Abstract
Radiation-attenuated (RA) schistosome larvae are potent stimulators of innate immune responses at the skin site of exposure (pinna) that are likely to be important factors in the development of Th1-mediated protective immunity. In addition to causing an influx of neutrophils, macrophages, and dendritic cells (DCs) into the dermis, RA larvae induced a cascade of chemokine and cytokine secretion following in vitro culture of pinna biopsy samples. While macrophage inflammatory protein 1α and interleukin-1β (IL-1β) were produced transiently within the first few days, the Th1-promoting cytokines IL-12 and IL-18 were secreted at high levels until at least day 14. Assay of C3H/HeJ mice confirmed that IL-12 secretion was not due to lipopolysaccharide contaminants binding Toll-like receptor 4. Significantly, IL-12 p40 secretion was sustained in pinnae from vaccinated mice but not in those from nonprotected infected mice. In contrast, IL-10 was produced from both vaccinated and infected mice. This cytokine regulates IL-12-associated dermal inflammation, since in vaccinated IL-10−/− mice, pinna thickness was greatly increased concurrent with elevated levels of IL-12 p40. A significant number of IL-12 p40+ cells were detected as emigrants from in vitro-cultured pinnae, and most were within a population of rare large granular cells that were Ia+, consistent with their being antigen-presenting cells. Labeling of IL-12+ cells for CD11c, CD205, CD8α, CD11b, and F4/80 indicated that the majority were myeloid DCs, although a proportion were CD11c− F4/80+, suggesting that macrophages were an additional source of IL-12 in the skin.
The processes leading to the induction of polarized T-helper lymphocyte populations following exposure of the host to infectious pathogens are believed to involve interactions between the innate and acquired immune systems at the initial site of infection (24). In this context, the skin is a major barrier across which many infectious agents, including the parasitic helminth Schistosoma mansoni, gain entry to the host. This site is populated by an array of immune-competent accessory cells acting as sentinels, which are an important source of cytokines and chemokines responsible for amplifying the inflammatory response by activating resident cells and recruiting additional cells (9, 49). Cytokines also have an important influence on Th cell development, with interleukin-12 (IL-12), IL-18, tumor necrosis factor alpha, and IL-1β being key factors in the differentiation of Th1 cells (20, 29).
In our study, we examined innate immune responses elicited in the skin of mice exposed to radiation-attenuated (RA) larvae of S. mansoni. In this vaccine model, RA larvae induce Th1-type acquired immunity, which is characterized by the persistence of gamma interferon (IFN-γ)-secreting Th cells in the skin-draining lymph nodes (sdLN) (26) and confers 60 to 70% protection against a challenge infection (11). There is clear evidence that IL-12 is a critical component of this Th1-mediated protection (2, 47). In contrast, normal larvae fail to support sustained production of IFN-γ in sdLN (30) and do not induce significant levels of immunologically mediated protection (6, 45). Despite the obvious potential for interaction between schistosomes and the innate immune system in the skin, remarkably little is known about events at this site. It is also unclear how RA and normal larvae differ in their effects on the innate immune response and how this influences the nature of the ensuing acquired immune response.
Schistosome cercariae are well adapted for infection of the host by a percutaneous route (23). In murine skin, larvae quickly reach the epidermal basement membrane by using secretions from the pre- and postacetabular glands and remain there for approximately 48 h prior to entering the dermis (44). The parasites then exit via postcapillary venules, or lymphatic vessels, with less than 25% of normal larvae remaining in the skin by day 5 (25). In contrast, RA larvae exhibit severely retarded migration from the skin (22, 25) and are well placed to stimulate accessory cells of the innate immune response, including antigen-presenting cells (APCs) (33). Although RA larvae in the skin cause an increase in the thickness of the epidermal and dermal layers (18, 44), secretion of cytokines in the skin of vaccinated mice has never been studied despite its likely relevance to the development of protective Th1-mediated immunity.
The aim of our studies was to analyze the innate response to RA larvae in order to define the cellular constituents and cytokines in the skin that may promote a biased Th1-type immune response. We show that the production of IL-12 p40 is a key feature of the innate response in the skin after exposure to RA larvae and that cells positive for dendritic cell (DC) and macrophage markers are the source of this molecule. Moreover, we report that RA larvae, contrary to previous findings (32), induce the production of IL-10, which regulates inflammation and the production of IL-12.
MATERIALS AND METHODS
Parasite and host.
Female C57BL/6 mice (8 to 12 weeks old) were exposed to 500 RA (20-kilorad 60Co source) or normal S. mansoni cercariae via the pinna as described in detail previously (27). By counting the nonpenetrant cercariae, we determined that 65 to 75% of the applied cercariae successfully entered the pinna and that the variation between individual mice for any vaccination was 5 to 10%. C3H/HeJ mice, which are hyporesponsive to lipopolysaccharide (LPS) because of a genetic defect in Toll-like receptor 4 (TLR-4) (31), and their normal C3H/HeN cohorts were obtained from Harlan United Kingdom Ltd. Protective immunity in these two strains was determined following exposure to challenge larvae (2). IL-12 p40−/− (21) and IL-10−/− (19) (Taconic Farms) mice were bred and maintained alongside wild-type (WT; C57BL/6 background) cohorts at the University of York. All animal work was carried out in accordance with the guidelines of the United Kingdom Animals (Scientific Procedures) Act 1986.
Sampling regimen.
On days 0 (naive), 1, 2, 4, 8, and 14 postvaccination (p.v.), groups of mice (providing four to six pinnae) were killed and the extent of inflammation was determined by measurement of pinna thickness with a dial gauge micrometer (Mitutoyo). Pinnae were carefully removed to ensure that the biopsy samples were of a consistent size and then prepared for culture in vitro or histologic analysis.
Histologic analysis of dermal immune responses.
