In plants with alternately arranged foliage, such as the coconut palm (Cocos nucifera), leaves are attached to the stem in either an ascending clockwise (left handed [L]) or counterclockwise (right handed [R]) spiral (Fig. 1). Foliar spiral direction (FSD) is not genetically determined in coconut palms: All crosses (R × R, R × L, L × R, L × L) yield R and L progeny in approximately equal numbers (Davis, 1962; Louis and Chidambaram, 1976; Toar et al., 1979). FSD is, thus, a classic case of morphological asymmetry in which dextral and sinistral forms are not inherited and are equally common within a species (Palmer, 2005). FSD would seem a simple stochastic process unworthy of further study if not for the observation by T.A. Davis, based on data collected from over 70,000 coconut palms in over 40 locations around the world, that the FSD of coconut palms varies with latitude: R trees predominate in the northern hemisphere and L trees predominate in the southern hemisphere (Davis and Davis, 1987). A reanalysis of Davis's data indicated that these hemispheric asymmetries in FSD are significantly better correlated with magnetic (dip) latitude than with geographic or geomagnetic (centered dipole) latitude, suggesting that latitudinal asymmetries in FSD might be associated with the temporally varying component of the Earth's magnetic field (Minorsky, 1998). Here, we report that asymmetries in FSD are also evident in populations of coconut palms on opposite sides of islands and that asymmetries between cohorts vary with an 11-year periodicity—two novel discoveries consistent with the hypothesis that geomagnetic variations underlie asymmetries in coconut palm FSD.
Figure 1.
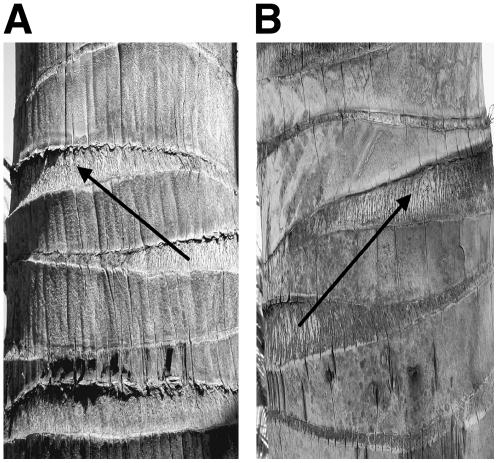
FSD of coconut palms is easily discernible by examination of the leaf scars on the stem. If the next highest (youngest) leaf scar is approximately 140° to the left, it is an L palm (left photo); if it is approximately 140° to the right, it is an R palm (right photo).
Whereas the effects of the geomagnetic field on the orientation of magnetotactic bacteria and various animals, particularly insects and migratory birds, has been extensively studied, relatively little is known about the effects of geomagnetism on plants (Belyavskaya, 2004; Galland and Pazur, 2005). Important questions remain unanswered, such as whether or not plants perceive the geomagnetic field and, if they do, by what mechanisms and to what possible advantage, if any? At this early stage in our understanding of magnetoreception, several alternative mechanisms are being discussed by biophysicists concerning how cells might sense weak electromagnetic fields. Among the proposed modes of action are (1) torque on ferromagnetic particles; (2) modulation of biochemical reactions that involve spin-correlated radical pairs (radical-pair mechanism); (3) modulation of the transport rates and binding by ion-cyclotron resonance; and (4) quantum coherence mechanisms (for review, see Galland and Pazur, 2005). Regardless of the exact mechanisms involved in the physical reception of magnetic stimulation, considerable evidence suggests the involvement of biological membranes, in general (Balcavage et al., 1996; Volpe, 2003), and of Ca2+ fluxes, in particular (Belova and Lednev, 2001; Bauréus Koch et al., 2003; Belyavskaya, 2004; Pazur et al., 2006), in magnetoreception.
