Abstract
Recent studies suggest that the toxicity of familial amyotrophic lateral sclerosis mutant Cu, Zn superoxide dismutase (SOD1) arises from its selective recruitment to mitochondria. Here we demonstrate that each of 12 different familial ALS-mutant SOD1s with widely differing biophysical properties are associated with mitochondria of motoneuronal cells to a much greater extent than wild-type SOD1, and that this effect may depend on the oxidation of Cys residues. We demonstrate further that mutant SOD1 proteins associated with the mitochondria tend to form cross-linked oligomers and that their presence causes a shift in the redox state of these organelles and results in impairment of respiratory complexes. The observation that such a diverse set of mutant SOD1 proteins behave so similarly in mitochondria of motoneuronal cells and so differently from wild-type SOD1 suggests that this behavior may explain the toxicity of ALS-mutant SOD1 proteins, which causes motor neurons to die.
Keywords: motor neuron, neurodegeneration, amyotrophic lateral sclerosis
In the familial form of ALS (fALS), which is linked to mutations in the Cu, Zn superoxide dismutase (SOD1) gene, it is generally considered that the pathological phenotype is because of the acquisition by the mutant SOD1 protein of new properties that transform it from a highly stable, dimeric antioxidant enzyme into a protein with a propensity to form toxic aggregates and cause oxidative damage to neuronal tissue (1). More than 100 different ALS-causing mutations in the SOD1 gene have been reported to date, and the properties of many of the fALS-linked mutant SOD1 proteins (mutSOD1s) have been studied (2–8) in a concerted effort to identify properties common to the mutant proteins but not shared by wild-type SOD1 (wtSOD1), which might explain their toxicity. Instead of similarities, however, biochemical and biophysical studies of the mutSOD1s have revealed a wide range of differences; in fact, some of the mutSOD1s have been found to be very similar to wtSOD1 in all of the properties that have been measured (1). For example, mutSOD1s with substitutions at or near the metal-binding region have altered metal-binding properties and a tendency to crystallize in filamentous structures, but other mutSOD1s with substitutions remote from the metal-binding region are very similar to wtSOD1 in their crystal structures (9–11). Likewise, the global stabilities of some of the metal ion-free mutSOD1 apoproteins are severely compromised, but others have apoproteins that are nearly identical to or even more stable than apo-wtSOD1 (7). Therefore, other abnormal properties of the mutant proteins must be sought to explain the toxicity of all of the mutSOD1s.
We reasoned that the common properties shared by the ALS-mutSOD1 proteins and not by wtSOD1 protein might only become apparent when the mutant proteins were studied within the cellular context and also, possibly, only within motoneuronal cells. We have therefore built a collection of inducible cell lines, derived from a mouse motoneuronal line (neuroblastoma × spinal cord-34; NSC-34) (12), which expresses a wide panel of mutSOD1s under control of the inducible Tet-On promoter. Our studies, reported here, indicate that each of those cell lines expressing a diverse range of mutSOD1s contain a significantly higher proportion of the cellular SOD1 protein associated with mitochondria and that the redox potential in that compartment has become significantly more oxidizing, relative to the cell lines expressing wtSOD1. These properties, which are held in common by all of the diverse mutSOD1s studied, may be the cause of their toxicity in ALS.
Results
MutSOD1s Show Diverse Properties with Respect to Superoxide Dismutase Activity and Propensities to Monomerize.
The 12 mutSOD1s that were chosen for the study are ones whose biophysical properties have been found to be highly diverse. Seven of the mutations are located at or near the metal-binding region: three with amino acid substitutions at either Cu (H46R and H48Q) or Zn (H80R) ligands, three (S134N, D124V, and D125H) with substitutions in the electrostatic loop, and one (G85R) that is known to lower substantially the metal-binding affinity. Five mutations remote from the metal-binding region were also chosen: one (A4V) with a substitution at the dimer interface, three (G37R, D90A, and G93A) in the β strands, and one (E133Δ) in the loop connecting two β strands. Each of these latter mutSOD1s in the metallated state has properties very similar to wtSOD1; however, the stabilities of their apoproteins differ widely. wtSOD1 and mutSOD1s show different immunoreactivities against commercial antibodies both in denaturing and reducing conditions (13) and nonreducing conditions (A.F., M.C., and M.T.C., unpublished work). Therefore, for this study, we made C-terminus myc-tagged (Fig. 1) and untagged versions (Fig. 6, which is published as supporting information on the PNAS web site) of the wtSOD1 and most of the mutants; where it was possible to assay both, the two sets of proteins showed similar behavior. In addition, the wild type was different from the mutSOD1s in both cases, encouraging us to believe that the myc tag is not influencing the observed behavior.
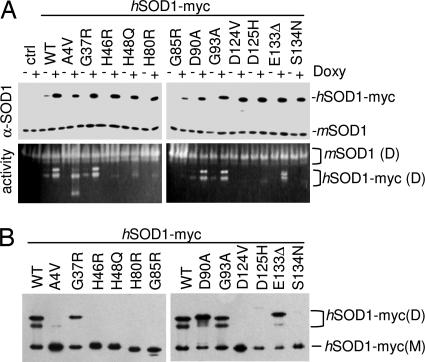
Fig. 1.
