Abstract
Advances in fetal magnetic resonance imaging (MRI) allow detection of subtle anatomic anomalies of unclear long-term clinical significance.
Objectives
To examine the accuracy of fetal MRI in diagnosing isolated inferior vermian hypoplasia (IIVH) and to describe the neurodevelopmental outcome.
Study Design
We reviewed all cases with fetal and postnatal MRI studies between 1999–2003 and identified 19 cases with diagnosis of IIVH. We compared prenatal and postnatal MRI studies and evaluated subjects using developmental scales.
Results
IIVH was confirmed by postnatal MRI in 68% (13/19), the remaining 6 having normal postnatal MRI. On developmental testing at mean age 19.8±4.9 months, 3 (23%) infants with confirmed postnatal diagnosis demonstrated motor and language delays and functional difficulties, and 2 (15%) had behavioral problems; none of the infants with normal postnatal MRI studies were delayed.
Conclusions
IIVH in the second trimester may be overdiagnosed by fetal MRI and therefore warrants postnatal MRI confirmation.
Keywords: Fetal, Magnetic resonance (MR), Inferior vermian hypoplasia, Developmental, Outcome
Abbreviations: MRI: magnetic resonance imaging, IIVH: isolated inferior vermian hypoplasia, CNS: central nervous system, VABS: Vineland Adaptive Behavior Scales, CBCL: Child Behavior Checklist, PSI: Parental Stress Index, SD: standard deviation
Introduction
Advances in fetal magnetic resonance imaging (MRI) have allowed detection of increasingly subtle cerebral anomalies, particularly in the posterior cranial fossa.1 The long-term clinical significance of posterior fossa anomalies is often incompletely understood and yet their consideration requires stressful and critical decisions from clinicians and families, and may frequently lead to termination of pregnancy, in some reports at a rate of up to 80%.1 Moreover, in cases where a viable fetus is delivered, misdiagnosis of brain malformations and resulting errors in prognostication have serious consequences for parents.2
One such fetal brain lesion, incomplete development of the cerebellar vermis, is the subject of this report. Some have used the term inferior vermian hypoplasia interchangeably with Dandy Walker variant,3, 4 while others consider this a normal variant.5, 6 These inconsistencies have prevented the meaningful comparison of diagnosis and outcome among published series, thereby compromising accurate prognostication. In studies comparing the diagnostic accuracy of prenatal ultrasound to pathologic evaluation at autopsy, the most common disparity involved the Dandy Walker complex and inferior vermian hypoplasia or agenesis, among which only 43% of the prenatal diagnoses were confirmed by autopsy.7, 8 This inaccuracy was primarily due to false-positive diagnoses. Furthermore, available evidence regarding the outcome of children with inferior vermian hypoplasia is conflicting, with some studies suggesting a favorable outcome6, 9 and others a more guarded prognosis.4, 10 A critical factor in the outcome of inferior vermian hypoplasia is the presence of associated malformations within and outside the central nervous system (CNS), and abnormal karyotype.4 To our knowledge, no study to date has systematically examined the developmental progress of infants with a fetal diagnosis of isolated inferior vermian hypoplasia (IIVH).
We therefore elected to study the outcome of this specific, clearly defined lesion. We aimed to establish the accuracy of fetal MRI compared with early postnatal MRI in identifying IIVH, and to determine whether this relatively minor anatomic “variant”, now increasingly identified by MRI, was associated with a normal developmental outcome.
Material and Methods
Selection criteria and procedures
Retrospective electronic database review of records from October 1999 through September 2003 identified 19 cases referred for prenatal counseling at Children’s Hospital Boston with a diagnosis of IIVH on fetal MRI studies, obtained at Children’s Hospital Boston or Beth Israel Deaconess Medical Center, who had undergone a postnatal MRI. We compared prenatal and postnatal MRI studies and sought informed consent for neurodevelopmental outcome assessment. We also administered a parental stress measure to examine the impact of the fetal diagnosis on family functioning. This study was approved by the Committee on Clinical Investigation at Children’s Hospital Boston.
Medical record review
Through medical record review we obtained the referral diagnosis, presence of extra CNS findings, gestational age at prenatal MRI (based on last menstrual period or, if unknown, on earliest fetal ultrasound), karyotype (when available), gender, birthweight and gestational age at postnatal MRI, and calendar year of prenatal MRI.
