Abstract
The Arabidopsis (Arabidopsis thaliana) GSTF8 gene is a member of the glutathione S-transferase (GST) family whose expression is induced by defense signals, certain chemical stresses, and some pathogens. Here, we have used transgenic plants and an in vivo imaging system to demonstrate that GSTF8 expression is subject to a distinct desensitization phenomenon because prior chemical treatment significantly reduces reactivation of the GSTF8 promoter by hydrogen peroxide, auxin, and salicylic acid. A GSTF8 null line had similar desensitization properties to wild type, demonstrating that GSTF8 protein levels are not responsible for desensitization. The resulting refractory period is unusually long lasting, with full recovery taking 4 d. Expression of the GSTF8 promoter following a second treatment occurred predominantly in newly formed tissue at the root tip, suggesting that desensitization is lost upon cell division. Expression of the endogenous GSTF8 gene and another GST gene, GSTF6, is also desensitized following treatment with hydrogen peroxide. The desensitization phenomenon can be activated by a very low concentration of inducer that is not sufficient to activate the GSTF8 promoter. These results demonstrate that activation of the GSTF8 promoter is not essential for eliciting desensitization. A key promoter sequence within the GSTF8 gene, the ocs element, is also affected by desensitization. Treatment with a phosphatase inhibitor prevents desensitization of GSTF8 expression and ocs element activity, suggesting that dephosphorylation of one or more proteins is required for desensitization to occur.
Glutathione S-transferases (GSTs) are a diverse group of multifunctional proteins that catalyze the conjugation of glutathione to a range of electrophilic substrates. In plants, GSTs appear to play critical roles in the protection of tissues against oxidative damage as well as other roles, for example, in flavanoid synthesis (Marrs, 1996; Edwards et al., 2000; Frova, 2003). The regulation of expression of the Arabidopsis (Arabidopsis thaliana) GSTF8 gene, previously called GST6, has been well studied. GSTF8 promoter activity, RNA expression, and protein levels are under tissue-specific control and induced following treatment with auxin, salicylic acid (SA), hydrogen peroxide (H2O2), and by specific strains of the necrotropic fungus, Rhizoctonia solani (Chen and Singh, 1999; Perl-Treves et al., 2004; Sappl et al., 2004; Uquillas et al., 2004). The ocs element, a well-characterized promoter element (Bouchez et al., 1989; Ulmasov et al., 1994) within the GSTF8 promoter is responsible for part, but not all, of the induction by SA and H2O2 (Chen and Singh, 1999). In the case of SA, GSTF8 is considered to be an SA early-responsive gene whose expression is independent of NPR1, a key regulator of some SA-mediated responses, including pathogenesis-related gene expression (Uquillas et al., 2004).
Whereas considerable research has been directed at understanding how gene expression is regulated in plants in response to various stimuli, less work has been undertaken to understand what happens after gene induction has peaked. A number of signal transduction pathways in animals are regulated by desensitization, a process by which components in the pathway, in many cases the receptor, become refractory to prolonged or repeated exposure to a signal (for review, see Oppermann, 2004; Gainetdinov et al., 2004). Desensitization can be the result of repeated treatments with the same signal (homologous desensitization) or by different signals (heterologous or cross desensitization).
Desensitization has also been observed in plants, for example, with cell cultures in response to light, chitin, and other elicitors (Bowler et al., 1994; Felix et al., 1998; Chandra et al., 2000). However, there have been technical limitations to these studies, particularly in whole plants and when studying the effects of desensitization on gene expression, because it has been hard to study both gene induction and postgene induction phases in the same plant. The use of in vivo imaging systems that permit noninvasive approaches can help circumvent these problems and allow detailed temporal and spatial studies to be performed on individual plants during all phases of gene induction. In this article, we have used such an approach with transgenic Arabidopsis plants containing different GSTF8 promoter constructs fused to the luciferase (LUC) reporter gene. Using this system, we have uncovered additional complexity in the regulation of GSTF8 expression. We demonstrate that the GSTF8 promoter is subject to a distinct desensitization phenomenon with novel properties, which significantly reduces reactivation of GSTF8 expression following treatment with an initial stimulus. Furthermore, we present evidence that activation and desensitization of the GSTF8 promoter are not directly linked, that the GSTF8 protein is not involved, and that dephosphorylation of one or more proteins is required for desensitization.
RESULTS
GSTF8 Expression Is Desensitized following H2O2 Treatment and Is Regulated Spatially in Roots
We were interested in analyzing whether there was a difference in the kinetics of the response of the GSTF8 promoter to SA and H2O2, which may help elucidate whether these plant defense signals were working through similar or distinct mechanisms to induce GSTF8 expression. For these experiments, we used transgenic Arabidopsis plants containing a 792-bp GSTF8 promoter linked to the LUC reporter gene (GSTF8∷LUC) and a noninvasive imaging system, as previously reported (Perl-Treves et al., 2004), to perform detailed time course experiments. As shown in Figure 1A, we observed differences both in the magnitude and the induction kinetics of the GSTF8 promoter in response to 1 mm SA versus 1 mm H2O2.
Figure 1.
Response of the GSTF8 promoter to SA and H2O2 treatments. A, Four-day-old GSTF8∷LUC seedlings were treated with 1 mm H2O2 or 1 mm SA for 40 min and the bioluminescence over a 19-h time course was measured using a CCD camera. The average light units per seedling for each treatment for each hour, with ses, are presented. B, Four-day-old GSTF8∷LUC seedlings were treated at time 0 and then again at 13 h with 1 mm H2O2 for 40 min and then decanted. The average relative light units per GSTF8∷LUC seedling with ses is presented for each hour over a 19-h time course. C, In vivo imaging of a representative, 4-d-old GSTF8∷LUC seedling treated with 1 mm H2O2 at time 0 and 13 h and visualized using a CCD camera. The white signal comes from the cotyledons and represents the transient chlorophyll fluorescence, which was imaged prior to measuring the bioluminescence. Bioluminescence was monitored after 10 min in the dark and is imaged in blue. Bioluminescence images were captured at 0, 3, 6, and 13 h after the first treatment and 3 and 6 h after the second treatment. The fluorescence and bioluminescence images were superimposed in the figure. The left- and right-most sections are black and white photographs of the same seedling taken before the first and second H2O2 treatment, respectively.