MutSOD1s expressed in motoneuronal cells show diverse properties with respect to superoxide dismutase activity and propensity to monomerize. (A) NSC-34-derived cell lines expressing myc-tagged human wtSOD1 or mutSOD1 were left untreated (−) or treated (+) for 48 h with 1 μg/ml of doxycycline to induce expression of transgenic SOD1. Cell lysates were subjected to reducing SDS/PAGE (Upper) or in-gel SOD1 activity assay (Lower). The anti-SOD1 antibody used for immunodetection recognizes both human (hSOD1-myc) and mouse SOD1 (mSOD1). Position of the bands corresponding to mSOD1 dimers (D) and hSOD1-myc dimers (D) are indicated. Untransfected NSC-34 cells were used as control. (B) NSC-34-derived cell lines were induced as in A, but the extracts were subjected to a mildly denaturing (0.1% SDS), nonreducing PAGE. Western blot analysis was performed with an anti-myc antibody. Position of the bands corresponding to hSOD1-myc dimers (D) and monomers (M) are indicated.
Under the conditions used in this study, i.e., inducible expression for 48 h, the mutSOD1s did not induce a marked death phenotype (data not shown), an increase in the cell level of oxidative stress (as determined by reaction with dihydroethidium; see Fig. 7, which is published as supporting information on the PNAS web site), or an imbalance in the total ratio reduced glutathione/oxidized glutathione (GSH/GSSG) (Table 1, which is published as supporting information on the PNAS web site). In total cell extracts, the mutSOD1s displayed a wide range of superoxide dismutase activities and propensities to monomerize, as had been seen in previous studies (8): Some of them exhibited behavior similar to that of wtSOD1 and retained near-to-full SOD activity and the typical two-banded pattern in the activity gel assay (14), whereas some readily lost SOD activity in nondenaturing conditions (Fig. 1A). Interestingly, a fraction of the native A4V mutSOD1 was observed to be present as a SOD-active monomer. The other mutSOD1s showed a marked tendency to form monomers in mild denaturing, nonreducing conditions (0.1% SDS), but they differed widely in this property also, with some very prone to monomerization and others (e.g., G93A) acting very much like the wild-type protein (Fig. 1B). Similarly diverse results were obtained when the accessibility of cysteine residues was assayed by modification of total cell extracts with polyethyleneglycol maleimide (malPEG). The accessibility of the cysteines to malPEG modification was found to be the same as that of wtSOD1 for some of the mutSOD1s (A4V, G37R, D90A, and G93A), whereas others (G85R, H46R, H48Q, H80R, S134N, D125H, and D124V) were found to be more reactive (Fig. 8, which is published as supporting information on the PNAS web site).
MutSOD1s Accumulated in the Mitochondrial Fraction of NSC-34 Cells Are Partially Oxidized and Thus Less Accessible to Modification by malPEG.
As had been reported both in vitro and in vivo in the brain and spinal cord of transgenic mice and in human spinal cord (15–20), we found that some of the SOD1 protein, wild type or mutant, was associated with the mitochondria, but we found further that the amount of SOD1 associated with the mitochondria fraction appeared to be much higher for all of the mutSOD1s (Fig. 2A and B). This observation was reproduced in several independent clones expressing either untagged or myc-tagged wtSOD1 and mutSOD1s (data not shown). A precise quantization of the mitochondrial fraction was made difficult by the fact that the level of expression of the proteins in different cell lines was similar but not identical and by the fact that we observed some variation among different experiments. Nonetheless, in Western blot experiments, a 10-fold excess of total protein loading was needed to get a very weak signal for wtSOD1in the mitochondrial fraction as compared with a strong signal observed for the wtSOD1 in the cytosolic fraction. For each of the mutSOD1s, the same excess loading of mitochondrial proteins yielded a signal comparable with that in total cytosolic proteins. A semiquantitative densitometric analysis indicates that a significantly greater fraction of the mutSOD1 is associated with the mitochondria relative to wtSOD1 (Fig. 2B).
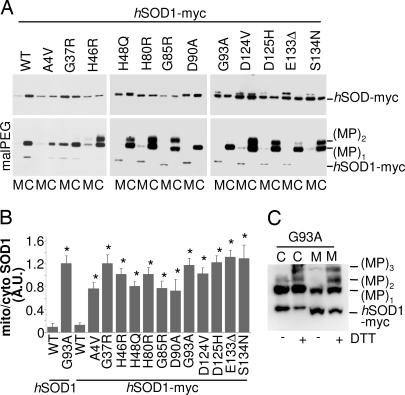
Fig. 2.