For conventional histologic analysis, pinnae were fixed in 10% neutral buffered formalin (Sigma), wax embedded, sectioned at 6 μm, and stained with hematoxylin and eosin (Eastalt Histology Laboratories, Warrington, United Kingdom). For immunohistochemical analysis, pinnae were embedded in OCT compound (H&E Ltd., Nottingham, United Kingdom), frozen with liquid N2, and then stored at −80°C prior to sectioning at 7 μm (Slee Cryostat) on poly-l-lysine-coated glass slides (Superfrost Plus; Invitrogen, Paisley, United Kingdom). Sections were fixed in 3.7% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min, washed, and air dried. Following rehydration in PBS, sections were incubated with 3% bovine serum albumin and then endogenous peroxidase and biotin blocked with 1% H2O2 in PBS for 15 min and a biotin blocking kit, respectively (Vector Labs, Peterborough, United Kingdom). Sections were subsequently incubated with unlabeled or biotinylated primary antibodies against cell surface markers (details below) for 30 min at room temperature and then, when required, with a biotinylated secondary antibody for 30 min at room temperature. For intracellular IL-12 p40 staining, 0.1% saponin was incorporated at each incubation step after fixing; sections were labeled with biotinylated anti-IL-12 p40 (C17.8; BD PharMingen, Oxford, United Kingdom). Immunostaining was completed with the Vectastain Elite ABC peroxidase reagent combined with the Vector VIP or NovaRED enzyme substrate and counterstained with methyl green or hematoxylin (Vector Labs).
In vitro culture of pinna biopsy samples and sdLN cells.
Pinnae were cultured in vitro with a technique adapted from that described by Belkaid et al. (4) as described in detail elsewhere (27). Briefly, each pinna was split and floated on 0.5 ml of RPMI medium containing 10% fetal calf serum (low endotoxin; Seralab, Oxon, United Kingdom), 2 mM l-glutamine, 200 U of penicillin per ml, and 100 μg of streptomycin per ml (RPMI/10; Sigma, Poole, United Kingdom) in 24-well hydrophobic culture plates (Greiner Labortechnik, Frickenhausen, Germany). Pinnae were cultured in the absence of added parasite antigen for 6 to 18 h at 37°C in 5% CO2. Nonadherent cells were recovered from culture supernatants by centrifugation at 800 × g for 5 min, while loosely adherent cells were obtained after incubation with Mg2+- and Ca2+-free PBS supplemented with 2 mg of glucose per ml for 20 min at 37°C. Cells were pooled and resuspended in phenobuffer (PBS containing 0.01% CaCl2, 0.01% MgCl2, and 0.1% bovine serum albumin). Cells from sdLN of vaccinated and infected mice (n = 5) were recovered on days 4, 8, and 14 after exposure and cultured for 72 h in the presence or absence of a soluble antigen preparation (50 μg/ml) from larval schistosomes (27). Cell-free supernatants from the dermal and sdLN cultures were collected and frozen at −20°C prior to analysis by enzyme-linked immunosorbent assay (ELISA). In all experiments, tissues from naive mice were similarly prepared to establish baseline levels for immune responses.
Phenotypic analysis of cells from skin cultures.
Aliquots of up to 5 × 105 cells were incubated with anti-CD16/CD32 (0.25 μg; BD PharMingen) to block Fc receptors and then with optimum dilutions of antibodies against surface markers for 30 min on ice. Different cell types were identified with antibodies against surface markers as follows: hematopoietic cells, CD45 (M1-9.3HL; Sigma); major histocompatibility complex class II+ APCs, Iab,d (B21.2; Caltag-MedSystems, Towcester, United Kingdom); macrophages/myeloid cells, CD11b (Mac-1, M1/70; Serotec) and F4-80 (C1:A3-1; Serotec); DCs, CD11c (N418; BD PharMingen) and CD205 (NLDC-145; Serotec); lymphoid DCs, CD8α (53-6.7; BD PharMingen). Antibodies were conjugated with fluorochromes (fluorescein isothiocyanate or phycoerythrin) or biotin, in which case Streptavidin-QR (Serotec) was used as a detection probe. Irrelevant isotype-matched antibodies were used to determine levels of nonspecific binding. Labeled cells were then analyzed by two-color flow cytometry (Coulter XL). For intracellular staining of IL-12 p40, cells were cultured as described above but in the presence of 1 μg of GolgiPlug (BD PharMingen) per ml for 12 h to block cytokine secretion. After surface staining, cells were treated with Cytofix/Cytoperm (BD PharMingen) and incubated with phycoerythrin-labeled IL-12 p40 monoclonal antibody (MAb) (C15.6; BD PharMingen) or a rat immunoglobulin G1 isotype control. At least 1,000 IL-12+ events were analyzed for each sample.
Cytokine and chemokine ELISAs.
Paired antibody capture ELISAs with a biotinylated detection step were used to measure cytokines and chemokines in dermal and sdLN culture supernatants as follows (all antibodies were from BD PharMingen, except where specified otherwise): anti-IL-1β MAb (30311) with an anti-IL-1β polyclonal antibody (PAb) (both R&D Systems, Oxon, United Kingdom); an anti-IL-4 MAb (BVD4-1D11) with an anti-IL-4 MAb (BVD6-24G2); an anti-IL-6 MAb (MP5-20F3) with an anti-IL-6 MAb (MP5-32C11); an anti-IL-10 MAb (JES5 2A5) with an anti-IL-10 MAb (SXC-1); an anti-IL-12 p40 MAb (C15-6) with an anti-IL-12 p40 MAb (C17.8); and an anti-IFN-γ MAb (R4-6A2) with an anti-IFN-γ MAb (XMG1.2). All biotinylated antibodies were detected with streptavidin peroxidase conjugate. IL-18 was detected with an rabbit anti-IL-18 PAb (gift from C. Locht, Institut Pasteur, Lille, France) with an anti-IL-18 PAb (R&D Systems) detected with anti-goat peroxidase conjugate (Sigma). Recombinant standards were IL-1β, IL-10, IL-18 (R&D Systems), IL-6, IL-4 (BD PharMingen), IFN-γ (211A CHO cell supernatant), and IL-12 (S. Wolf, Genetics Institute, Cambridge, Mass.). The lower limits of detection were 100 pg/ml (IL-18), 25 pg/ml (IL-1β and IFN-γ), 20 pg/ml (IL-12 p40), 10 pg/ml (IL-4 and IL-10), and 5 pg/ml (IL-6). Macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and eotaxin were measured with Quantikine kits (R&D Systems); the lower limit of detection was 5 pg/ml. Reactions were detected with TMB substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) and read at 630 nm (MRX II plate reader; Dynex Technologies, Billinghurst, United Kingdom).
Statistics.
Comparisons of data were tested for significance with Student's t test. (***, P < 0.001; **, P < 0.01; *, P < 0.05; nonsignificant, P > 0.05). Arithmetic means ± the standard error of the mean (SEM) are shown. The data shown are representative of three to eight different experiments.