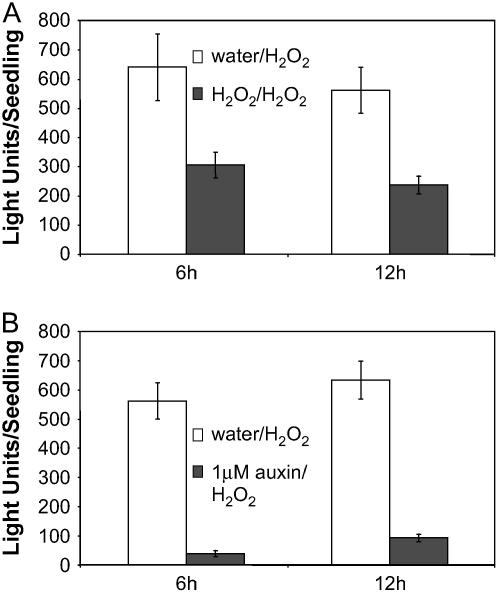
Because the response of the GSTF8 promoter to H2O2 peaked earlier than the SA response, we were interested in analyzing whether this was because H2O2 was more labile than SA. If this was the case, one may expect that the induction would be restored by a second treatment of H2O2. However, as shown in Figure 1B, we observed that a second H2O2 treatment, administered after the expression following the first treatment, had returned close to basal levels, resulting in a much smaller induction of the GSTF8 promoter compared to the first treatment. The graph in Figure 1B shows the relative light units averaged from 22 4-d-old seedlings after treatment with 1 mm H2O2 at 0 h and a second treatment at 13 h. The second induction peaks at 4 h after the second treatment and is only about 30% as strong as the induction seen with the first treatment. These results bear analogy to the refractory phenomenon described for some signaling pathways in plants and animals, where transient desensitization of the pathway occurs following an initial stimulus (e.g. Bowler et al., 1994; Felix et al., 1998; Oppermann, 2004). We cannot rule out a reduction in H2O2 concentration after the first treatment. If so, this may contribute to GSTF8 expression returning to basal levels by 13 h after the first chemical treatment. However, what these experiments demonstrate is that the expression of the GSTF8 promoter and the endogenous gene are subject to desensitization following treatment with H2O2 because restimulation after the expression levels from an initial induction had returned to a basal level, resulted in a much smaller induction than the initial treatment.
We also found that desensitization of the GSTF8 promoter caused by H2O2 also reduced the response to other stimuli such as 1 mm SA and 50 μm of the synthetic auxin, 2,4-dichlorophenoxyacetic acid (2,4-D; data not shown). To rule out the possibility that LUC activity itself was being affected by treatment with any of these stimuli, we used plants expressing the cauliflower mosaic virus 35S promoter fused to LUC and found that LUC activity was not affected by any of these stimuli (see Supplemental Fig. 1).
We used the in vivo imaging system to examine the temporal expression patterns of the GSTF8 promoter in young seedlings in response to consecutive H2O2 treatments and these results are shown in Figure 1C. For both the first and second H2O2 treatments, expression is predominantly in the root. Interestingly, the second H2O2 treatment only resulted in GSTF8 expression in newly formed root tissue at the root tip or in regions of the root where GSTF8 expression was no longer visible by the end of the first H2O2 treatment, as observed for the middle of the root at the 3- and 6-h time points after the second treatment.
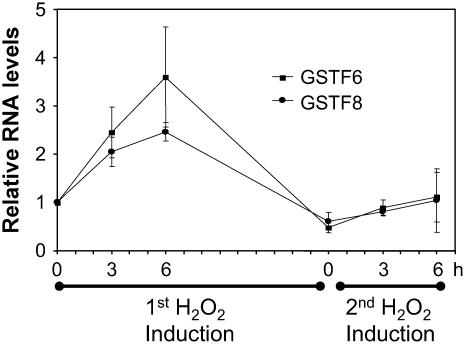
To determine whether the desensitization phenomenon observed with the GSTF8 promoter also occurred for the native GSTF8 gene, we looked at GSTF8 RNA levels in 8-d-old seedlings following consecutive H2O2 treatments. Figure 2 shows the results of reverse transcription (RT)-PCR performed with specific primers for GSTF8 and a second Arabidopsis GST gene called GSTF6, originally called GST1, also linked to defense responses (Grant et al., 2000). β-Tubulin RNA levels were used as a control to normalize the RT-PCR results. RNA levels for both GSTF6 and GSTF8 increased after treatment with 1 mm H2O2 and were at their highest levels at the 6-h time point. After 13 h, they were back to basal levels. A second H2O2 treatment at 13 h resulted in only a small induction of GSTF6 and GSTF8 expression, demonstrating that the regulation of these GST genes by H2O2 also involves desensitization.
Figure 2.
Expression of the endogenous GSTF8 gene is also regulated by desensitization. Eight-day-old GSTF8∷LUC seedlings were treated with 1 mm H2O2 and RNA was extracted at 0, 3, and 6 h after the first H2O2 treatment and 0, 3, and 6 h after the second H2O2 treatment. RT-PCR was performed using GSTF6, GSTF8, and β-tubulin-specific primers and the results for GSTF6 and GSTF8 were standardized to the β-tubulin control. The average and se of two biological repeats are graphed.