A much higher fraction of the mutSOD1s copurify with mitochondria of NSC-34 cells, relative to wtSOD1, and the mitochondrial SOD1 is partially oxidized and thus inaccessible to modification by malPEG. (A) NSC-34-derived cell lines were treated for 48 h with 1 μg/ml of doxycycline. Lysates from mitochondrial (M) and cytosolic (C) fractions were analyzed in Western blot for SOD1 expression with an anti-myc antibody (half of the sample; Upper) or subjected to malPEG modification and then analyzed in Western blot with the same antibody (other half of the sample; Lower). One-half microgram of cytosolic proteins was loaded to obtain levels of SOD1s similar to that from 5 μg of mitochondria proteins in both Upper and Lower, but a short exposure was chosen for Lower to help appreciate the pattern of MP bands. Note that the anti-myc antibody recognizes malPEG-modified SOD1s more efficiently than the unmodified form. (B) Histogram of the mean ratio ± SD of mitochondrial versus cytosolic SOD1s levels (expressed in densitometric arbitrary units) in NSC-34-cells as determined in four independent Western blot experiments. Values significantly different from the relative wtSOD1 (both myc-tagged and untagged) are indicated with an asterisk when P < 0.01. (C) NSC-34 cells expressing the mutSOD1 G93A-myc were treated for 48 h with doxycycline. Lysates from cytosolic (C) and mitochondrial (M) fractions were incubated for 16 h in the absence or in the presence of 2 mM DTT, treated with malPEG, and then subjected to SDS/PAGE and Western blot with an anti-myc antibody.
Surprisingly, we also found that the SOD1 protein in the mitochondrial fraction differed from the bulk of the SOD1 protein in the cytosolic fraction in that it was less reactive with malPEG. Quantization of the amount of malPEG-modifiable SOD1 was made difficult by the fact that malPEG modification produced both size shifts in the SOD1 protein and, at times, enhanced the intensity of the immunoreactive bands on immunoblots (Fig. 2A Lower). Nonetheless, the intensity of the band indicated as (MP)1, corresponding to SOD1 modified with one malPEG, was usually less intense in protein associated with mitochondria than in the corresponding cytosolic sample (Fig. 2A Lower and Fig. 9,which is published as supporting information on the PNAS web site). Treatment of both cytosolic and mitochondrial fractions with DTT before malPEG treatment significantly increased the reactivity of SOD1 to this reagent (Fig. 2C), suggesting the presence of oxidized cysteines in SOD1 in these preparations.
Cys-111 Is Necessary for Association with Mitochondria of C6F mutSOD1.
Human SOD1 has four cysteine residues that have different reactivities: Cys-111, which is exposed on the protein surface near the dimer interface, is expected to be the most prone to modification with malPEG, followed by Cys-6, which is packed tightly within the interior of the β barrel. By contrast, Cys-57 and Cys-146, which are involved in the disulfide bridge, are expected to be unreactive to malPEG unless the SOD1 core is disrupted and the disulfide bridge reduced (21, 22), and, indeed, we barely detected bands corresponding to (MP)3 and (MP)4 for a few of the mutSOD1s under the conditions used in this study. We analyzed the malPEG reactivities of mutSOD1s C111S and C6F. As shown in Fig. 10A, which is published as supporting information on the PNAS web site, C6F mutSOD1 is modified by malPEG despite the absence of Cys-6, but removal of the Cys-111 residue in C111S greatly lowered reactivity with malPEG, suggesting that Cys-111 is a primary site of malPEG modification.
We also observed that the fraction of C111S mutSOD1 associated with mitochondria was similar to that of wtSOD1, whereas C6F mutSOD1 was similar to G93A mutSOD1 in relative association with mitochondria (Fig. 3). Interestingly, the double mutant C6F/C111S SOD1 also did not copurify with mitochondria of NSC34 cells (Fig. 3) further implicating Cys-111 as a mediator of association with mitochondria. Thus human wtSOD1 appears to be unique in that it contains Cys-111, yet it is less abundant in the mitochondrial fraction than mutSOD1.
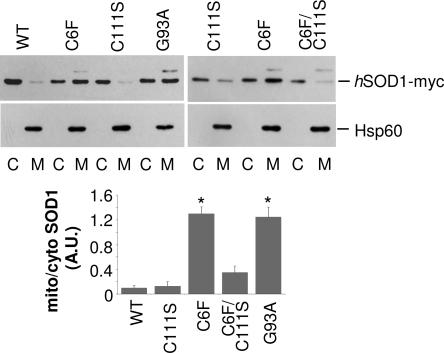
Fig. 3.
Oxidation of Cys-111 is necessary for C6F mutSOD1 association with mitochondria. NSC-34 cell lines expressing myc-tagged hSOD1s (WT, C6F, C111S, and the double SOD1 mutant C6F/C111S) were treated for 48 h with 1 μg/ml of doxycycline. Lysates from mitochondrial (M) and cytosolic (C) fractions were analyzed in Western blot with an anti-myc antibody (Top) or with an anti-Hsp60 antibody to check for equal loading of mitochondrial fractions (Middle). One-half microgram of cytosolic proteins was loaded to obtain levels of SOD1s similar to that from 5 μg of mitochondria proteins. Histogram of the mean ratio ± SD of mitochondrial versus cytosolic SOD1s levels in NSC-34-cells as determined in three independent Western blot experiments. Values significantly different from wtSOD1 are indicated with an asterisk when P < 0.01 (Bottom).