RESULTS
RA larvae elicit inflammation of the exposure site.
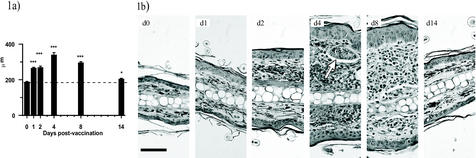
Immediately following exposure to RA larvae, the site of exposure increased in thickness, as measured with a gauge micrometer at autopsy (day 1; +41.9%; P < 0.001) and the thickness continued to increase to a peak on day 4 before declining to near baseline (naive) values by day 14 (Fig. 1a). Although shrinkage occurred following sample preparation, the increase in pinna thickness was also evident by histological analysis (Fig. 1b). Both the epidermis and dermis exhibited increased cellularity, particularly between days 2 and 8. An influx of cells into the dermis was apparent immediately after vaccination on either side of the central zone of cartilage and by day 4 had formed large foci, some surrounding parasites (e.g., day 4). However, by day 14, the inflammatory response had largely subsided, with only a residual cellular influx remaining.
FIG. 1.
Inflammation of the skin after exposure to RA larvae. (a) Pinna thickness at autopsy on days 1, 2, 4, 8, and 14 p.v. The data are means + SEM for 8 to 10 pinnae. (b) Micrographs showing inflammatory reactions through transverse sections of pinnae stained with hematoxylin and eosin. Scale bar, 50 μm. The arrow indicates the position of a parasite.
RA larvae stimulate a cascade of inflammatory cytokines and chemokines in dermal tissues.
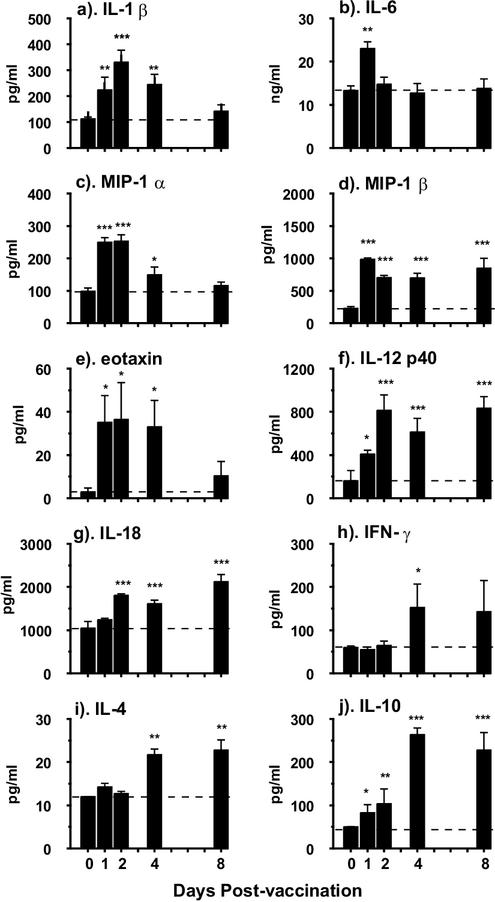
We first established that the pattern of cytokine production detected at 6 and 18 h after in vitro culture of pinnae did not differ, although quantitatively more of each cytokine was produced by the latter sampling time (data not shown); consequently, all of the data shown are for cytokine production at 18 h. Cytokine secretion is expressed per pinna, not on the basis of an arbitrary number of cells, since it is more relevant to consider the total amount of cytokine expressed at any particular time by the whole organ, which would influence the immune priming process. One of the first cytokines to be detected was IL-1β, which increased significantly by day 1 (P < 0.01) above the level in naive skin and peaked on day 2 (Fig. 2a). IL-1β was still elevated on day 4, although it declined to baseline levels by day 8. IL-6 was by far the most abundant cytokine detected, even in cultures from naive mice (Fig. 2b), but exposure to RA cercariae led to a transient increase on day 1. Highly elevated levels of MIP-1α were also detected immediately after vaccination on days 1 and 2, which were followed by a decline to the baseline on day 8 (Fig. 2c). Production of MIP-1β too increased rapidly after vaccination, but in this case, secretion remained elevated until day 8 (Fig. 2d). The levels of eotaxin were low at all time points, although they were significantly above the baseline until day 4 (P < 0.05; Fig. 2e).
FIG. 2.
Cytokine and chemokine production by in vitro-cultured pinnae at 18 h obtained from naive mice and on days 1,2, 4, and 8 p.v. Bars represent cytokine production in picograms or nanograms per milliliter, which is equivalent to the total per pinna. Data are means + SEM for four to six pinnae. Statistical significance is for data compared to naive values. Horizontal dashed line shows the level of cytokine production in naive mice.
Cytokines that are involved in the development of Th1 cell populations were notable constituents of dermal supernatants from vaccinated mice. Elevated levels of IL-12 p40 were recorded from day 1 (P < 0.05), and production increased to a plateau between days 2 and 8 (P < 0.001; Fig. 2f). We were unable to detect significant quantities of the p70 heterodimer because it is present at levels 1,000-fold less than that of the p40 molecule (S. J. Jenkins and A. P. Mountford, unpublished data) and commercial ELISA reagents are insufficiently sensitive to permit detection in our dermal supernatants. IL-18 was also produced at significantly increased levels (P < 0.001) between days 2 and 8 (Fig. 2g). In contrast, very low levels of IFN-γ were detected and only on days 4 and 8 (Fig. 2h). IL-4 secretion also remained at naive levels until days 4 and 8, when there was an approximately twofold increase (Fig. 2i; P < 0.01). Finally, IL-10, which may have an inhibitory effect on cellular reactivity in the skin, was detected as early as day 1 (P < 0.05), although peak values occurred later than for most other monokines between days 4 and 8 (P < 0.001; Fig. 2j).
IL-12 p40 secretion is sustained in vaccinated but not infected mice.