Feedback Regulation Is Not Involved in Desensitization of GSTF8 Expression
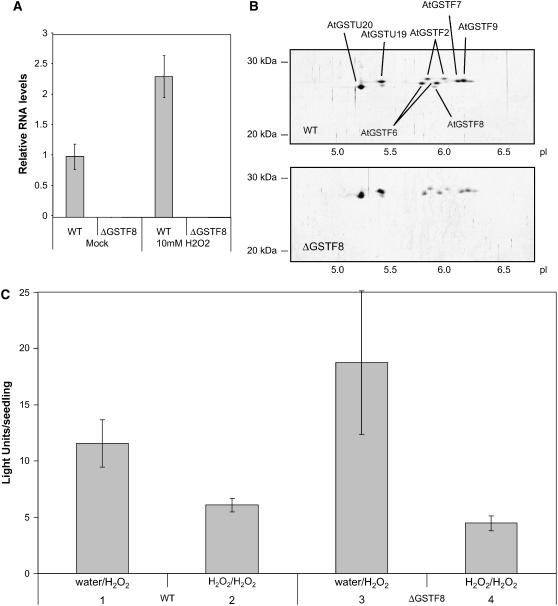
To explore whether GSTF8 protein levels played a role in desensitization of the GSTF8 promoter, we used a T-DNA insertion SALK line (039887), developed by Alonso et al. (2003), which contains a T-DNA insertion in the coding region of GSTF8 (At2g47730). A line homozygous for the T-DNA insertion, as determined by PCR, was selected. This line (called ΔGSTF8) had no detectable expression of either AtGSTF8 mRNA (Fig. 3A) or AtGSTF8 protein (Fig. 3B). ΔGSTF8 was crossed into the GSTF8∷LUC background and F3 plants homozygous for ΔGSTF8 and GSTF8∷LUC were examined for desensitization of GSTF8 expression. For these experiments, 4-d-old GSTF8∷LUC seedlings and homozygous ΔGSTF8 × GSTF8∷LUC lines were treated for 18 h with water or 1 mm H2O2. The seedlings were then treated with 1 mm H2O2 and bioluminescence was measured for each seedling 6 h after the second treatment (approximately 10–20 seedlings/treatment). As shown in Figure 3C, there was no significant difference between the desensitization of GSTF8 promoter activity in wild-type plants versus the ΔGSTF8 plants, suggesting that feedback regulation through changes in GSTF8 protein levels are not involved in regulating desensitization.
Figure 3.
GSTF8 protein does not regulate desensitization. A, AtGSTF8 mRNA is undetectable in ΔGSTF8 plants. Eight-day-old seedlings were mock treated with water or 10 mm H2O2 for 30 min. Whole seedlings were harvested 7 h after commencing the treatments and gene expression was measured using real-time PCR. Relative AtGSTF8 mRNA expression levels were normalized to cyclophilin. The average of three biological replicates is shown along with ses. B, AtGSTF8 protein is undetectable in ΔGSTF8 plants. Ten-day-old seedlings were mock treated with water or 1 mm SA for 30 min. Whole soluble cell extracts were prepared from whole seedlings harvested 24 h after commencing the treatments. GSTs were purified by glutathione affinity chromatography and resolved by isoelectric focusing/SDS-PAGE over a pI range of 3 to 10. The resulting two-dimensional gels were silver stained and GSTs were identified based on their relative mobilities according to our previous mass spectrometry analysis of Arabidopsis GSTs (Sappl et al., 2004). C, Plates containing 4-d-old wild-type (columns 1 and 2) and ΔGSTF8 (columns 3 and 4) seedlings in the GSTF8∷LUC background were pretreated with water (columns 1 and 3) or 1 mm H2O2 (columns 2 and 4). The seedlings were treated again with 1 mm H2O2 18 h later. For each treatment, 3 mL were added to a 55-mm Murashige and Skoog plate containing 50 μm luciferin and decanted after 40 min. Bioluminescence was measured for each seedling (approximately 20 seedlings/treatment) 6 h after the second treatment using a CCD camera and the average light units per seedling with ses are presented.
Desensitization of GSTF8 Expression Lasts for about 4 d
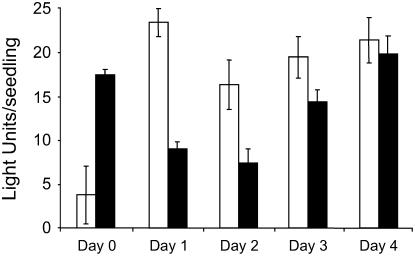
In other systems where desensitization was reported, the refractory period is transient and often lasts for minutes and rarely more than a few hours (Gainetdinov et al., 2004; Oppermann, 2004). We examined the length of the desensitization period for the GSTF8 promoter following an initial treatment with H2O2. Ten plates containing approximately 28 seedlings (4-d-old) were either treated with water or 1 mm H2O2 at day 0, and bioluminescence was measured at 0 and 4 h. At days 1, 2, 3, and 4, two plates (one previously treated with water and one previously treated with H2O2) were given a second 1 mm H2O2 treatment and bioluminescence was measured 4 h later. As shown in Figure 4, the second H2O2 treatment resulted in only a small induction at days 1 and 2 compared to the first H2O2 treatment. By day 3, the response of the GSTF8 promoter to a second H2O2 treatment had been restored to 70% of the original induction and, by day 4, the response had been fully restored.
Figure 4.