Evidence does exist that the electrical potentials of trees change in parallel, even in fine detail, with earth currents induced by variations in the Earth's magnetic field. Pc1-type geomagnetic pulsations (0.2–5 Hz) of very small amplitude (0.05–0.1 nT), for example, have been recorded in oak (Quercus lobata) trees (Fraser-Smith, 1978). These extremely weak geomagnetic pulsations gave rise to electrical potential oscillations of approximately 100-μV amplitude (Fraser-Smith, 1978). These electrical signals were not artifactual: They were not found when the tree was replaced with a resistor or when a dead tree was used. Similar electrical periodicities had been measured previously in plants and correlated with 1- to 10-Hz leaf movements (Semenenko, 1972), suggesting that plants are not simply passive antennae for geomagnetic variations, but that membrane functioning may be affected (Minorsky, 2001). Conceivably, a membrane transport process that might be affected by induced currents is the polar transport of auxin, a plant hormone that determines the phyllotaxy of plants (Reinhardt et al., 2003). Plant cells use electric currents to control their physiological polarity and direction of growth (Morris, 1980; Bandurski et al., 1992; Mina and Goldsworthy, 1992). Individual plant cells generate their own polar electric currents, but the direction of these currents can be changed by a brief application of a weak external current, after which the cell's new current is in the same direction as the one that was applied (Mina and Goldsworthy, 1992). It is hypothesized that canalized physiological polarity involves the electrophoretic distribution of differently charged membrane proteins (e.g. auxin transport proteins) along the cell's electrical axis (Mina and Goldsworthy, 1992). The induced current hypothesis proposes that asymmetries in coconut palm FSD result from earth currents in trees that are induced by variations in the vertical Z component of the geomagnetic field, and that these earth currents consequently cause a rotational bias in the axial electrophoresis of morphogens (e.g. auxin transporters) in coconut palm embryos (Minorsky, 1998).
The prediction that asymmetries in coconut palm FSDs of opposite sign should exist on opposite sides of islands arises from the fact that, because seawater is more electrically conductive than land, induced earth currents tend to divide and stream past an island in a pattern determined by the surrounding bathymetry. The geomagnetic island effect is characterized by a complete reversal of the vertical Z component of short-period geomagnetic field variations at observation points on opposite sides of islands (Elvers and Perkins, 1964; Sasai, 1967; Honkura, 1972; Klein, 1972; Yamaguchi et al., 1992). To examine whether coconut palm FSD varies around the circumferences of islands, data were collected on two Caribbean islands (Puerto Rico, n = 4,850; Antigua, n = 2,038), two Hawaiian islands (Hawaii, n = 3,552; Maui, n = 2,175), and two French Polynesian islands (Tahiti, n = 1,635; Moorea, n = 2,116). It should be noted that the convention we use for designating palms as L or R in this contribution is in agreement with the Descriptors for Coconut established by the International Plant Genetic Resources Institute (IPGRI, 1995) and opposite to that used previously (Davis, 1962, 1963; Davis and Davis, 1987; Minorsky, 1998). Insofar as our research is concerned, Tahiti and nearby Moorea behaved as a single island that we refer to as Tahiti/Moorea: Thus, we have five datasets. No effort was made to distinguish between different varieties of coconut palms because previous research by Davis (1962) suggested that this is not an important factor. Data were collected in a wide variety of locales, including beaches, parks, groves, resorts, plantations, and private properties: Only urban (e.g. sidewalk) populations were excluded. For each population, the degree of asymmetry for trees with easily distinguishable leaf scars was determined by calculating an asymmetry quotient (AQ) based on the formula:
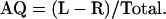 |
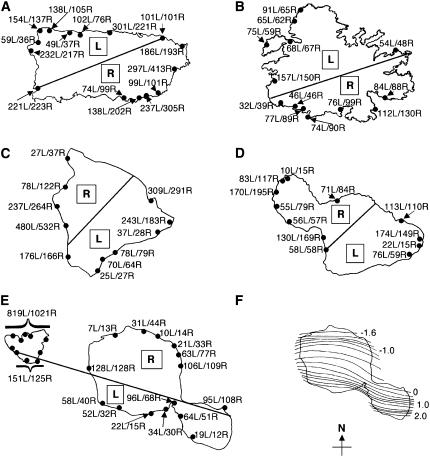
Asymmetries in FSD were evident on opposite sides of all five islands studied (Fig. 2, A–E). The azimuths of maximal AQ varied between the three island groups: AQs were maximum at the following bearings (from geographic N): −20° in the Caribbean islands; 125° in the Hawaiian islands; and −165° in Tahiti/Moorea.
Figure 2.
Numbers of L and R coconut palms counted around the circumferences of islands. A, Puerto Rico. B, Antigua. C, Hawaii. D, Maui. E, Tahiti/Moorea. F, Map of Pz, a scaled parameter representing the Z component of the anomalous geomagnetic field that arises from the distortion of electric current flowing in the ocean around Tahiti (Fig. 2F redrawn from Yamaguchi et al., 1992). Lines that bisect the islands in Figure 2, A to E, represent lines of symmetry connecting the estimated azimuths of zero asymmetry.