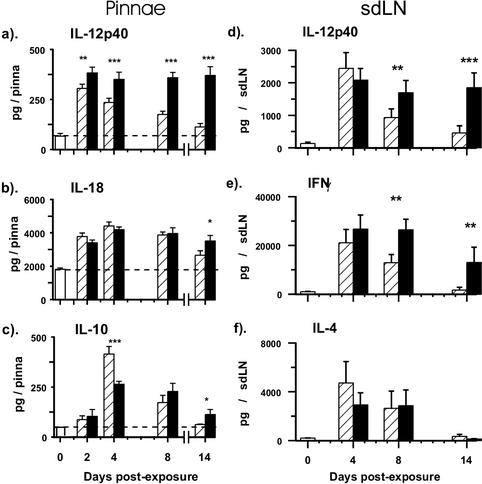
Analysis of a number of early-phase cytokines (e.g., IL-1β, IL-6, MIP-1α, and eotaxin) secreted by in vitro-cultured skin biopsy samples showed that there was little or no difference in the quantities detected by ELISA between mice exposed to RA or normal larvae (data not shown). The most obvious difference was in the sustained production of IL-12 p40 by pinnae from vaccinated mice, which was significantly higher than for infected pinnae at every time point (Fig. 3a; P < 0.01 and P < 0.001). Moreover, the production of IL-12 p40 by infected pinnae declined from a peak on day 2, such that on day 14, pinnae from vaccinated mice secreted more than three times the amount produced by infected skin. In contrast, the secretion of IL-18 by in vitro-cultured pinnae was not substantially different between infected and vaccinated mice between days 2 and 8; only on day 14 was the level of IL-18 slightly higher for vaccinated mice (P < 0.05; Fig. 3b). IL-10 was also secreted by skin biopsy samples from both infected and vaccinated mice, although its production lagged behind that of IL-12 p40 (Fig. 3c). In infected mice, peak values of IL-10 were detected on day 4 before declining to the baseline values by day 14. In comparison, vaccinated pinnae secreted less IL-10 on day 4 but its production persisted until at least day 8. Moreover, by day 14, the amount secreted by vaccinated pinnae was significantly higher than that produced by infected pinnae.
FIG. 3.
IL-12 p40 production by in vitro-cultured pinnae and sdLN cells is sustained in vaccinated mice compared to that in infected mice. Skin biopsy samples and sdLN cells were obtained from naive mice and on days 2, 4, 8, and 14 after exposure to RA (solid) or normal larvae (hatched). Production of IL-12 p40 (a), IL-18 (b), and IL-10 (c) by pinnae at 18 h and production of IL-12 p40 (d) by sdLN at 72 h were determined in the absence of added antigen. IFN-γ (e) and IL-4 (f) production at 72 h was determined after stimulation with parasite antigen. Data are means + SEM for four to six pinnae and five sdLN. Statistical significance of differences between vaccinated and infected mice is shown. The horizontal dashed line shows dermal cytokine production in naive mice.
A divergent pattern of cytokine secretion was also observed in the supernatants from in vitro-cultured cells from the sdLN of vaccinated mice compared to those of infected mice. Although early on (day 4), there was no difference in the quantities of IL-12 p40 produced by sdLN cells between the two groups, the production of abundant IL-12 was sustained on days 8 (P < 0.01) and 14 (P < 0.001) in the vaccinated group but not in the infected group (Fig. 3d). Antigen-stimulated production of IFN-γ by sdLN cells had a similar profile and remained highly elevated in the vaccinated group on days 8 and 14, while in the infected group, secretion of IFN-γ quickly declined and was hardly greater than naive levels by day 14 (Fig. 3e). Meanwhile, production of IL-4 declined rapidly from a peak on day 4 in both groups of mice. These data show that there is a strong Th1 bias in cytokine production in the sdLN of vaccinated mice.
Inflammation and IL-12 production by dermal cells is regulated by the presence of IL-10.
Since significant amounts of IL-10 were produced by skin biopsy samples from vaccinated mice, we examined its effect on inflammation and cytokine production in vaccinated IL-10−/− and IL-12 p40−/− mice. Inflammation of the skin on day 4, as judged by pinna thickness, was significantly greater in IL-10−/− mice than the increase in either WT (normal) or IL-12 p40−/− mice (P < 0.001; Fig. 4a). Secretion of IL-12 p40 by skin biopsy samples obtained from IL-10−/− mice was also significantly greater (P < 0.001) than that from pinnae of WT mice (Fig. 4b). Conversely, secretion of IL-10 by pinnae from IL-12 p40−/− mice was highly elevated over the level in WT mice (P < 0.001; Fig. 4d). The production of IL-18 was not significantly different in IL-12 p40−/− or IL-10−/− mice compared to that in WT cohorts (P > 0.05; Fig. 4c).
FIG. 4.
Dermal inflammation (a) and production of IL-12 p40 (b), IL-18 (c), and IL-10 (d) in WT (solid), IL-12 p40−/− (hatched), and IL-10−/− (cross-hatched) mice on day 4 p.v. Inflammation is expressed as the increase in pinna thickness in each group of mice. The horizontal dashed line shows pinna thickness or cytokine production in naive mice for each group of mice. Data are means ± SEM for four to six pinnae. Statistical significance of differences between WT and cytokine-deficient mice is shown.
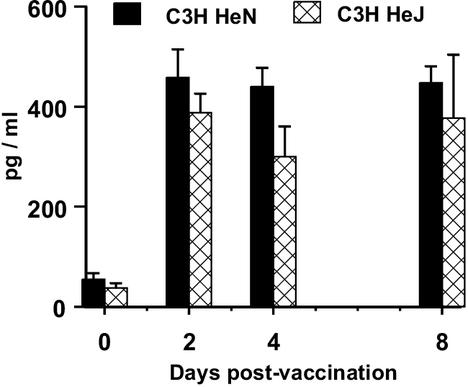
IL-12 production in response to RA larvae is not dependent on TLR-4.
IL-12 p40 secretion was not significantly lower in the culture supernatants from C3H/HeJ mice than that of their normal C3H/HeN cohorts on day 2, 4, or 8 (all P > 0.05; Fig. 5). No other cytokine tested differed between the two strains, and the pinnae were similar in thickness (data not shown). The mean worm burdens in vaccinated and challenged animals were 45.4 ± 4.2 for strain C3H/HeN and 40.2 ± 6.1 for strain C3H/HeJ, which were not significantly different (P > 0.05). Protective immunity was 63.2 and 66.7% for C3H/HeN and C3H/HeJ mice, respectively.
FIG. 5.
IL-12 p40 production by in vitro-cultured pinnae of vaccinated C3H/HeN (solid bars) and C3H/HeJ (cross-hatched bars) mice. Data are means + SEM for four to six pinnae. Values of the two strains are not statistically significantly different at any time point (P > 0.05).
Identification of IL-12 p40+ cells in skin tissue.
