1995) and adjusted to a concentration of 0.1 μg RNA μL−1. Five-week-old Arabidopsis Di-0 plants (n = 15) were challenge inoculated by applying 3-μL droplets of TCV RNA (0.1 μg μL−1) in bentonite buffer (0.05 m Gly, 0.03 m K2HPO4, 0.02 g bentonite mL−1) on three damaged and three undamaged leaves per plant. On mock-induced plants, six undamaged leaves were inoculated with TCV. Droplets were rubbed across the leaf surface with a glass rod and the inoculated leaves were marked. Five days after challenge, the number and diameter of the lesions were determined under a dissection microscope and viral RNA accumulation was assessed by RNA-blot analysis, as described below.
Chemical Treatments
Treatments with SA, MeJA, and the ET precursor ACC were performed by dipping the leaves into a solution of 0.015% (v/v) Silwet L77, containing either 1 mm SA, 0.1 mm MeJA, 0.1 mm ACC, or a combination of 1 mm SA and MeJA (0.01, 0.1, or 1 mm) or ACC (0.001, 0.01, or 0.1 mm). Control treatments were dipped into a solution containing 0.015% (v/v) Silwet L77.
RNA Extraction and RNA-Blot Analysis
Total RNA was extracted as described previously (Van Wees et al., 2000). For RNA-blot analysis, 10 μg of RNA were denatured using glyoxal and dimethyl sulfoxide (Sambrook et al., 1989), electrophoretically separated on 1.5% agarose gel, and blotted onto Hybond-N+ membranes (Amersham) by capillary transfer. The electrophoresis and blotting buffer consisted of 10 and 25 mm sodium phosphate (pH 7.0), respectively. RNA blots were hybridized with a gene-specific probe for PR-1, as described previously (Van Wees et al., 2000). To check for equal loading, rRNA bands were stained with ethidium bromide or the blots were stripped and hybridized with a probe for either 18S ribosomal RNA or β-tubulin (TUB). The Arabidopsis Genome Initiative numbers for the genes studied are At2g14610 (PR-1) and At5g44340 (TUB). Probes for 18S rRNA and TCV were derived from cDNA clones as described (Ton et al., 2002; Verhagen et al., 2004).
Acknowledgments
We gratefully acknowledge Adriaan Verhage and Hans van Pelt for technical assistance and Leo Koopman, Frans van Aggelen, and André Gidding for insect rearing.
This work was supported in part by the Earth and Life Sciences Foundation (grant nos. 865–04–002 and 813–06–002), which is subsidized by the Netherlands Organization of Scientific Research.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Corné M.J. Pieterse (c.m.j.pieterse@bio.uu.nl).
References
- Agrawal AA, Kurashige NS (2003) A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J Chem Ecol 29: 1403–1415 [DOI] [PubMed] [Google Scholar]
- Bostock RM (1999) Signal conflicts and synergies in induced resistance to multiple attackers. Physiol Mol Plant Pathol 55: 99–109 [Google Scholar]
- Bostock RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43: 545–580 [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Terras FRG, Cammue BPA, Vanderleyden J (1990) An automated quantitative assay for fungal growth. FEMS Microbiol Lett 69: 55–60 [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant-pathogen interactions. Trends Plant Sci 7: 210–216 [DOI] [PubMed] [Google Scholar]
- Cui J, Bahrami AK, Pringle EG, Hernandez-Guzman G, Bender CL, Pierce NE, Ausubel FM (2005) Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci USA 102: 1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Jander G, Racki LR, Kim PD, Pierce NE, Ausubel FM (2002) Signals involved in Arabidopsis resistance to Trichoplusia ni caterpillars induced by virulent and avirulent strains of the phytopathogen Pseudomonas syringae. Plant Physiol 129: 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, Van Loon LC, Dicke M, et al (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18: 923–937 [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Pathirana MS, Wobbe KK, Klessig DF (1997) Identification of an Arabidopsis locus required for resistance to turnip crinkle virus. Plant J 11: 301–311 [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis L, Turner JG (2002) Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol Plant Microbe Interact 15: 1025–1030 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Almeras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6: 372–378 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton GW, Korth KL (2000) Trade-offs between pathogen and herbivore resistance. Curr Opin Plant Biol 3: 309–314 [DOI] [PubMed] [Google Scholar]
- Feussner I, Wasternack C (2002) The lipoxygenase pathway. Annu Rev Plant Biol 53: 275–297 [DOI] [PubMed] [Google Scholar]
- Feys BJ, Parker JE (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet 16: 449–455 [DOI] [PubMed] [Google Scholar]
- Glazebrook J (2001) Genes controlling expression of defense responses in Arabidopsis—2001 status. Curr Opin Plant Biol 4: 301–308 [DOI] [PubMed] [Google Scholar]
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Ausubel FM (1994) Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA 91: 8955–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Chen W, Estes B, Chang H-S, Nawrath C, Métraux J-P, Zhu T, Katagiri F (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J 34: 217–228 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125: 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1938) The water culture method for growing plants without soil. Calif Agric Exp Stn Bull 347: 36–39 [Google Scholar]
- Howe GA (2005) Jasmonates as signals in the wound response. J Plant Growth Regul 23: 223–237 [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo P, Yoshioka K, Shah J, Dooner KD, Klessig DF (2000) Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299–328 [DOI] [PubMed] [Google Scholar]
- King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of phycocyanin and fluorescin. J Lab Clin Med 44: 301–307 [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5: 325–331 [DOI] [PubMed] [Google Scholar]