We also forced wtSOD1 and mutSOD1 G93A to reach the mitochondria in motoneuronal cells by fusion with a proper import peptide (see Supporting Text, which is published as supporting information on the PNAS web site). Because mitochondria-targeted mutSOD1 is quite toxic for cells (23), we could obtain only cell lines with low-level expression of the mutant protein (Fig. 10B Left). Both proteins were found to be virtually absent in the cytosol (Fig. 10B Right). The mitochondria-targeted G93A mutSOD1 was observed to be largely unreactive with malPEG, whereas mito-wtSOD1 appeared to be more reactive (Fig. 10B Right). It is notable that there is little evidence of malPEG reactivity with mouse SOD1 in cytosolic fractions, reinforcing the potential role of Cys-111 in malPEG reactivity; murine SOD1 encodes Ser at codon 111. Together, these results provide additional evidence for the role of Cys-111 in the reactivity of human SOD1 to malPEG and for the idea that mutSOD1 in mitochondrial fractions is inherently less reactive to malPEG.
Oxidized mutSOD1s Associated with Mitochondria Impair Mitochondrial Function and Form Cross-Linked Oligomers That Disappear upon Thiol Reduction.
Because of its well known role in redox homeostasis, the couple GSH/GSSG is a good candidate for performing the oxidation of mutSOD1 in vivo (24). GSSG is virtually absent in the cytoplasm of both neuronal and nonneuronal cells in the absence of stress; however, we have ascertained that mitochondria of motoneuronal NSC-34 cells do contain detectable levels of GSSG, with the ratio GSH/GSSG dramatically decreased (5:1) relative to HEPG2 liver cells (where GSSG is undetectable) or other neuronal or nonneuronal cells (10:1 in SH-SY5Y neuroblastoma cells and 20:1 in N9 microglia cells). This property of motoneurons may help to explain their vulnerability in ALS: If mitochondria of motoneurons (at variance with those of any other cell type) are a microenvironment more favorable to cysteine oxidation than (any) other cell compartment, they could be the only site where accessible cysteines become oxidized, possibly causing formation of oligomers in mitochondria.
MutSOD1s themselves may contribute to creating a prooxidant environment, because expression of many of the mutSOD1s was found to shift the GSH/GSSG ratio toward the oxidized form in mitochondria of motoneuronal cells, irrespective of the basal level (Fig. 4A and Table 2, which is published as supporting information on the PNAS web site). This effect was less pronounced or absent for at least two of the mutations typical of mild forms of fALS (H46R and D90A) (and therefore seems to correlate with severity of symptoms) and be completely absent for C111S and the C6F/C111S double mutant, which did not accumulate in the mitochondrial fraction; C6F mutSOD1 behaves like most of mutSOD1s (Fig. 4B). As a confirmation that cysteine residues of SOD1 are specifically sensitive to modification of the redox environment created by the couple GSH/GSSG, preincubation of the recombinant purified G93A and H46R mutSOD1s with the oxidized form of glutathione (GSSG) prevented their derivatization by malPEG, whereas preincubation with the reduced form of glutathione (GSH) facilitated derivatization relative to control (Fig. 11A Right, which is published as supporting information on the PNAS web site). Furthermore, treatment of mutSOD1 with hydrogen peroxide in vitro did not interfere with cysteine accessibility (Fig. 11A Left), suggesting that oxidative modification of Cys residues, rather than some other type of oxidation, is involved in modification of mutSOD1s.
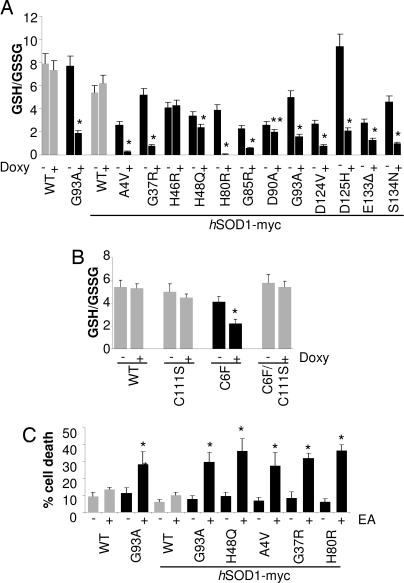
Fig. 4.
Association of oxidized mutSOD1s with mitochondria decreases reduced/oxidized glutathione ratio (GSH/GSSG). (A) Mitochondrial GSH/GSSG ratio in NSC-34 cell lines uninduced (−) or induced (+) for the expression of the various hSOD1s. (B) Mitochondrial GSH/GSSG ratio in NSC-34 expressing C111S, C6F, or C6F/C111S mutSOD1s. (C) Cell death was measured by trypan blue dye exclusion in induced NSC-34-derived cell lines treated with 50 μM ethacrynic acid for 1 h. Values significantly different from the relative control are indicated with an asterisk when P < 0.01.
In line with our observations, and supporting the concept that mitochondria levels of GSSG are crucial in establishing the toxic phenotype, if GSH was lowered in mitochondria by acute treatment with ethacrynic acid (Fig. 11B), one-third of neuronal cells expressing any of the mutSOD1s analyzed were induced to die, whereas cells expressing wtSOD1 were unaffected by the treatment (Fig. 4C). Acute depletion (1 h) of cytoplasmic GSH with l-buthionine-(S,R)-sulfoximine (BSO) had no effect on mitochondria GSH level (Fig. 11B), indicating that the exchange between the mitochondria glutathione pool and the cytosol is slow.