To identify the source of IL-12 in the skin, immunohistochemical analysis was used to identify the constituent cell types. The dermis of C57BL/6 mice contains melanin, as shown by small brown flecks in transverse cryosections of pinnae stained with a representative antibody isotype control (Fig. 6a). Cells positive for the neutrophil-specific marker 7/4 antigen (purple-black staining) were detected as early as day 2 p.v. adjacent to the epidermis (data not shown), but the peak influx was detected on day 4 (Fig. 6b). The density of CD11b+ cells in the cellular foci appeared less than for 7/4+ cells, and their distribution was more dispersed (Fig. 6c), while CD11c+ cells were rare (Fig. 6d). We also observed zones of IL-12+ cells in the dermis on day 4 (red-pink staining in Fig. 6e), which colocalized with the regions of greatest cellular infiltration. However, we were unable to accurately identify the phenotype of the individual IL-12 p40+ cells involved with peroxidase staining because of the heterogeneity and close proximity of the cells within the inflammatory foci. As an alternative, we opted to analyze cells that spontaneously emigrate from cultured pinnae.
FIG. 6.
Immunohistochemical analysis of cellular influx into pinnae on day 4 after vaccination. Micrographs of positive staining (Vector VIP; black-purple) counterstained with methyl green illustrated by a representative isotype control (a), the neutrophil marker 7/4 (b), the macrophage marker CD11b (c), and the DC marker CD11c (d). (e) Zones of IL-12 p40+ cells colocalize with the cellular influx in the dermis (pink-red; arrow). Melanin can be distinguished as a brown marking in the upper dermis; see panel a. Scale bars, 50 μm.
IL-12 p40+ cells in the dermal exudate population are CD11c+ and F4/80+.
Cells which detached from the pinnae during in vitro culture were taken to be indicators of those that contribute to dermal inflammation. They also indicate which types are capable of migrating to the sdLN. No enzymes were used to ensure that cell surface markers were not degraded.
By focusing on pinnae obtained on day 4 after vaccination (approximate peak of cell efflux), we identified three groups of cells in the exudate on the basis of size and granularity (Fig. 7a). Two populations, containing nearly 90% (16.1 and 71.6%) of the cells, were both classified as small but differed in their granularity; a third group comprising very large granular cells (LGC) accounted for only 11.0% of the total (Fig. 7a). While only 3.9% of the CD45+ cell population was IL-12+ (Fig. 7b and c), nearly 70% of them were within the LGC population (Fig. 7d). A smaller proportion (23%) of IL-12+ cells was in the small and granular population, while only 7.8% was in the highly abundant but small nongranular population (Fig. 7d). The flow cytometric scatter distribution of IL-12 p40+ cells based on their size and granularity is similar, regardless of the day after exposure (i.e., 2, 4, or 8; data not shown). Moreover, analysis of the results from several experiments (n = 2 to 4) showed that the percentage of all cells that were IL-12+ remained constant at all time points (day 2, 2.9% ± 0.4%; day 4, 3.4% ± 0.3%; day 8, 2.3% ± 0.7%). The proportions of LGC that were IL-12+ at different times were also very similar (day 2, 11.6% ± 0.6%; day 4, 12.2% ± 0.8%; day 8, 11.5% ± 1.2%). Because of the low number of cells recoverable from naive mice, it was not possible to accurately detect IL-12+ cells in the exudate population on day 0.
FIG. 7.
Flow cytometric analysis of dermal exudate cells. For a representative experiment, panel a illustrates the size (FS) and granularity (SS) of the total unstained exudate cell population on day 4 p.v. composed of three major groupings of cells; percentages define the proportions of cells within the three groups according to Quadstat analysis. An LGC population (circled) makes up 11% of the cells recovered. The total exudate population double stained for CD45 (FL3) with intracellular IL-12 p40 (FL2) is shown in panel c, and CD45 with an intracellular isotype control is shown in panel b. The distribution of IL-12 p40+ cells on the basis of their size and granularity is shown in panel d; percentages define the proportions of IL-12 p40+ cells located within the three groups of cells by Quadstat analysis.
Analysis of the IL-12+ cells that were present in the LGC population showed that most were Ia+ on days 2, 4, and 8 (Table 1). A large number of IL-12+ cells were also positive for CD11c or CD205, which is supportive of the hypothesis that most IL-12+ cells were DCs. Less than 2% of the IL-12+ cells in the LGC population were CD8α+, but nearly all (>95%) were CD11b+, demonstrating that the DCs were myeloid and not lymphoid (34). Further, we report that about two-thirds of the IL-12+ exudate population was positive for the macrophage marker F4/80; this molecule is present on some DCs from the skin (14), and in our study, most CD11c+ cells in the LGC region coexpressed F4/80 (80.3%; data not shown). However, approximately 40 to 50% of IL-12+ cells were CD11c− or CD205−. Since more than 95% of the IL-12+ cells were CD11b+ and most (63 to 74%) were F4/80+, the IL-12+ CD11c− population may represent mature macrophages.
TABLE 1.
Analysis of the surface of IL-12 p40+ cells in the LGC regiona
| Phenotype | Day 2 | Day 4 | Day 8 |
|---|---|---|---|
| Ia | 70.4 ± 3.1 | 77.1 ± 4.1 | 79.7b |
| CD11c | 58.5 ± 1.9 | 49.4 ± 5.2 | ND |
| CD205 | 60.1 ± 3.3c | 59.7 ± 0.5c | ND |
| CD8α | <2.0 | <2.0 | ND |
| CD11b | 96.4 ± 3.8 | 97.8 ± 4.1 | 94.7b |
| F4/80 | 63.3 ± 2.4 | 67.7 ± 3.7 | 74.4b |
The data shown are the mean percent positive ± SEM from three or four experiments. ND, not done.
n = 1.
n = 2.
DISCUSSION
Our studies document, for the first time, the inflammatory innate immune response and the cytokine profile in murine skin exposed to RA schistosome larvae. We highlight the role of IL-12 in the skin during the first 14 days, which is important in the development of Th1-type immune responses. Despite being the first site that encounters parasite antigen, the skin has not previously been considered an important organ involved in the initiation of immunity to schistosomes.