Based on the facts that the trade winds in Puerto Rico are northeasterly and that the line of zero asymmetry runs along a northeast-to-southwest diagonal in Puerto Rico, one might reasonably formulate the working hypothesis that positive AQs (high left handedness) are associated with the counterclockwise flow of wind around the island, and negative AQs are associated with a clockwise flow of wind. Unfortunately, the wind hypothesis is completely dashed by our findings on Tahiti/Moorea (Fig. 2E), where the trade winds blow from the southeast and the cross-island asymmetry is completely opposite from what would be predicted by the wind hypothesis based on the case in Puerto Rico (i.e. the data from Puerto Rico and Tahiti/Moorea when considered together show an antiparallel relation to the trade winds rather than a parallel one). Thus, no correspondence exists between the cross-island asymmetries of AQ and the directions of the trade winds.
The effects we report were observed most strongly in natural populations; for example, the highest X2 value (assuming L = R) we found for any population (n = 710; X2 = 18.95; P < 0.001) was from a natural grove in Humaçao, Puerto Rico. Thus, the activities of man (e.g. transplantation or the differential culling of trees of opposite handedness) would appear to obfuscate, rather than create, the differences seen on opposite sides of islands.
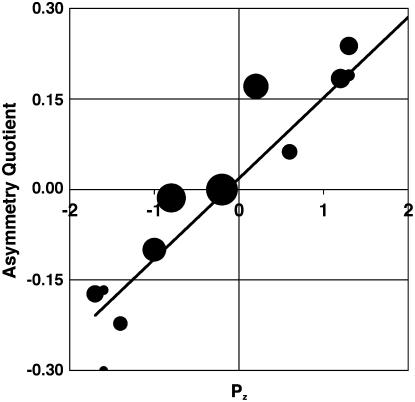
It is of interest to consider whether the palm island effect described here bears any relation to the geomagnetic island effect described by geophysicists. Yamaguchi et al. (1992) have provided the most detailed map of the geomagnetic island effect for a tropical island, namely, Tahiti. In their map, which is reproduced in Figure 2F, Pz is a scaled parameter representing the vertical Z component of the anomalous geomagnetic field that arises from the distortion of electric current flowing in the ocean around Tahiti. There was a strong correlation (r = 0.87; n= 1,635; P < 0.001) between the AQs of coconut palm populations on Tahiti weighted for population size and Pz (Fig. 3).
Figure 3.
A close correlation (r = 0.87) exists between the AQ of coconut palm populations around the circumference of Tahiti and Pz, a scaled parameter representing the Z component of the anomalous geomagnetic field that arises from the distortion of electric current flowing in the ocean around Tahiti. Area of largest circle represents 256 trees.
A consequence of the palm island effect is that the interpretation of about one-half of the locations in the Davis and Davis (1987) dataset, namely, all those designated as islands or island nations, is rendered ambiguous. Culling of the island data points from the Davis and Davis dataset (1987), which constituted nearly all of the southern hemisphere data and more than one-half the trees (n = 71,596 to n = 32,954), slightly improved, albeit insignificantly, the correlation coefficients between AQ and magnetic latitude (r = 0.62; P < 0.001 to r = 0.64; P < 0.005). In contrast, the respective correlations between AQ and geomagnetic (r = 0.57; P < 0.001 to r = 0.41; P = 0.097) and geographic latitude (r = 0.50; P < 0.002 to r = 0.18; P = 0.487) were rendered insignificant. This is further testament to the robustness of the correlation between FSD asymmetry and magnetic latitude.