Degeneration of mitochondria, which are the main site of generation of reactive oxygen species (ROS) in the cell, has been strongly implicated in the mechanism of motoneuron death (25). In our motoneuronal model, part of the mutSOD1s associated with the mitochondrial fraction was observed to form disulfide cross-linked oligomers (Fig. 5A), in analogy to what was observed in vitro (6). Furthermore, cross-linked mutSOD1s disappeared on treatment with 2-mercaptoethanol, an additional indication that oxidative modification of their Cys residues occurs in mitochondria.
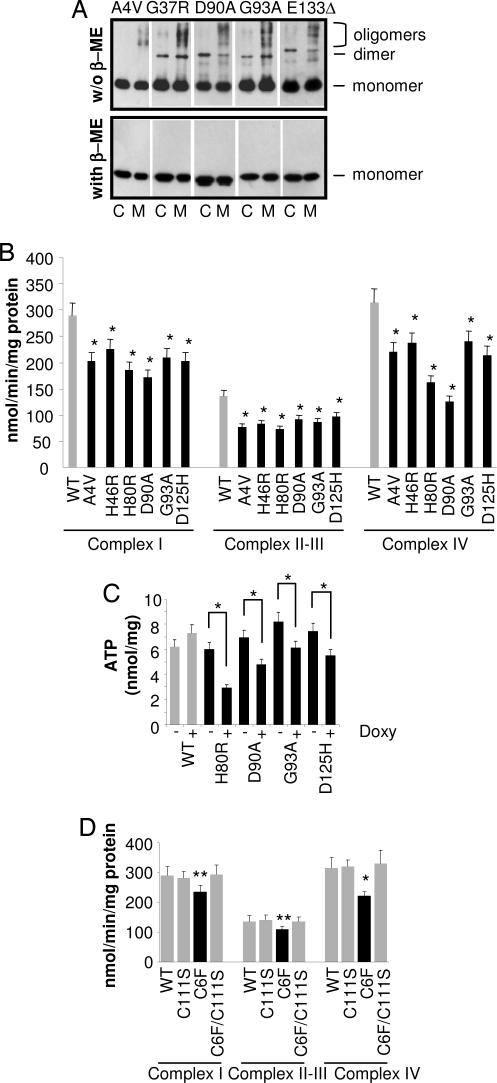
Fig. 5.
Oxidized mutSOD1s associated with mitochondria form disulfide cross-linked oligomers and impair mitochondrial function. (A) Cytosolic (C) and mitochondrial (M) fractions from induced NSC-34 cells expressing the indicated SOD1 were lysed and subjected to a denaturing PAGE, either nonreducing (without 2-mercaptoethanol, β-ME; Upper) or reducing (with β-ME; Lower). One-half microgram of cytosolic proteins was loaded to obtain levels of SOD1s similar to that from 5 μg of mitochondria proteins. Western blot analysis was performed with an anti-myc antibody. Positions of the bands corresponding to hSOD1-myc monomers, dimers, and oligomers are indicated. (B) Activities of electron transport chain complexes I, II-III, and IV were measured by spectrophotometric assays on the crude mitochondrial fraction from doxycycline-treated NSC-34-derived cells. Activities are normalized for protein content. (C) ATP content in NSC-34 cells uninduced (−) or induced (+) for the expression of the various hSOD1s. Values significantly different from the relative control are indicated with an asterisk when P < 0.01. (D) Activities of electron transport chain complexes I, II-III, and IV were measured by spectro-photometric assays on the crude mitochondrial fraction from doxycycline-treated NSC-34-derived cells expressing wild type, C6F, C111S SOD1, and the double SOD1 mutant C6F/C111S. Values significantly different from the relative control are indicated with an asterisk when P < 0.01 and with two asterisks when P < 0.05.
All mutSOD1s analyzed were found to induce primary mitochondrial damage as evidenced by impairment of respiratory complexes (Fig. 5B and Fig. 12 A and B, which is published as supporting information on the PNAS web site) and a decrease in ATP content that depends on mutSOD1 level of expression (Figs. 5C and 12C), in line with the notion that mitochondria are an early site of damage in both familial and sporadic ALS and represent a pathological hallmark in ALS (26, 27). Again, C111S mutSOD1 and C6F/C111S double mutant SOD1 did not affect mitochondria metabolism (Fig. 5D).
Discussion
Several studies have reported metabolic and morphological alterations of mitochondria in a variety of experimental models of SOD1-linked fALS and in ALS patients as well (17, 26, 27). Moreover, there is considerable evidence of elevated oxidative damage in mouse models of SOD1-linked ALS and in patients (28), and malfunctioning mitochondria might explain such damage. A small fraction of the wtSOD1, and the mutSOD1s, associates with various mitochondria compartments both in vitro and in vivo in the brain and spinal cord of transgenic mice and in human spinal cord (15–20). The mechanisms by which mutSOD1s association with mitochondria affects cell viability are only partly understood. Pasinelli et al. (19) have reported evidence that both wtSOD1 and mutSOD1 bind the antiapoptotic protein Bcl-2 in ALS models and patients, and it is known that cytochrome c in the brain of G93A-SOD1 mice has a reduced association with the inner mitochondrial membrane (29). In other studies, it has been reported that aberrant macromolecular aggregates of mutSOD1 accumulate in the mitochondria matrix of brain but not in the liver (16). However, little was known about the properties of either wtSOD1 or mutSOD1 associated with the mitochondria.