The influx of cells into the skin contributing to its increased thickness is liable to be initiated by the secretion of chemokines and cytokines detected immediately after vaccination. The earliest molecules to be detected are three CC chemokines, MIP-1α (CCL3), MIP-1β (CCL4), and eotaxin (CCL11), all of which are released at peak levels within 24 h of parasite exposure. MIP-1α and MIP-1β are attractants for monocytes, lymphocytes, and immature DCs into the skin (36, 38, 48), and while the former chemokine is only expressed transiently, production of the latter is maintained. Interestingly, both MIP-1α and MIP-1β have been linked to the development of Th1-type responses (10). These studies illustrate the rapidity of the host's response to parasite penetration forming the initial step in the inflammatory cascade leading to the transient production of acute-phase cytokines, such as IL-1β and IL-6. Such cytokines are known to recruit additional cells and direct the migration of some (e.g., Langerhans cells) out of the skin (9, 43). Indeed, the greatest production of chemokines and acute-phase cytokines occurs before the peak of cell infiltration observed on days 4 to 8. In addition, these mediators are potent activators of candidate APCs by increasing the expression of major histocompatibility complex class II and aid the production of IL-12 and IL-18.
One of the major findings of our studies is that the skin is an important site for the production of IL-12. The persistent production of IL-12 p40 in the skin until day 14 is clearly conducive to the promotion of IFN-γ-secreting Th cells present in the sdLN (26). Indeed, the absence of this molecule in IL-12 p40−/− mice leads to the development of Th2-type responses and a significant reduction in protective immunity (2). Although the p40 molecule detected by ELISA may be an antagonist homodimer (p40)2 (12), others have reported that p40 in the skin functions like the heterodimer (18). Another Th1-inducing cytokine, IL-18, is also detected in the dermal supernatants and is known to be produced by keratinocytes (37) and Langerhans cells (42). It can act in concert with IL-12 and other cytokines, such as IL-1β, tumor necrosis factor alpha, and IL-6, in the proinflammatory immune response (29). However, we show that IL-18 production is not diminished in IL-12 p40−/− mice, which do not to develop Th1-type responses (2). Moreover, the level of IL-18 production is similar in infected and vaccinated mice, reinforcing the conclusion that IL-18 does not play a major role in aiding the generation of Th1 cells in this model. The absence of significant secretion of IFN-γ in the skin appears to rules out this cytokine (and, by implication, natural killer cells as a possible source of early IFN-γ) as an important factor in priming for a Th1-type response. Instead, we conclude that sustained IL-12 p40 in the skin is an important feature in protectively vaccinated mice. Indeed, in mice exposed to normal (nonprotective) larvae, IL-12 production p40 declines rapidly after the first few days, correlating with the rapid exit of normal parasites from the skin (25). The transient production of IL-12 p40 in the skin is undoubtedly a major factor in the failure of infected mice to develop substantive and persistent populations of IL-12 p40+ APCs and IFN-γ+ CD4+ cells in the sdLN and hence the inability of these mice to develop a protective immune response.
One significant issue is whether IL-12 production is induced by the parasite itself or a bacterial contaminant that may enter the host's skin following cercarial penetration. LPS binding to TLR-4 is a major cause of IL-12 production by macrophages and DCs (1). In this respect, C3H/HeJ strain mice have a natural TLR-4 gene mutation and are unresponsive to LPS (31). However, the production of IL-12 p40 by in vitro-cultured pinnae from vaccinated mice is unaffected by the absence of TLR-4 and protective immunity is not reduced, indicating that TLR-4 is not responsible for the induction of IL-12 in our model.
Another important cytokine produced by skin biopsy samples is IL-10. One theory is that, in contrast to normal parasites, RA larvae in the skin fail to induce immunoregulatory prostaglandin E2 and IL-10 (32). Normal parasites are also known to stimulate the production of prostaglandin D2 and IL-7, which are implicated in the regulation of inflammatory immune responses (3, 46). Since prostaglandin E2 inhibits IL-12 production (40) via its effect on IL-10 (13), this could restrict the development of Th1-type responses. However, our data show that abundant IL-10 is secreted in response to RA larvae. IL-10 clearly has an important immunoregulatory effect since the skin of vaccinated IL-10−/− mice was more inflamed than normal (WT) and skin biopsy samples secreted much higher levels of IL-12 p40. Therefore, IL-10 appears to protect the host against an excess of IL-12 p40, which causes gross inflammation of the skin. We concluded that, in normal vaccinated mice, the quantities of IL-10 are sufficient to regulate dermal responses to an acceptable level but an excess, such as in IL-12 p40−/− mice, leads to no further dampening of inflammation. Since vaccinated IL-10−/− and IL-12 p40−/− mice develop highly polarized Th1- and Th2-type responses, leading to elevated and reduced levels of protection, respectively (2, 17), we conclude that the ability of RA larvae to induce Th1-type responses in normal (WT) mice reflects the induction of a fine balance between proinflammatory IL-12 and regulatory IL-10 in the skin shortly after exposure rather than simply a failure to induce IL-10.
A technically demanding but important aim of our studies has been to identify the type of cell in the skin that is responsible for IL-12 secretion. Small numbers of IL-12 p40+ cells were detected in the dermis, but it is not possible to identify them on the basis of surface marker expression because of the close apposition of different cell types within the foci. However, approximately 3% of the cells that emigrate from in vitro-cultured pinnae are IL-12 p40+ and although this value may be thought low, IL-12 is a very potent cytokine in vivo. Moreover, the significance of the IL-12+ dermal exudate population is that it comprises cells capable of migrating to the sdLN, where priming and differentiation of Th cells occur.
Neutrophils are among the most abundant cells in inflammatory foci, but although they can produce IL-12 (7), on a per-cell basis, neutrophils produce less than monocytes (8) and only a small number of IL-12 p40+ cells in the dermal exudate are detected within the small granular leukocyte (neutrophil) population. On the other hand, most IL-12+ cells are very large and granular (i.e., LGC) and are Ia+, which suggests that they are antigen-experienced DCs and/or macrophages. Indeed, although LGC represent only 11 to 12% of the total cell exudate, nearly 70% of IL-12+ cells occur within this population. DCs are potent producers of IL-12, particularly the CD8α+ lymphoid subset (15). However, we could not detect a CD8α+ population of CD11c+ DCs; rather, the CD11c+ DCs are CD11b+, which is supportive of their being myeloid DCs. In addition, a significant proportion of the CD11c+ cells in this study is positive for F4/80, which is present on many dermal exudate DCs (14). Therefore, although myeloid DCs are not widely accepted as a potent source of IL-12, the data presented here show that they represent the major population of IL-12 p40+ cells in the dermal exudate. Such cells could be migrating Langerhans cells from the epidermis since Langerhans cell-like DCs produce IL-12 in response to leishmania infection (41).