Desensitization of GSTF8 expression is regulated temporally. Four-day-old GSTF8∷LUC seedlings were pretreated with either water (white bars) or 1 mm H2O2 (black bars) on day 0. Bioluminescence was measured 4 h after a second 1 mm H2O2 treatment delivered either on days 1, 2, 3, or 4 after the first treatment. Separate seedlings were used for each treatment and discarded after each day's measurements. The average bioluminescence values of seedlings are presented with ses.
Desensitization of GSTF8 Expression Can Occur without Detectable Preinduction
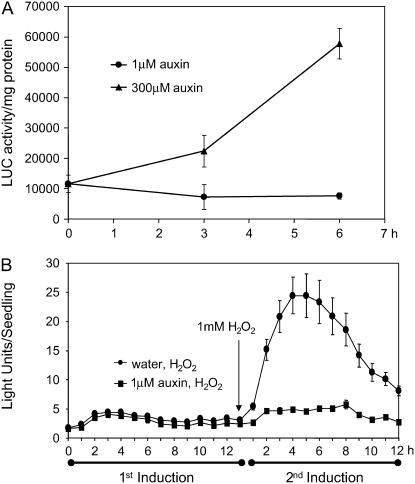
We were interested in examining whether lower concentrations of an inducer could also cause desensitization of GSTF8 expression. In the case of H2O2, we found that desensitization only occurred at H2O2 concentrations that were able to induce the GSTF8 promoter (see Supplemental Fig. 2). The synthetic auxin 2,4-D has been shown to induce the GSTF8 promoter at concentrations of 100 μm (Chen and Singh, 1999). However, as shown in Figure 5, lower concentrations that failed to induce the GSTF8 promoter (Fig. 5A) could still activate the desensitization response (Fig. 5B). For these experiments, 4-d-old GSTF8∷LUC seedlings were treated for 4 h with water or 1 μm auxin, an auxin concentration that does not induce GSTF8 promoter activity. The seedlings were then treated with 1 mm H2O2 the following day and bioluminescence was measured for each seedling (approximately 20 seedlings/treatment). As shown in Figure 5B, low concentrations of auxin (1 μm) also caused desensitization. Therefore, regulation of GSTF8 expression appears to be complex and activation and desensitization of GSTF8 promoter activity are not directly linked.
Figure 5.
Low concentrations of auxin can activate the desensitization phenomenon. A, Four-day-old plants were treated with 1 or 300 μm auxin and plant extracts were assayed biochemically for LUC activity and graphed as LUC activity per milligrams protein with sds. Samples were tested with technical and biological repeats. The averages of the biological means and sd are graphed. B, Four-day-old GSTF8∷LUC seedlings were pretreated with water or 1 μm auxin and 13 h later all seedlings were treated with 1 mm H2O2. Bioluminescence was measured for each seedling (approximately 20 seedlings/treatment) using a CCD camera and the average light units per seedling with ses are presented.
Activity of the ocs Element Is Also Regulated through Desensitization
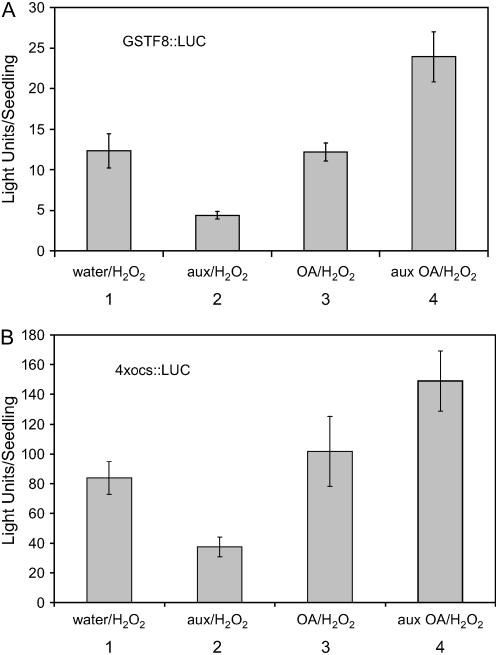
The GSTF8 promoter contains an ocs element (TTATGTCATTGATGACGACC), also referred to as an as-1 site, that is responsive to SA, H2O2, auxin, and R. solani (Lam et al., 1989; Xiang et al., 1996; Chen and Singh, 1999; Perl-Treves et al., 2004). It was of interest to see whether the ocs element is involved in the desensitization phenomenon. For these experiments, we used an Arabidopsis transgenic line in which a tetramer of the GSTF8 ocs element was fused to a LUC reporter gene and transformed into Arabidopsis (Perl-Treves et al., 2004). As shown in Figure 5, pretreatment with 1 mm H2O2 (Fig. 6A) or 1 μm auxin (Fig. 6B) resulted in a substantial reduction in the response to a subsequent treatment with H2O2, compared to plants that had been pretreated with water. Therefore, activity of the ocs element within the GSTF8 promoter, which plays a major role in defense signal induction, is also regulated by desensitization.
Figure 6.
Ocs element activity is also desensitized following treatment with 1 mm H2O2 or 1 μm auxin. Four-day-old 4 × ocs∷LUC seedlings were pretreated with water (white columns), 1 mm H2O2 (black columns in A), or 1 μm auxin (black columns in B) and 14 h later the seedlings were treated with 1 mm H2O2. Bioluminescence was measured for each seedling (approximately 20 seedlings/treatment) using a CCD camera. The relative bioluminescence for each seedling was recorded at 6 and 12 h after the second treatment. The average light units per seedling with ses are presented.