- Lawton KA, Potter SL, Uknes S, Ryals J (1994) Acquired resistance signal transduction in Arabidopsis is ethylene independent. Plant Cell 6: 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Williams MM, Loh YT, Lee GI, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130: 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16: 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15: 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA (1995) β-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA 92: 2036–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn J, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94: 5473–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer A, Wanner G, Boland W (2005) Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol 137: 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Herbivory: caterpillar saliva beats plant defences—a new weapon emerges in the evolutionary arms race between plants and herbivores. Nature 416: 599–600 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Métraux J-P (2002) EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Métraux J-P (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MA, von Roepenack-Lahaye E, Parr A, Daniels MJ, Dow JM (2002) Prior exposure to lipopolysaccharide potentiates expression of plant defenses in response to bacteria. Plant J 29: 487–495 [DOI] [PubMed] [Google Scholar]
- Oh J-W, Kong Q, Song C, Carpenter C, Simon AE (1995) Open reading frames of turnip crinkle virus involved in satellite symptom expression and incompatibility with Arabidopsis thaliana ecotype Dijon. Mol Plant Microbe Interact 6: 979–987 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Ton J, Van Loon LC (2001) Cross-talk between plant defence signalling pathways: boost or burden? AgBiotechNet 3: ABN 068
- Pieterse CMJ, Van Loon LC (1999) Salicylic acid-independent plant defence pathways. Trends Plant Sci 4: 52–58 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, Van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo MJ, Cordier C, Dumas-Gaudot E, Gianinazzi S, Barea JM, Azcón-Aguilar C (2002) Localized vs systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J Exp Bot 53: 525–534 [DOI] [PubMed] [Google Scholar]
- Pozo MJ, Van Loon LC, Pieterse CMJ (2005) Jasmonates—signals in plant-microbe interactions. J Plant Growth Regul 23: 211–222 [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE (2004) A conserved transcriptional pattern in response to a specialist and a generalist herbivore. Plant Cell 16: 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1: 404–411 [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Ausubel FM (1997) Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell 9: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989). Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Simon AE, Li XH, Lew JE, Stange R, Zhang C, Polacco M, Carpenter CD (1992) Susceptibility and resistance of Arabidopsis thaliana to turnip crinkle virus. Mol Plant Microbe Interact 5: 496–503 [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux J-P, Brown R, Kazan K, et al (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89: 6837–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz HU, Koch T, Biedermann A, Weniger K, Boland W, Mitchell-Olds T (2002) Evidence for regulation of resistance in Arabidopsis to Egyptian cotton worm by salicylic and jasmonic acid signaling pathways. Planta 214: 648–652 [DOI] [PubMed] [Google Scholar]
- Stout MJ, Fidantsef AL, Duffey SS, Bostock RM (1999) Signal interactions in pathogen and insect attack: systemic plant-mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol 54: 115–130 [Google Scholar]
- Thaler JS, Farag MA, Pare PW, Dicke M (2002. a) Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol Lett 5: 764–774 [Google Scholar]
- Thaler JS, Karban R, Ullman DE, Boege K, Bostock RM (2002. b) Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia 131: 227–235 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Owen B, Higgins VJ (2004) The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol 135: 530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Nelissen I, Eggermont K, Broekaert WF (1999) Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J 19: 163–171 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Penninckx IAMA, Broekaert WF, Cammue BPA (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13: 63–68 [DOI] [PubMed] [Google Scholar]
- Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, Maeder MN, Métraux J-P, Mauch-Mani B (2005) Dissecting the β-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant Cell 17: 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Van Pelt JA, Van Loon LC, Pieterse CMJ (2002) Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis. Mol Plant Microbe Interact 15: 27–34 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH (2000) Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: isolation and bioactivity. J Chem Ecol 26: 189–202 [Google Scholar]
- Van Hulten M, Pelser M, Van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103: 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon JJA, De Boer JG, Dicke M (2000) Parasitoid-plant mutualism: parasitoid attack of herbivore increases plant reproduction. Entomol Exp Appl 97: 219–227 [Google Scholar]
- Van Loon LC (2000) Systemic induced resistance. In AJ Slusarenko, RSS Fraser, LC Van Loon, eds, Mechanisms of Resistance to Plant Diseases. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 521–574
- Van Poecke RMP, Dicke M (2002) Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. J Exp Bot 53: 1793–1799 [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Dicke M (2004) Indirect defence of plants against herbivores: using Arabidopsis thaliana as a model plant. Plant Biol 6: 387–401 [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Posthumus MA, Dicke M (2001) Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J Chem Ecol 27: 1911–1928 [DOI] [PubMed] [Google Scholar]
- Van Wees SCM, Chang H-S, Zhu T, Glazebrook J (2003) Characterization of the early response of Arabidopsis to Alternaria brassicicola infection using expression profiling. Plant Physiol 132: 606–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wees SCM, De Swart EAM, Van Pelt JA, Van Loon LC, Pieterse CMJ (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA 97: 8711–8716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen BWM, Glazebrook J, Zhu T, Chang H-S, Van Loon LC, Pieterse CMJ (2004) The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant Microbe Interact 17: 895–908 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Jakab G, Métraux J-P, Mauch-Mani B (2000) Potentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acid. Proc Natl Acad Sci USA 97: 12920–12925 [DOI] [PMC free article] [PubMed] [Google Scholar]