As described, when we were looking at total cellular SOD1, most of the properties of the mutSOD1s that we studied within motoneuronal cells in culture were found to be highly diverse (Table 3, which is published as supporting information on the PNAS web site). By contrast, however, when we compared the properties of the SOD1s in the mitochondrial versus the cytosolic fractions, we identified properties of the mutSOD1s that were strikingly similar to each other and different from those of wtSOD1. In particular, the mutSOD1s analyzed tended to associate with the mitochondria and form cross-linked oligomers and aggregate, in analogy to what has been observed in vitro (6, 30), and they induced a primary mitochondria damage in terms of net impairment of respiratory complexes and decrease in ATP content. Thus, despite their different properties in the cytosolic reducing environment, all mutSOD1s found associated with the mitochondria behaved similarly.
As discussed by Wang et al. (8, 31), the tendency to aggregate of mutSOD1s is very likely explained by the occurrence of interactions between specific domains that are exposed only when nonnative structures are adopted in misfolded monomers. ALS-related mutations may increase the burden of partially unfolded SOD1 species in vivo because of impaired metal ion-binding, abnormal hydrophobic behavior (30), or aberrant covalent modification such as oxidative damage. Alteration of cellular redox balance could also cause reduction of the intrasubunit disulfide bond in mutSOD1s (32) and thus profoundly affect the biochemistry and the structural features of mutSOD1s. Indeed, recent experiments (carried out exclusively in vitro) demonstrate a connection between the redox state of cysteine residues and alterations of quaternary structure and aggregation of SOD1 (6, 33).
It has been suggested that mitochondrial mutSOD1 acquires a conformation different from wtSOD1; for instance, the fact that mutSOD1 G93A, which is resistant to proteinase K digestion elsewhere, exhibits partial sensitivity to this enzyme in the mitochondria matrix, where it forms age-dependent aggregates (16), suggests the existence of a biochemical difference between wtSOD1 and mutSOD1s in this mitochondrial compartment. However, whether these aggregates are the cause or the consequence of mitochondrial dysfunction associated with mutant SOD1 was not known, and the role of the complex compartmentalization of mutSOD1 in the pathogenesis of fALS remained to be determined.
Our data suggest that the mechanism underlying the mutSOD1 accumulation in the mitochondrial fraction is mediated by cysteine residues. The observation that treatment with DTT caused the cysteines in mitochondrial mutSOD1 to become more accessible to modification by malPEG suggests that normally accessible cysteine residues have become oxidized. The evidence that mitochondrial mutSOD1 oligomers disappeared under reducing condition further indicates that oxidation of cysteines residues is involved in mutSOD1 aggregation in vivo.
As mentioned earlier, human SOD1 has four cysteine residues that have different accessibilities, with Cys-111, exposed on the protein surface near the dimer interface, being the most reactive. Contrary to what was observed for the other mutSOD1s analyzed, the Cys-111 mutant was less abundant in mitochondrial fractions: Its level was more similar to that of wtSOD1. Removal of the Cys-111 residue from C6F mutSOD1 prevented its association with the mitochondria, suggesting a role for Cys-111 in the mechanism of human mutSOD1 localization to mitochondrial fractions. Alternatively, removal of two cysteine residues (Cys-111 and Cys-6) may suppress oligomerization of mutSOD1 by reducing the protein's ability to form intermolecular disulfide bonds, which could be essential for mutSOD1 association with mitochondrial fractions; substitution of one Cys residue only (as in the C6F mutSOD1) did not significantly reduce the ability to associate with mitochondria. On the other hand, Cys-111 (which is the least conserved residue among the four cysteines of human SOD1) is absent in mouse SOD1, and mice that express murine SOD1-G85R do develop ALS-like symptoms (34). Moreover, a mutation in Cys-111 has been found in one human ALS case, but it is not clear whether this mutation is a single isolated variant or is causative in ALS because there is no data on familial inheritance. Therefore, although our data implicate Cys-111 as a key mediator of mitochondrial association and subsequent dysfunction, it is unclear whether Cys-111 mediates all aspects of toxicity.
The fact that alteration of the GSH/GSSG ratio in the mitochondria and impairment of the respiratory chain are rescued in NSC-34 cells expressing C6F/C111S mutSOD1, which did not associate with mitochondria, clearly suggests a strong correlation between mitochondrial localization and a toxic property of mutSOD1. It is possible that mutant SOD1 toxicity involves injury to multiple cellular compartments and that Cys-111 is a key mediator of injury to mitochondria.
Because of the significant level of GSSG in the mitochondria of our motoneuronal cells, we hypothesize that formation of cross-linked mutSOD1s is because of aberrant reactivity of cysteines driven by the more oxidizing redox environment of these mitochondria. Previous data support the idea that mitochondrial glutathione levels are related to mutSOD1 toxicity. The acetylated variant of the amino acid l-cysteine is able to restore viability and ATP production in human neuroblastoma cells expressing mutSOD1 G93A (35) and to prolong survival of G93A transgenic mice (36). Therefore, this result is probably related not only to the well known antioxidant activity of the acetylated variant of the amino acid l-cysteine, but also to the fact that this drug is an excellent source of sulfhydryl groups and in vivo is converted into metabolites capable of stimulating glutathione synthesis.