Our results also support a role for F4/80+ CD11c− macrophages in the skin as a source of IL-12. Indeed, macrophages stimulated by listeriae were among the first cell types identified as a source of IL-12 (35). However, it is not known whether IL-12 production is restricted to a specific type or class of macrophages. For example, reductive macrophages are better producers of IL-12 than are oxidative ones, which secrete IL-10 and IL-6 (28). Alternatively, the balance of IL-12 versus IL-10 may result from differences in the relative dominance of DCs versus macrophages should they be the primary sources of these two cytokines, respectively (16). The cellular source of the IL-10 in our model is not known, but it could derive from keratinocytes (32), F4/80+ Gr1+ suppressor macrophages (39), and/or regulatory T cells (5). Further studies on the dermal sources of this cytokine will provide an important insight into how cutaneous inflammation is controlled in this experimental model and how this may be manipulated to enhance the priming of the acquired immune response.
Acknowledgments
This work was supported by a Wellcome Trust University Fellowship to A.P.M. (grant 056213). S.K. was supported by a Ph.D. studentship awarded by the Royal Thai Government.
The following are thanked: R. Alan Wilson and Patricia S. Coulson for critical review of the manuscript and for advice on flow cytometry; George Pitchford and Niall MacDougall at Cookridge Hospital, Leeds, for access to the 60Co source; and Ann Bamford for maintaining the parasite life cycle.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S., V. L. Shires, R. A. Wilson, and A. P. Mountford. 1998. In the absence of IL-12, the induction of Th1-mediated protective immunity by the attenuated schistosome vaccine is impaired, revealing an alternative pathway with Th2-type characteristics. Eur. J. Immunol. 28:2827-2838. [DOI] [PubMed] [Google Scholar]
- 3.Angeli, V., C. Faveeuw, O. Roye, J. Fontaine, E. Teissier, A. Capron, I. Wolowczuk, M. Capron, and F. Trottein. 2001. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 193:1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkaid, Y., H. Jouin, and G. Milon. 1996. A method to recover, enumerate and identify lymphomyeloid cells present in an inflammatory dermal site: a study in laboratory mice. J. Immunol. Methods 199:5-25. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickle, Q., J. Bain, A. McGregor, and M. Doenhoff. 1979. Factors affecting the acquisition of resistance against Schistosoma mansoni in the mouse. III The failure of primary infections with cercariae of one sex to induce resistance to reinfection. Trans. R. Soc. Trop. Med. Hyg. 73:37-41. [DOI] [PubMed] [Google Scholar]
- 7.Bliss, S. K., B. A. Butcher, and E. Y. Denkers. 2000. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 165:4515-4521. [DOI] [PubMed] [Google Scholar]
- 8.Cassatella, M. A., L. Meda, S. Gasperini, A. D'Andrea, X. Ma, and G. Trinchieri. 1995. Interleukin-12 production by human polymorphonuclear leukocytes. Eur. J. Immunol. 25:1-5. [DOI] [PubMed] [Google Scholar]
- 9.Cumberbatch, M., R. J. Dearman, and I. Kimber. 1997. Stimulation of Langerhans cell migration in mice by tumour necrosis factor alpha and interleukin 1 beta. Adv. Exp. Med. Biol. 417:121-124. [DOI] [PubMed] [Google Scholar]
- 10.Dorner, B. G., A. Scheffold, M. S. Rolph, M. B. Huser, S. H. Kaufmann, A. Radbruch, I. E. Flesch, and R. A. Kroczek. 2002. MIP-1α, MIP-1β, RANTES, and ATAC/lymphotactin function together with IFN-γ as type 1 cytokines. Proc. Natl. Acad. Sci. USA 99:6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne, D. W., and A. P. Mountford. 2001. Resistance to infection in humans and experimental models, p. 133-212. In A. F. F. Mahmoud (ed.), Schistosomiasis. Imperial College Press, London, England.
- 12.Gately, M. K., D. M. Carvajal, S. E. Connaughton, S. Gillessen, R. R. Warrier, K. D. Kolinsky, V. L. Wilkinson, C. M. Dwyer, G. F. Higgins, Jr., F. J. Podlaski, D. A. Faherty, P. C. Familletti, A. S. Stern, and D. H. Presky. 1996. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann. N. Y. Acad. Sci. 795:1-12. [DOI] [PubMed] [Google Scholar]
- 13.Harizi, H., M. Juzan, V. Pitard, J. F. Moreau, and N. Gualde. 2002. Cyclooxygenase-2-issued prostaglandin e2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J. Immunol. 168:2255-2263. [DOI] [PubMed] [Google Scholar]
- 14.Henri, S., D. Vremec, A. Kamath, J. Waithman, S. Williams, C. Benoist, K. Burnham, S. Saeland, E. Handman, and K. Shortman. 2001. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167:741-748. [DOI] [PubMed] [Google Scholar]
- 15.Henri, S., J. Curtis, H. Hochrein, D. Vremec, K. Shortman, and E. Handman. 2002. Hierarchy of Susceptibility of dendritic cell subsets to infection by Leishmania major: inverse relationship to interleukin-12 production. Infect. Immun. 70:3874-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickman, S. P., J. Chan, and P. Salgame. 2002. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J. Immunol. 168:4636-4642. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, K. F., S. L., James, A. W., Cheever, and T. A. Wynn. 1999. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163:927-938. [PubMed] [Google Scholar]
- 18.Kopp, T., J. D. Kieffer, A. Rot, S. Strommer, G. Stingl, and T. S. Kupper. 2001. Inflammatory skin disease in K14/p40 transgenic mice: evidence for interleukin-12-like activities of p40. J. Investig. Dermatol. 117:618-626. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 20.Ma, X., and G. Trinchieri. 2001. Regulation of interleukin-12 production in antigen-presenting cells. Adv. Immunol. 79:55-92. [DOI] [PubMed] [Google Scholar]
- 21.Magram. J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 22.Mastin, A. J., Q. D. Bickle, and R. A. Wilson. 1983. Schistosoma mansoni: migration and attrition of irradiated and challenge schistosomula in the mouse. Parasitology 87:87-102. [DOI] [PubMed] [Google Scholar]