Treatment with a Phosphatase Inhibitor Prevents Desensitization of GSTF8 and ocs Element Expression
In a number of mammalian systems, phosphorylation of a receptor is one of the early steps in desensitization (e.g. Gainetdinov et al., 2004; Oppermann, 2004). To begin to analyze the mechanisms underlying the desensitization of GSTF8 expression, we asked whether phosphorylation/dephosphorylation events played any role in this system. For the experiments shown in Figure 7A, we used 1 μm auxin, which is unable to induce the GSTF8 promoter, but is able to desensitize the response of the promoter to a subsequent treatment with H2O2. Treatment with 1 μm okadaic acid, an inhibitor of type 1 and type 2A protein phosphatases, is not able to induce the GSTF8 promoter (see Supplemental Fig. 3) or lead to desensitization of the GSTF8 promoter because a subsequent treatment with H2O2 gives a normal level of induction (Fig. 7A, compare columns 1 and 3). However, when okadaic acid is administered together with 1 μm auxin in the first treatment, desensitization of the GSTF8 promoter is prevented (Fig. 7A, compare columns 2 and 4). Okadaic acid was also able to prevent desensitization of the ocs element (Fig. 7B, compare columns 2 and 4). These results suggest that dephosphorylation of one of more proteins is required for desensitization of GSTF8 and ocs element expression.
Figure 7.
Okadaic acid releases desensitization of the GSTF8 promoter and ocs element activity. A, Plates containing 4-d-old GSTF8∷LUC seedlings were pretreated with water (column 1), 1 μm auxin (column 2), 1 μm okadaic acid (column 3), or both 1 μm auxin and 1 μm okadaic acid (column 4). The seedlings were treated with 1 mm H2O2 17 h later. For each treatment, 1 mL was added to a 55-mm Murashige and Skoog plate containing 50 μm luciferin and decanted after 40 min. Bioluminescence was measured for each seedling (approximately 20 seedlings/treatment) 6 h after the second treatment and the average light units per seedling with ses are presented. B, Plates containing 4-d-old 4 × ocs∷LUC seedlings were pretreated as in Figure 7A. The seedlings were treated with 1 mm H2O2 24 h later. Bioluminescence was measured for each seedling (approximately 20 seedlings/treatment) 12 h after the second treatment and the average light units per seedling with ses are presented.
DISCUSSION
The Arabidopsis GSTF8 promoter is induced by a range of stimuli, including defense signals, auxin, and some pathogens. In this article, we report the use of an in vivo imaging system coupled with promoter/reporter constructs to study desensitization. We demonstrate that activity of the GSTF8 promoter and expression of the endogenous GSTF8 gene are regulated by desensitization, as reactivation is significantly reduced following pretreatment with a stimulus. A T-DNA knockout line for GSTF8 was used to demonstrate that failure to reactivate is not due to feedback regulation via GSTF8 protein levels.
Desensitization of GSTF8 expression occurred following repeated treatment with the same or different stimuli and is therefore an example of both homologous and heterologous desensitization. Desensitization assays have been used as a research tool by others to determine whether different plant signaling pathways share components, for example, UVB, oligosaccharide elicitors, and systemin (Yalamanchili and Stratmann, 2002; Holley et al., 2003). Our results suggest that different inducers of the GSTF8 promoter share one or more components in their respective signal transduction pathways and are consistent with the fact that many inducers of plant GSTs cause oxidative stress (Marrs, 1996). These results are also consistent with our previous findings that a specific promoter sequence, the ocs element, mediates in part the response of the GSTF8 promoter to different inducers (Chen and Singh, 1999; Perl-Treves et al., 2004) and the observations reported here that the ocs element is also regulated by desensitization.
There are a number of interesting features associated with the desensitization of GSTF8 expression. One is the length of the desensitization period, which lasts several days, in contrast to the desensitization periods observed in many other signaling pathways. For example, in some light responses, desensitization ranges from seconds to minutes (e.g. Govorunova et al., 1997; Mathieu et al., 1998; Baum et al., 1999), whereas desensitization of the mitogen-activated protein kinase response in tomato (Lycopersicon esculentum) to elicitors lasted less than 120 min (Holley et al., 2003). In soybean (Glycine max), cultured cells pretreated with a chemical elicitor were able to be quickly restimulated by the same or different elicitors, with the refractory period having a half-life of 20 min (Chandra et al., 2000).
Another interesting feature associated with desensitization of GSTF8 expression involved the site of reinduction; whereas a second induction of the GSTF8 promoter was about 30% as strong as the first induction, spatial analysis revealed that much of the initial response to a second treatment occurred in newly formed tissue at the root tip. This demonstrates that the desensitization of GSTF8 expression is more pronounced in older parts of the plant and suggests that desensitization may be lost upon cell division. Perhaps upon cell division the concentration of a key component involved in desensitization falls below a critical level in the daughter cells and consequently desensitization no longer occurs. We also observed that specific regions of the root responded differently to repeated treatments. The response of the middle region of the root to an initial stimulus was short lived compared to the rest of the root. Consequently, the middle region was able to respond much more rapidly to a second treatment (Fig. 1C). The reason for such an altered desensitization response for GSTF8 expression in this part of the root compared to other parts is unknown, but highly specific patterns of GST expression in different parts of the root have been reported previously (Smith et al., 2003).
It was also interesting to observe that very low concentrations of auxin (1 μm), which were not able to detectably induce GSTF8 expression, were able to desensitize the GSTF8 promoter. These results bear some similarity to the response of tomato cells with chitin treatment, which results in a rapid and transient alkalinization of the medium and involves a desensitization phenomenon (Felix et al., 1998). The concentration of chitin oligomers required for desensitization was about 20 times lower than that required for alkalinization. In contrast to the results obtained with low concentrations of auxin, we were not able to identify a concentration of H2O2 that could cause desensitization of GSTF8 expression while also not acting as an inducer (see Supplemental Fig. 2), and similar results were observed with SA (data not shown). At this stage, the reasons for these differences between auxin and H2O2/SA are unclear, but they raise the interesting possibility that desensitization of GSTF8 expression can occur by more than one mechanism. From the auxin results, it is clear that detectable activation of the GSTF8 promoter is not essential for eliciting desensitization.