Neuroimaging diagnostic criteria
All prenatal and postnatal MRI studies were interpreted by a pediatric neuroradiologist. A diagnosis of IIVH was made when there was partial absence of the inferior portion of the cerebellar vermis with normal or near-normal shaped cerebellar hemispheres, a normal-sized posterior fossa without obvious cystic lesions, and normal supratentorial structures. Vermian development was assessed in the midline sagittal view from the caudal extent of the inferior vermis over the 4th ventricle (Figure 1). Confirmation of IIVH was also sought on axial and coronal imaging.
Figure 1.
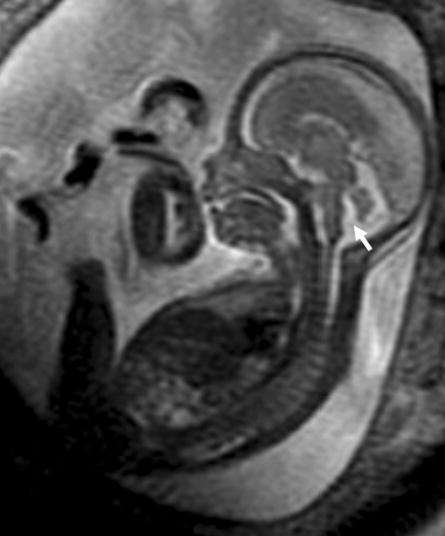
MRI image of a 20 week fetus with IIVH (arrow).
Prenatal and early postnatal neuroimaging techniques
Prenatal MRI studies were performed at both centers using a 1.5 Tesla MR scanner. At Children’s Hospital Boston, prenatal imaging was performed using an 8 channel phased array cardiac coil. Multiplanar single shot fast spin echo (SSFSE) imaging sequences were performed using the following parameters: TEeff=80 msec; NEX=0.5; field of view, 40 cm × 40 cm; section thickness, 4 mm with no interslice gap; and acquisition matrix 256 × 256. At Beth Israel Deaconess Medical Center, prenatal imaging was performed with a 4 or 8 element surface coil and a similar SSFSE imaging sequence with the following parameters: TEeff =90 msec; NEX = 0.5; field of view, 26 cm × 30 cm; slice thickness, 4 mm with no interslice gap; and acquisition matrix of 256 × 256.
Postnatal imaging studies were performed at Children’s Hospital Boston using a quadrature head coil. Sagittal and axial T1-weighted conventional spin echo sequences (TR/TE/NEX =600/20/2; field of view, 20 cm × 20 cm; section thickness, 4 mm with 1 mmgap; and acquisition matrix, 256 × 192) and fast spin-echo axial T2-weighted axial images (TR/TE/NEX = 3200/85/1; field of view, 20 cm × 15 cm; section thickness, 4 mm with 1 mm gap; acquisition matrix, 256 × 192; echo train length, 8 were obtained.
Testing procedures
All examiners were blinded to past medical history, the infants’ MRI findings, and to each other’s clinical findings.
Neurologic examination
A pediatric neurologist performed a formal neurologic examination including assessment of cranial size, cranial nerves, special senses, and motor function (i.e., deep tendon reflexes, muscle tone, muscle strength, coordination, and gait).
Developmental testing
A licensed pediatric psychologist administered the Mullen Scales of Early Learning11 which included assessment of motor, language, and visual reception skills. Infants scoring below 35 (i.e., 1.5 standard deviations [SD] below the mean of 50) were considered delayed in that subdomain.
The Vineland Adaptive Behavior Scale (VABS) is a measure of functional performance in communication, daily living, socialization, and motor skills.12 Scores below 78 (1.5 SD cutoff) were indicative of a functional delay in each subdomain.
The Child Behavior Checklist 1.5–5 (CBCL) is a measure of child behavioral and emotional problems which was completed by the parents.13 Scores were summed to yield a total problem score as well as externalizing and internalizing behavior problem scores, with higher scores reflecting more behavioral symptoms. Scores were dichotomized to normal versus borderline or clinical range.
The Parenting Stress Index (PSI) is a parental questionnaire consisting of three scales: parental distress, parent-child dysfunction, and difficult child.14 Parental stress scores were dichotomized as highly stressed (scores >85th percentile) or normal range. As part of their questionnaires, parents also were specifically asked whether the option of termination of pregnancy was discussed with them at the time of the prenatal diagnosis of IIVH.
Results
One case of IIVH was identified by prenatal MRI in 1999 and one in 2000; the majority (n=17) were identified between 2001–2003 (4 in 2001, 7 in 2002 and 6 in 2003). The prenatal diagnosis was made at a median gestational age of 22 weeks (range 19–26 weeks). All families were offered fetal karyotyping. Three families declined karyotype testing; in the remaining 16 cases, the karyotype was found to be normal. Mean gestational age at delivery was 39.1 ± 1.4 weeks.