- 23.McKerrow, J. H., and J. Salter. 2002. Invasion of skin by Schistosoma cercariae. Trends Parasitol. 18:193-195. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 25.Mountford, A. P., P. S. Coulson, and R. A. Wilson. 1988. Antigen localization and the induction of resistance in mice vaccinated with irradiated cercariae of Schistosoma mansoni. Parasitology 97:11-25. [DOI] [PubMed] [Google Scholar]
- 26.Mountford, A. P., P. S. Coulson, R. M. Pemberton, L. E. Smythies, and R. A. Wilson. 1992. The generation of interferon-gamma-producing T lymphocytes in skin-draining lymph nodes, and their recruitment to the lungs, is associated with protective immunity to Schistosoma mansoni. Immunology 75:250-256. [PMC free article] [PubMed] [Google Scholar]
- 27.Mountford, A. P., K. G. Hogg, P. S. Coulson, and F. Brombacher. 2001. Signalling via interleukin-4 receptor alpha chain is required for successful vaccination against schistosomiasis in BALB/c mice. Infect. Immun. 69:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata, Y., T. Shimamura, and J. Hamuro. 2002. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int. Immunol. 14:201-212. [DOI] [PubMed] [Google Scholar]
- 29.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275-283. [DOI] [PubMed] [Google Scholar]
- 30.Pemberton, R. M., L. E. Smythies, A. P. Mountford, and R. A. Wilson. 1991. Patterns of cytokine production and proliferation by T lymphocytes differ in mice vaccinated or infected with Schistosoma mansoni. Immunology 73:327-333. [PMC free article] [PubMed] [Google Scholar]
- 31.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 32.Ramaswamy, K., P. Kumar, and Y. X. He. 2000. A role for parasite-induced PGE2 in IL-10-mediated host immunoregulation by skin stage schistosomula of Schistosoma mansoni. J. Immunol. 165:4567-4574. [DOI] [PubMed] [Google Scholar]
- 33.Riengrojpitak, S., S. Anderson, and R. A. Wilson. 1998. Induction of immunity to Schistosoma mansoni: interaction of schistosomula with accessory leucocytes in murine skin and draining lymph nodes. Parasitology 117:301-309. [DOI] [PubMed] [Google Scholar]
- 34.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 35.Skeen, M. J., M. A. Miller, T. M. Shinnick, and H. K. Ziegler. 1996. Regulation of murine macrophage IL-12 production: activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J. Immunol. 156:1196-1206. [PubMed] [Google Scholar]
- 36.Sozzani, S., W. Luini, A. Borsatti, N. Polentarutti, D. Zhou, L. Piemonti, G. D'Amico, C. A. Power, T. N. Wells, M. Gobbi, P. Allavena, and A. Mantovani. 1997. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J. Immunol. 159:1993-2000. [PubMed] [Google Scholar]
- 37.Stoll, S., G. Muller, M. Kurimoto, J. Saloga, T. Tanimoto, H. Yamauchi, H. Okamura, J. Knop, and A. H. Enk. 1997. Production of IL-18 (IFN-γ-inducing factor) messenger RNA and functional protein by murine keratinocytes. J. Immunol. 159:298-302. [PubMed] [Google Scholar]
- 38.Taub, D. D., K. Conlon, A. R. Lloyd, J. J. Oppenheim, and D. J. Kelvin. 1993. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science 260:355-358. [DOI] [PubMed] [Google Scholar]
- 39.Terrazas, L. I., K. L. Walsh, D. Piskorska, E. McGuire, and D. A. Harn, Jr. 2001. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1+ cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4+ cells: a potential mechanism for immune polarization in helminth infections. J. Immunol. 167:5294-5303. [DOI] [PubMed] [Google Scholar]
- 40.van der Pouw Kraan, T. C., L. C. Boeije, R. J. Smeenk, J. Wijdenes, and L. A. Aarden. 1995. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J. Exp. Med. 181:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Stebut, E., Y. Belkaid, B. V. Nguyen, M. Cushing, D. L. Sacks, and M. C. Udey. 2000. Leishmania major-infected murine Langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous leishmaniasis. Eur. J. Immunol. 30:3498-3506. [DOI] [PubMed] [Google Scholar]
- 42.Wang, B., C. Feliciani, B. G. Howell, I. Freed, Q. Cai, H. Watanabe, and D. N. Sauder. 2002. Contribution of Langerhans cell-derived IL-18 to contact hypersensitivity. J. Immunol. 168:3303-3308. [DOI] [PubMed] [Google Scholar]
- 43.Wang, B., P. Amerio, and D. N. Sauder. 1999. Role of cytokines in epidermal Langerhans cell migration. J. Leukoc. Biol. 66:33-39. [DOI] [PubMed] [Google Scholar]
- 44.Wheater, P. R., and R. A. Wilson. 1979. Schistosoma mansoni: a histological study of migration in the laboratory mouse. Parasitology 79:49-62. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, R. A. 1990. Leaky livers, portal shunting and immunity to schistosomes. Parasitology Today. 6:354-358. [DOI] [PubMed] [Google Scholar]
- 46.Wolowczuk, I., M. Delacre, O. Roye, S. L. Giannini, and C. Auriault. 1997. Interleukin-7 in the skin of Schistosoma mansoni-infected mice is associated with a decrease in interferon-gamma production and leads to an aggravation of the disease. Immunology 91:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wynn, T. A., A. Reynolds, S. James, A. W. Cheever, P. Caspar, S. Hieny, D. Jankovic, M. Strand, and A. Sher. 1996. IL-12 enhances vaccine-induced immunity to schistosomes by augmenting both humoral and cell-mediated immune responses against the parasite. J. Immunol. 157:4068-4078. [PubMed] [Google Scholar]
- 48.Ying, S., Q. Meng, L. T. Barata, and A. B. Kay. 2001. Macrophage inflammatory protein-1α and C-C chemokine receptor-1 in allergen-induced skin late-phase reactions: relationship to macrophages, neutrophils, basophils, eosinophils and T lymphocytes. Clin. Exp. Allergy 31:1724-1731. [DOI] [PubMed] [Google Scholar]
- 49.Yoshie, O., T. Imai, and H. Nomiyama. 2001. Chemokines in immunity. Adv. Immunol. 78:57-110. [DOI] [PubMed] [Google Scholar]