Whereas desensitization of signaling pathways has been observed in both animals and plants, much of our knowledge of the underlying mechanisms comes from animal studies. A well-studied example of desensitization in animals is called agonist-induced desensitization and involves phosphorylation of G-protein-coupled receptors by Ser-Thr protein kinases. Subsequent binding of a class of β-arrestins prevents G protein from binding to the receptors and helps initiate their endocytosis (for review, see Gainetdinov et al., 2004; Oppermann, 2004). In some cases, receptors can be subjected to both homologous and heterologous desensitization via receptor phosphorylation (Xin et al., 2004; Avendano-Vazquez et al., 2005) and, in a number of studies, treatment with okadaic acid blocks the resensitization response (e.g. McLaughlin et al., 2003). In a few cases, heterologous desensitization in animal systems involves second messenger-dependent kinases that phosphorylate a range of proteins and lead to suppression of signaling pathways induced by different stimuli (Freedman and Lefkowitz, 1996).
In plants there have been only limited studies on the mechanisms involved in desensitization. In the case of chitin signaling, desensitization was not associated with inactivation of the stimulus or with the disappearance of high-affinity chitin-binding sites from the cell surface (Felix et al., 1998). In oligogalacturonide-activated signaling of soybean cells, there is evidence that phosphorylation events are involved in establishing a refractory state following an initial treatment with oligogalacturonides (Navazio et al., 2002). We examined desensitization of GSTF8 expression in different SA, jasmonic acid, or ethylene signal transduction mutant backgrounds, but found no evidence for a role of the SA, jasmonic acid, or ethylene pathways (data not shown). However, we did find evidence for a role of phosphorylation in the desensitization of GSTF8 expression with okadaic acid treatment, suggesting that dephosphorylation of one of more proteins is required for desensitization of the ocs element and the GSTF8 promoter. Whereas these results are in contrast with those reported for oligogalacturonide-activated desensitization, where okadaic acid acted to prolong desensitization (Navazio et al., 2002), they are consistent with results from the wound-induced SAMK pathway, where a Ser-Thr protein phosphatase 2C is one of the factors responsible for resetting the SAMK pathway in preparation for subsequent signaling (Meskiene et al., 1998). One possibility is that okadaic acid, which does not induce GSTF8 expression, may be acting to inhibit the dephosphorylation of an activator and/or repressor that is required for desensitization. For example, the CK2 enzyme phosphorylates and affects the DNA binding of several transcription factors (Klimczak et al., 1992; Tjaden and Coruzzi, 1994; Ciceri et al., 1997; Sugano et al., 1999), including TGACG-sequence-specific binding protein (TGA) family members that bind to the ocs element (Kang and Klessig, 2005).
Desensitization of signaling pathways has been suggested to allow an increase in the dynamic range of the sensory system (Armitage, 1992). Desensitization may also play a protective role in containing the extent of a response and preventing potentially deleterious effects of overstimulation such as can occur via the overproduction of toxic compounds during the oxidative burst associated with the plant defense response (Chandra et al., 2000). Because a number of the stimuli that induce GSTF8 expression are linked to defense, namely, the defense signals H2O2 and SA, a similar explanation may hold for the desensitization reported here.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) transgenic lines contained the −783 GSTF8 promoter fused to the LUC reporter gene (GSTF8∷LUC) in the Columbia ecotype background as previously described (Chen and Singh, 1999). All data presented are from a single T4 line homozygous for the transgene. The 4 × ocs element promoter construct was made by ligating four copies of the GSTF8 ocs element in front of the −58 GSTF8 minimal promoter (Perl-Treves et al., 2004) and a homozygous T4 line was used. For both the GSTF8 and ocs element promoters, all experiments were repeated with a second independent line and similar results were obtained. The T-DNA insertion line, SALK_039887, was obtained from the Salk Institute Genomic Analysis laboratory and contained a T-DNA insertion in the third exon of GSTF8 (A2g47730). This T-DNA insertion was confirmed by using PCR on genomic DNA with the primers 5′-GGGCGAGAGTCAAAGAGCACC-3′ and 5′-TGCAATAAATAAAGGGGACCCAA-3′. The homozygous seed line, SALK_039887C, will be available through the Arabidopsis Biological Resource Center at Ohio State University.
Growth of Arabidopsis on Agar Plates
Agar plates contained 1× Murashige and Skoog salts (4.3 g/L; Gibco) and 0.8% agar, with 3% Suc, pH adjusted to 5.7, with 1 m KOH. Plates for the LUC assay were supplemented with 50 μm luciferin (Biosynth AG) added after autoclaving the medium. Arabidopsis seeds were surface sterilized for 15 min with 70% ethanol followed by 2 d at 4°C. Seeds were germinated on Murashige and Skoog plates containing 50 μm luciferin and grown vertically for 4 d. Approximately 30 seeds were plated on round 55-mm plates, sealed with Micropore tape, and incubated vertically in the growth room (22°C, 16-h light/8-h dark photoperiod).
Plant Treatment
All auxin treatments used the synthetic auxin, 2,4-D (Sigma). Okadaic acid was from Sigma. Unless specified otherwise, 3 mL of treatment were pipetted onto the agar plates containing 4-d-old seedlings grown vertically. The solution was drained off after 40 min. The plates were left in the dark chamber in the EG & G Berthold molecular light imager during the time course experiments.