On the whole, our data suggest that an important toxic property of most mutSOD1s derives from their association with the mitochondria, and that this association is because of glutathione-mediated modification of cysteine residues, causing mutSOD1s to accumulate in a oxidized, aggregate state. Further, the presence of mutSOD1s leads to impairment of the respiratory chain and a shift in the mitochondrial redox balance (GSH/GSSG ratio) toward more oxidizing. Understanding the mechanism of association of mutSOD1 with mitochondria should help to develop methods to prevent this phenomenon to decrease toxicity in patients.
Materials and Methods
Detailed experimental procedures are provided in Supporting Text.
Cell Lines.
Mouse motoneuronal cell line NSC-34 (a gift of N. R. Cashman, University of Toronto, Toronto, ON, Canada; see ref. 12) is a hybrid NSC cell line that is considered the best stable motoneuronal cell line model system available (see Supporting Text). This line was stably transfected with the pTet-ON plasmid (Clontech, Palo Alto, CA) coding for the reverse tetracycline controlled transactivator (rtTA), by using lipofectamine reagent (Invitrogen, Paisley, U.K.) according to the manufacturer's guideline. Single clones were isolated after 3 weeks of selection with 400 μg/ml G418 (Gibco, Paisley, U.K.) and screened according to their ability to control the expression of an inducible reporter gene (pTRE-luc, Clontech). The line designed NSC-34-ON7, which displays a very low level of basal expression and high inducibility, was used to construct inducible cell lines expressing the cDNAs encoding human wtSOD1 or 1 of the 12 human fALS-typical mutSOD1s inserted in the pTRE2 or pTRE2–5myc plasmids. To allow the selection, we cotransfected the plasmid pTK-Hygro (Clontech). Multiple clones for each construction, isolated after selection with 400 μg/ml Hygromicin-B (Invitrogen), were analyzed by Western blot, and clones with similar level of expression were chosen. Stable clones expressing mitoSOD1 (wild type or G93A) were obtained by transfection of NSC-34 cells with pCMV-mito-hSOD1 wt/G93A plasmids and selection with 400 μg/ml Hygromicin-B. Conditions for growth and induction of cell cultures are reported in Supporting Text.
Subcellular Fractionation and Mitochondria Purification.
Mitochondria were isolated from NSC-34-derived cell lines as reported by ref. 15 with some modification (see Supporting Text).
Detection of Accessible Cysteines in SOD1.
Cytosolic and mitochondrial fractions were resuspended in mitochondrial buffer containing 0.1% SDS. malPEG (molecular mass of 5 kDa; NEKTAR, San Carlos, CA) was then added to a final concentration of 3 mM for covalent modification of accessible cysteines for 1 h at 25°C. The addition of malPEG to accessible cysteines increases the subunit mass of SOD1 by ≈5 kDa per modification. SDS/PAGE loading buffer containing 5% 2-mercaptoethanol was then added. Samples were boiled at 100°C for 3 min, separated by denaturing SDS/PAGE, and subject to Western blot analysis as described.
Determination of Glutathione Content.
Determination of intracellular glutathione was performed in HPLC as described (37).
Statistical Analysis.
The results are presented as means ± SD of n ≥ 3 independent experiments. Statistical evaluation was conducted by a simple Student's t test, and values significantly different from the relative control are indicated with an asterisk when P < 0.01 and with two asterisks when P < 0.05.
Supplementary Material
Acknowledgments
We thank Carla Russo, Laura Biondini, and Ilaria Amori for invaluable support in the construction of NSC-34-derived cell lines and Lilia Calabrese and Andrea Battistoni for critical reading of the manuscript. This work was supported by Telethon Grant GGP030066 (to M.T.C.), Ministero della Salute Ricerca Finalizzata Malattie Neurodegenerative and Progetto Finalizzato “Approcci neuroprotettivi nel danno da deprivazione energetica” (M.T.C.), and National Institutes of Health Grants DK46828 and GM28222 (to J.S.V.).
Abbreviations
- fALS
familial ALS
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- malPEG
maleimide-polyethylene glycol
- NSC
neuroblastoma x spinal cord
- SOD1
Cu, Zn superoxide dismutase
- mutSOD1
mutant SOD1
- wtSOD1
wild-type SOD1.
Note.