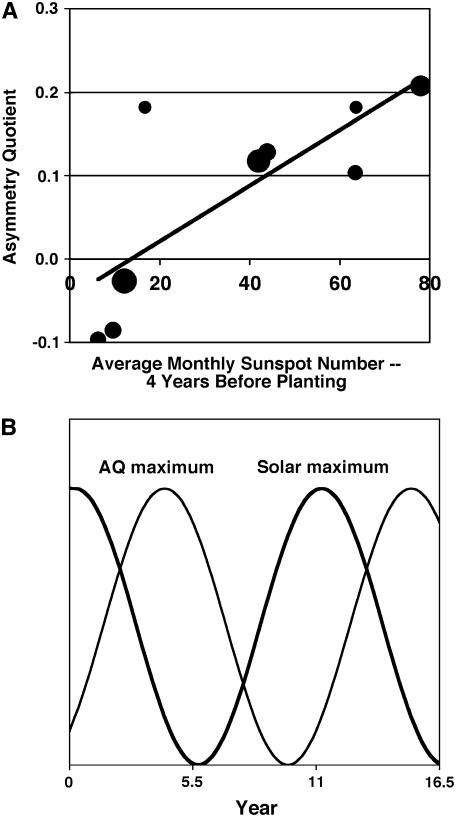
The frequencies of occurrence of many classes of geomagnetic variations change over the course of a sunspot cycle. Moreover, previous research by Sulima (1970) established a link between sunspot cycles and variations in the morphological asymmetry of cereal grains. Thus, it was of interest to determine whether asymmetry in coconut palm FSD also varies with the sunspot cycle. The age of coconut palms growing in nature, however, cannot be reliably estimated because palms, being monocots, do not produce annual growth rings. Thus, to examine the question of whether the AQs of palm cohorts vary with the sunspot cycle, it is necessary to examine data from research stations and plantations that have maintained records of the FSD and time of planting of each of their accessions. Davis (1963) published a suitable dataset concerning a population of coconut palms (n = 384 trees) growing in Kerala, India. Of these 384 trees, 375 fell into one of nine cohorts of 22 trees or more (planted at 5-year intervals from 1888–1928). There was a strong positive correlation between the AQs of the nine cohorts weighted for population size (r = 0.85; P < 0.001) and the total average monthly sunspot numbers 4 years prior to their respective years of planting (Fig. 4A). Our analysis of Davis's (1963) data suggests that maximal AQ is achieved during the late descending phase of the sunspot cycle (Fig. 4B), a time in the solar cycle typically characterized by the highest frequency of recurrent geomagnetic storms (Pérez-Peraza et al., 1997). Geomagnetic storms have the largest amplitudes of any geomagnetic variation—typically more than 1,000 times larger than those of Pc1 pulsations.
Figure 4.
A, AQs of different-aged cohorts of coconut palms are closely correlated with the mean annual sunspot numbers 4 years before planting (r = 0.85). Area of largest circle represents 76 trees. B, Idealized version of the relationship between sunspot numbers (thick line) and AQ (thin line). Oscillations in the AQ are proposed to be coincident with oscillations in the frequency of recurrent geomagnetic storms.
Natural experiments, such as those we have performed, have the inherent and well-recognized drawback that the researcher has no control over the situation being observed and, thus, there is always a possibility that some other factor is having an influence on the dependent variable. Clearly, the cause of the temporal and spatial variations in coconut palm FSD is not attributable to genetics, the hand of man, or the trade winds. Any role for the Coriolis force can also be ruled out because its strength at these dimensions would be below that of thermal noise, and any hemispheric asymmetry in FSD arising from the Coriolis force would be better correlated with geographic latitude than magnetic latitude. It is impossible to prove by natural experiments a role for geomagnetic variations in establishing asymmetries in coconut palm FSD, but by eliminating rival hypotheses, the induced current hypothesis gains in stature.
The argument could be made that coconut palms are unusual in their apparent sensitivity to geomagnetic storms. It is possible that the saline soils in which they typically grow may be especially efficient conductors of earth currents. Moreover, coconut palms appear to be unusually sensitive to electromagnetic fields. Leaves growing within 30 to 60 cm of power lines typically exhibit chlorosis or necrosis at the leaf tip and can even die from this disorder. Leaves do not have to be in physical contact with the power lines for injury to occur (Broschat and Meerow, 2000). However, a recent report that geomagnetic storms affect mitosis in onion (Allium cepa) root tips (Nanush'yan and Murashev, 2003) suggests that sensitivity to geomagnetic storms is not limited solely to coconut palms.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Peter V. Minorsky (pminorsky@mercy.edu).