In Vivo Bioluminescence Assay
Luminescence was measured in an EG & G Berthold molecular light imager using a 5-min exposure after a 10-min fluorescent decay delay. Plants were superimposed onto a fluorescent exposure for orientation as previously described (Perl-Treves et al., 2004). The luminescence from each plant was measured against background and the average reading and se were graphed. Results are presented with the bioluminescence (blue) image superimposed on the fluorescence (white) image or graphically as average light units per seedling as determined by the EG & G Berthold software, WinLight 32. Figure 1, A and B, shows different light units per seedling as the camera settings were modified to capture the different number of plates in each experiment.
Biochemical LUC Assay
Eight-day-old seedlings were treated with 1 μm auxin or 300 μm auxin. After 0, 3, and 6 h, seedlings were homogenized in 0.2 m KPO4, pH 7.2, 1 mm dithiothreitol, and 0.5 mm phenylmethanesulfonyl fluoride (Sigma). Samples were then tested for LUC activity on a FLUOstar optima microplate reader (BMG Labtech) using the manufacturer's protocol. LUC activity was standardized against protein concentrations in the extracts as determine by the Bio-Rad DC protein assay.
RT-PCR
Eight-day-old seedlings were harvested from Murashige and Skoog agar plates. RNA isolation, cDNA synthesis, and quantitative RT-PCR were performed as previously described (Kang et al., 2003; Lister et al., 2004), using the iCycler program described in Sappl et al. (2004). The gene-specific primer pairs used for real-time PCR were AtGSTF6 (At1g02930): 5′-CCCCGTCGATATGAGAGC-3′ and 5′-GAGAGAGGGTCACTACTGCTTCTGG-3′; ATGSTF8 (A2g47730): 5′-CACAGGCTTGGTGAGTCCAAG-3′ and 5′-CAAATCAAACACTCGGCAGCAG-3′; β-tubulin (At5g23860): 5′-CACAGCAATACAGAGCCTTAACC-3′ and 5′-CTGTTGTTATTGCTCCTCCTGCA-3′; and Cyclophilin (At2g29960): 5′-TCTTCCTCTTCGGAGCCATA-3′ and 5′-AAGCTGGGAATGATTCGATG-3′.
Two-Dimensional Gel Electrophoresis
Whole soluble cell extracts were prepared from 10-d-old seedlings (grown on Murashige and Skoog plants) treated with 1 mm SA for 24 h. GSTs were purified by glutathione affinity chromatography and resolved by isoelectric focusing/SDS-PAGE over a pI range of 3 to 10 as previously described (Sappl et al., 2004). The resolved proteins were visualized by silver staining according to standard protocols. AtGSTU19 (At1g78380) and AtGSTU20 (At1g78370) were identified by mass spectrometry as described previously (Sappl et al., 2004). The identities of the other GST proteins were inferred from their relative mobilities as described (Sappl et al., 2004).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM180148, NM100174, NM106485, NM106484, AY081473, and NM128550.
Supplementary Material
Acknowledgments
We thank Elaine Smith, Hayley Casarotto, and Linne Jenkins for expert technical assistance, Louise Thatcher for helpful comments on the manuscript, and members of the Singh laboratory for useful discussions. We also thank the Salk Institute Genomic Analysis laboratory and the Arabidopsis Biological Resource Center for the Arabidopsis line SALK_039887.
This work was supported in part by a Grains Research and Development Corporation Visiting Fellowship (VF63; to R.P.-T.), by a Grains Research and Development Corporation postgraduate scholarship (to P.G.S.), and by the Australian Research Council Centre of Excellence Program (Australian Research Council QEII research fellowship to A.H.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Karam B. Singh (karam.singh@csiro.au).
The online version of this article contains Web-only data.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Armitage JP (1992) Behavioral responses in bacteria. Annu Rev Physiol 54: 683–714 [DOI] [PubMed] [Google Scholar]
- Avendano-Vazquez SEA, Garcia-Caballero A, Garcia-Sainz JA (2005) Phosphorylation and desensitization of the lysophosphatidic acid receptor LPA(1). Biochem J 385: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum G, Long JC, Jenkins GI, Trewavas AJ (1999) Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc Natl Acad Sci USA 96: 13554–13559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchez D, Tokuhisa JG, Llewellyn DJ, Dennis ES, Ellis JG (1989) The ocs-element is a component of the promoters of several T-DNA and plant viral genes. EMBO J 8: 4197–4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Yagamata H, Newhaus G, Chua N-H (1994) Phytochrome signal transduction pathways are regulated by reciprocal control mechansims. Genes Dev 8: 2188–2202 [DOI] [PubMed] [Google Scholar]
- Chandra S, Cessna SG, Yahraus T, Devine R, Low PS (2000) Homologous and heterologous desensitization and synergy in pathways leading to the soybean oxidative burst. Planta 211: 736–742 [DOI] [PubMed] [Google Scholar]
- Chen WQ, Singh KB (1999) The auxin, hydrogen peroxide and salicylic acid induced expression of the Arabidopsis GST6 promoter is mediated in part by an ocs element. Plant J 19: 667–677 [DOI] [PubMed] [Google Scholar]
- Ciceri P, Gianazza E, Lazzari B, Lippoli G, Genga A, Hoschek G, Schmidt RJ, Viotti A (1997) Phosphorylation of Opaque2 changes diurnally and impacts its DNA binding activity. Plant Cell 9: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Dixon DP, Walbot V (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Felix G, Baureithel K, Boller T (1998) Desensitization of the perception system for chitin fragments in tomato cells. Plant Physiol 117: 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman NJ, Lefkowitz RJ (1996) Desensitization of G protein-coupled receptors. Recent Prog Horm Res 51: 319–353 [PubMed] [Google Scholar]