Deng et al. (38) have published a paper that supports a role for disulfide-linked SOD1 multimerization in mitochondria of mouse models of ALS.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Valentine JS, Doucette PA, Potter SZ. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 2.Hayward LJ, Rodriguez JA, Kim JW, Tiwari A, Goto JJ, Cabelli DE, Valentine JS, Brown RH., Jr J Biol Chem. 2002;277:15923–15931. doi: 10.1074/jbc.M112087200. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez JA, Valentine JS, Eggers DK, Roe JA, Tiwari A, Brown RH, Jr, Hayward LJ. J Biol Chem. 2002;277:15932–15937. doi: 10.1074/jbc.M112088200. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg MJ, Tibell L, Oliveberg M. Proc Natl Acad Sci USA. 2002;99:16607–16612. doi: 10.1073/pnas.262527099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindberg MJ, Bystrom R, Boknas N, Andersen PM, Oliveberg M. Proc Natl Acad Sci USA. 2005;102:9754–9759. doi: 10.1073/pnas.0501957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa Y, O'Halloran TV. J Biol Chem. 2005;280:17266–17274. doi: 10.1074/jbc.M500482200. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez JA, Shaw BF, Durazo A, Sohn SH, Doucette PA, Nersissian AM, Faull KF, Eggers DK, Tiwari A, Hayward LJ, Valentine JS. Proc Natl Acad Sci USA. 2005;102:10516–10521. doi: 10.1073/pnas.0502515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Hum Mol Genet. 2003;12:2753–2764. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- 9.Elam JS, Taylor AB, Strange R, Antonyuk S, Doucette PA, Rodriguez JA, Hasnain SS, Hayward LJ, Valentine JS, Yeates TO, Hart PJ. Nat Struct Biol. 2003;10:461–467. doi: 10.1038/nsb935. [DOI] [PubMed] [Google Scholar]
- 10.Hough MA, Grossmann JG, Antonyuk SV, Strange RW, Doucette PA, Rodriguez JA, Whitson LJ, Hart PJ, Hayward LJ, Valentine JS, Hasnain SS. Proc Natl Acad Sci USA. 2004;101:5976–5981. doi: 10.1073/pnas.0305143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonyuk S, Elam JS, Hough MA, Strange RW, Doucette PA, Rodriguez JA, Hayward LJ, Valentine JS, Hart PJ, Hasnain SS. Protein Sci. 2005;14:1201–1213. doi: 10.1110/ps.041256705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Dev Dyn. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara N, Miyamoto Y, Ogasahara K, Takahashi M, Ikegami T, Takamiya R, Suzuki K, Taniguchi N. J Biol Chem. 2005;280:5061–5070. doi: 10.1074/jbc.M406106200. [DOI] [PubMed] [Google Scholar]
- 14.Steinkuhler C, Sapora O, Carri MT, Nagel W, Marcocci L, Ciriolo MR, Weser U, Rotilio G. J Biol Chem. 1991;266:24580–24587. [PubMed] [Google Scholar]
- 15.Okado-Matsumoto A, Fridovich I. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 16.Vijayvergiya C, Beal MF, Buck J, Manfredi G. J Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. J Biol Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 18.Higgins CM, Jung C, Ding H, Xu Z. J Neurosci. 2002;22:RC215. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Lillo C, Jonsson PA, Vande Velde C, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, et al. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari A, Hayward LJ. J Biol Chem. 2003;278:5984–5992. doi: 10.1074/jbc.M210419200. [DOI] [PubMed] [Google Scholar]
- 22.Arnesano F, Banci L, Bertini I, Martinelli M, Furukawa Y, O'Halloran TV. J Biol Chem. 2004;279:47998–48003. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi H, Kobayashi Y, Ishigaki S, Doyu M, Sobue G. J Biol Chem. 2002;277:50966–50972. doi: 10.1074/jbc.M209356200. [DOI] [PubMed] [Google Scholar]
- 24.Schafer FQ, Buettner GR. Free Radical Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 25.Andersen JK. Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 26.Bendotti C, Carrì MT. Trends Mol Med. 2004;10:393–400. doi: 10.1016/j.molmed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, Jung C, Higgins C, Levine J, Kong J. J Bioenerg Biomembr. 2004;36:395–399. doi: 10.1023/B:JOBB.0000041774.12654.e1. [DOI] [PubMed] [Google Scholar]
- 28.Valentine JS. Free Radical Biol Med. 2002;33:1314–1320. doi: 10.1016/s0891-5849(02)01080-8. [DOI] [PubMed] [Google Scholar]
- 29.Kirkinezos IG, Barman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, Bradley WG, Moraes CT. J Neurosci. 2005;25:164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A. J Biol Chem. 2004;279:15499–15504. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Xu G, Borchelt DR. J Neurochem. 2006;96:1277–1288. doi: 10.1111/j.1471-4159.2005.03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonsson PA, Graffmo KS, Andersen PM, Brannstrom T, Lindberg M, Oliveberg M, Marklund SL. Brain. 2006;129:451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 33.Doucette PA, Whitson LJ, Cao X, Schirf V, Demeler B, Valentine JS, Hansen JC, Hart PJ. J Biol Chem. 2004;279:54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 34.Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW. Proc Natl Acad Sci USA. 1995;92:689–693. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beretta S, Sala G, Mattavelli L, Ceresa C, Casciati A, Ferri A, Carri MT, Ferrarese C. Neurobiol Dis. 2003;13:213–221. doi: 10.1016/s0969-9961(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 36.Andreassen OA, Dedeoglu A, Klivenyi P, Beal MF, Bush AI. NeuroReport. 2000;11:2491–2493. doi: 10.1097/00001756-200008030-00029. [DOI] [PubMed] [Google Scholar]
- 37.Ciriolo MR, Palamara AT, Incerpi S, Lafavia E, Bue MC, De Vito P, Garaci E, Rotilio G. J Biol Chem. 1997;272:2700–2708. doi: 10.1074/jbc.272.5.2700. [DOI] [PubMed] [Google Scholar]
- 38.Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, et al. Proc Natl Acad Sci USA. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.