References
- Balcavage WX, Alvager T, Swez J, Goff CW, Fox MT, Abdullyava S, King MW (1996) A mechanism for action of extremely low frequency electromagnetic fields on biological systems. Biochem Biophys Res Commun 222: 374–378 [DOI] [PubMed] [Google Scholar]
- Bandurski RS, Schulze A, Jensen P, Desrosiers M, Epel B, Kowalczyk S (1992) The mechanism by which an asymmetric distribution of plant growth hormone is attained. Adv Space Res 12: 203–210 [DOI] [PubMed] [Google Scholar]
- Bauréus Koch CLM, Sommarin M, Persson BRR, Salford LG, Eberhardt JL (2003) Interaction between weak low frequency magnetic fields and cell membranes. Bioelectromagnetics 24: 395–402 [DOI] [PubMed] [Google Scholar]
- Belova NA, Lednev VV (2001) Effects of extremely weak alternating magnetic fields on plant gravitropism. Biofizika 46: 122–125 [PubMed] [Google Scholar]
- Belyavskaya NA (2004) Biological effects due to weak magnetic fields on plants. Adv Space Res 34: 1566–1574 [DOI] [PubMed] [Google Scholar]
- Broschat TK, Meerow AW (2000) Ornamental Palm Horticulture. University Press of Florida, Gainesville, FL
- Davis TA (1962) The non-inheritance of asymmetry in Cocos nucifera L. J Genet 58: 42–50 [Google Scholar]
- Davis TA (1963) The dependence of yield on asymmetry in coconut palms. J Genet 58: 186–215 [Google Scholar]
- Davis TA, Davis B (1987) Association of coconut foliar spirality with latitude. Math Model 8: 730–733 [Google Scholar]
- Elvers D, Perkins D (1964) Geomagnetic research on spatial dependence of time variations across Puerto Rico. Trans Am Geophys Union 45: 46 [Google Scholar]
- Fraser-Smith AC (1978) ULF tree potentials and geomagnetic pulsations. Nature 271: 641–642 [Google Scholar]
- Galland P, Pazur A (2005) Magnetoreception in plants. J Plant Res 118: 371–389 [DOI] [PubMed] [Google Scholar]
- Honkura Y (1972) Geomagnetic anomaly on Miyake-jima island. J Geomagn Geoelec 23: 307–333 [Google Scholar]
- IPGRI (1995) Descriptors for Coconut (Cocos nucifera L.). International Plant Genetics Research Institute, Rome
- Klein DP (1972) Geomagnetic Time-Variations, The Island Effect and Electromagnetic Depth Sounding on Oceanic Islands: Results from the Analysis of Data Obtained in the Frequency Range of 0.5 to 10 Cycles per Hour on Oahu, Hawaii. Hawaii Institute of Physics Geophysics Publications, Oahu, HI, Publication 72–3
- Louis HH, Chidambaram A (1976) Inheritance studies on the phyllotaxis of coconut palm. Ceylon Coconut Q 27: 22–24 [Google Scholar]
- Mina MG, Goldsworthy A (1992) Electrical polarization of tobacco cells by Ca2+ ion channels. J Exp Bot 43: 449–454 [Google Scholar]
- Minorsky PV (1998) Latitudinal differences in coconut foliar spiral direction: a re-evaluation and hypothesis. Ann Bot (Lond) 82: 133–140 [Google Scholar]
- Minorsky PV (2001) News from the archives. Plant Physiol 126: 928–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA (1980) The influence of small direct electric currents on the transport of auxin in intact plants. Planta 150: 431–434 [DOI] [PubMed] [Google Scholar]
- Nanush'yan ER, Murashev VV (2003) Induction of multinuclear cells in the apical meristems of Allium cepa by geomagnetic outrages. Russ J Plant Physiol 50: 522–526 [Google Scholar]
- Palmer AR (2005) Asymmetry. In B Hallgrimmson, BK Hall, eds, Variation: A Central Concept in Biology, Academic Press, New York, pp 359–397
- Pazur A, Rassadina V, Dandler J, Zoller J (2006) Growth of etiolated barley plants in weak static and 50 Hz electromagnetic fields tuned to calcium ion cyclotron resonance. BioMagn Res Tech 4: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Peraza J, Leyva A, Libin IY, Fomichev V, Guschina RT, Yudakhin K, Jaani A (1997) Simulating the mechanism of the action of heliophysical parameters on atmospheric processes. Geofís Intl 36: 245–280 [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Sasai Y (1967) Spatial dependence of short-period geomagnetic fluctuations on Oshima Island. Bull Earthquake Res Inst 45: 137–157 [Google Scholar]
- Semenenko AD (1972) Electrophytogram of pulsation rhythm. Dokl Bot Sci 206: 124–126 [Google Scholar]
- Sulima YG (1970) Biosimmetricheskie i Bioritmicheskie Iavleniia i Priznaki u Sel'skokhoziaistvennykh Rastenii. Akad. Nauk Moldavskoi SSR, Kishinev, Moldova
- Toar RE, Rompas TM, Sudasrip H (1979) The non-inheritance of the direction of foliar spiral in coconut. Experientia 35: 1585–1587 [Google Scholar]
- Volpe P (2003) Interaction of zero-frequency and oscillating magnetic fields with biostructures and biosystems. Photochem Photobiol Sci 2: 637–648 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isezaki N, Yaskawa K (1992) Conductivity structure in the south-east Pacific inferred from the island effect on Tahiti island. J Geomagn Geoelec 44: 43–54 [Google Scholar]