- Frova C (2003) The plant glutathione transferase gene family: genomic structure, functions, expression and evolution. Physiol Plant 119: 469–479 [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27: 107–144 [DOI] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Hegemann P (1997) Desensitization and dark recovery of the photoreceptor current in Chlamydomonas reinhardtii. Plant Physiol 115: 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JJ, Yun BW, Loake GJ (2000) Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J 24: 569–582 [DOI] [PubMed] [Google Scholar]
- Holley SR, Yalamanchili RD, Moura DS, Ryan CA, Stratmann JW (2003) Convergence of signaling pathways induced by systemin, oligosaccharide elicitors, and ultraviolet-B radiation at the level of mitogen-activated protein kinases in Lycopersicon peruvianum suspension-cultured cells. Plant Physiol 132: 1728–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Foley RC, Onate-Sanchez L, Lin CGT, Singh KB (2003) Target genes for OBP3, a Dof transcription factor, include novel basic helix-loop-helix domain proteins inducible by salicylic acid. Plant J 35: 362–372 [DOI] [PubMed] [Google Scholar]
- Kang HG, Klessig DF (2005) Salicylic acid-inducible Arabidopsis CK2-like activity phosphorylates TGA2. Plant Mol Biol 57: 541–557 [DOI] [PubMed] [Google Scholar]
- Klimczak LJ, Schindler U, Cashmore AR (1992) DNA-binding activity of the Arabidopsis G-box binding-factor Gbf1 is stimulated by phosphorylation by casein kinase-Ii from broccoli. Plant Cell 4: 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E, Benfey PN, Gilmartin PM, Fang RX, Chua NH (1989) Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci USA 86: 7890–7894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Chew O, Lee MN, Heazlewood JL, Clifton R, Parker KL, Millar AH, Whelan J (2004) A transcriptomic and proteomic characterization of the Arabidopsis mitochondrial protein import apparatus and its response to mitochondrial dysfunction. Plant Physiol 134: 777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 127–158 [DOI] [PubMed] [Google Scholar]
- Mathieu Y, Guern J, Spiro MD, O'Neill MA, Kates K, Darvill AG, Albersheim P (1998) The transient nature of the oligogalaturonide-induced ion fluxes of tobacco cells is not correlated with fragmentation of the oligogalacturonides. Plant J 16: 305–311 [Google Scholar]
- McLaughlin JP, Xu M, Mackie K, Chavkin C (2003) Phosphorylation of a carboxyl-terminal serine within the kappa-opioid receptor produces desensitization and internalization. J Biol Chem 278: 34631–34640 [DOI] [PubMed] [Google Scholar]
- Meskiene I, Bogre L, Glaser W, Balog J, Brandstotter M, Zwerger K, Ammerer G, Hirt H (1998) MP2C, a plant protein phosphatase 2C, functions as a negative regulator of mitogen-activated protein kinase pathways in yeast and plants. Proc Natl Acad Sci USA 95: 1938–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Moscatiello R, Bellincampi D, Baldan B, Meggio F, Brini M, Bowler C, Mariani P (2002) The role of calcium in oligogalacturonide-activated signalling in soybean cells. Planta 215: 596–605 [DOI] [PubMed] [Google Scholar]
- Oppermann M (2004) Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal 16: 1201–1210 [DOI] [PubMed] [Google Scholar]
- Perl-Treves R, Foley RC, Chen WQ, Singh KB (2004) Early induction of the Arabidopsis GSTF8 promoter by specific strains of the fungal pathogen Rhizoctonia solani. Mol Plant Microbe Interact 17: 70–80 [DOI] [PubMed] [Google Scholar]
- Sappl PG, Onate-Sanchez L, Singh KB, Millar AH (2004) Proteomic analysis of glutathione S-transferases of Arabidopsis thaliana reveals differential salicylic acid-induced expression of the plant-specific phi and tau classes. Plant Mol Biol 54: 205–219 [DOI] [PubMed] [Google Scholar]
- Smith AP, Nourizadeh SD, Peer WA, Xu JH, Bandyopadhyay A, Murphy AS, Goldsbrough PB (2003) Arabidopsis AtGSTF2 is regulated by ethylene and auxin, and encodes a glutathione S-transferase that interacts with flavonoids. Plant J 36: 433–442 [DOI] [PubMed] [Google Scholar]
- Sugano S, Andronis C, Ong MS, Green RM, Tobin EM (1999) The protein kinase CK2 is involved in regulation of circadian rhythms in Arabidopsis. Proc Natl Acad Sci USA 96: 12362–12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden G, Coruzzi GM (1994) A novel at-rich DNA-binding protein that combines an Hmg I-like DNA-binding domain with a putative transcription domain. Plant Cell 6: 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle T (1994) The ocs element in the soybean Gh2/4 promoter is activated by both active and inactive auxin and salicylic acid analogs. Plant Mol Biol 26: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Uquillas C, Letelier I, Blanco F, Jordana X, Holuigue L (2004) NPR1-independent activation of immediate early salicylic acid-responsive genes in Arabidopsis. Mol Plant Microbe Interact 17: 34–42 [DOI] [PubMed] [Google Scholar]
- Xiang CB, Miao ZH, Lam E (1996) Coordinated activation of as-1-type elements and a tobacco glutathione S-transferase gene by auxins, salicylic acid, methyl-jasmonate and hydrogen peroxide. Plant Mol Biol 32: 415–426 [DOI] [PubMed] [Google Scholar]
- Xin CY, Ren SY, Pfeilschifter J, Huwiler A (2004) Heterologous desensitization of the sphingosine-1-phosphate receptors by purinoceptor activation in renal mesangial cells. Br J Pharmacol 143: 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili RD, Stratmann JW (2002) Ultraviolet-B activates components of the systemin signaling pathway in Lycopersicon peruvianum suspension-cultured cells. J Biol Chem 277: 28424–28430 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.