Postnatal MRI studies were performed at a median gestational age equivalent (i.e., gestational age + postnatal age) of 42 weeks (range 39–46 weeks). Postnatal MRI studies confirmed a prenatal diagnosis of IIVH in 13/19 (68%) cases, while 6/19 (32%) had normal postnatal MRI studies. Table I describes further characteristics of these two subject groups. There was no association between the year (i.e., 2000–2001 versus 2002–2003) in which the prenatal diagnosis of IIVH was made and the postnatal MRI findings.
Table I.
Characteristics of the study sample (N=19)
| Parameter | Normal Postnatal MRI (n=6) | IIVH (n=13) |
|---|---|---|
| Gestational age at birth, wk, mean ± SD | 39.4 ± 1.3 | 38.7 ± 1.5 |
| Birth weight, grams, mean ± SD | 3148.8 ± 345.4 | 3208.1 ± 324.0 |
| Gestational age at prenatal MRI, wk, median (range) | 22 (18–28) | 22 (19–26) |
| Gestational age equivalent at postnatal MRI, wk, mean ± SD | 44.1 ± 1.2 | 43.8 ± 1.6 |
| Age at testing, months, mean ± SD | 19.2 ± 4.9 | 20.4 ± 4.3 |
Of the 19 families identified and approached for consent, all agreed to participate in the follow-up study. Neurodevelopmental testing was carried out at a mean age of 19.2 ± 4.9 months and 20.4 ± 4.3 months for infants with normal MRI studies and those with IIVH, respectively.
Neurological examinations were normal in 6/6 infants with normal postnatal MRI studies and in 77% (10/13) with confirmed postnatal diagnosis. Three of the 13 infants with confirmed diagnosis had mild axial hypotonia with an otherwise normal examination.
Developmental performance on the Mullen Scales of Early Learning is summarized in Table II. Mean gross and fine motor, expressive language, and overall developmental performance scores were significantly higher in infants with normal postnatal MRI studies compared with those with confirmed diagnosis (p<0.05 for all). Furthermore, the same 3 infants (23%) with confirmed diagnosis of IIVH who had mild axial hypotonia also had gross and fine motor as well as expressive language delays (1.5 SD below the normative means), whereas none of the infants with normal postnatal MRI studies demonstrated developmental delays.
Table II.
Comparison of developmental and functional scores in infants with normal postnatal MRI studies and infants with IIVH
| Outcome Variable | Normal Postnatal MRI (n=6); mean ± SD | IIVH (n=13); mean ± SD | P-Value* |
|---|---|---|---|
| Mullen Scales of Early Learning | |||
| Gross motor | 49.9 ± 2.9 | 37.5 ± 3.6 | <0.01 |
| Fine motor | 51.2 ± 3.9 | 39.2 ± 4.9 | 0.01 |
| Visual reception | 48.6 ± 3.8 | 41.9 ± 4.5 | 0.21 |
| Receptive language | 49.2 ± 3.3 | 42.8 ± 4.3 | 0.28 |
| Expressive language | 51.8 ± 3.5 | 37.1 ± 4.2 | 0.002 |
| Vineland Adaptive Behavior Scale | |||
| Communication | 97.7 ± 3.1 | 84.1 ± 3.5 | 0.002 |
| Daily living skills | 98.3 ± 2.9 | 93.8 ± 3.1 | 0.31 |
| Socialization | 99.1 ± 3.4 | 86.5 ± 3.6 | 0.001 |
| Motor | 98.6 ± 2.7 | 85.3 ± 3.3 | 0.004 |
Differences between groups are assessed by the Wilcoxon rank-sum test
Similarly, functional performance on the VABS indicated that mean communication, socialization and motor scores were significantly lower among infants with confirmed diagnosis compared to those with normal postnatal MRI studies (p<0.01) (Table II). The 3 (23%) infants that exhibited developmental delays on the Mullen Scales also experienced functional difficulties in motor, social and communication skills (i.e., 1.5 SD cutoff). All infants with normal postnatal MRI studies demonstrated age-appropriate functional abilities.
Behavioral outcomes also differed between the two groups. Caregivers of children with confirmed diagnosis reported significantly more symptoms on the CBCL than did caregivers of children with normal postnatal MRI study. Moreover, 2 infants (15%) with confirmed diagnosis, who also demonstrated developmental delays and functional difficulties, scored in the clinical range for behavioral problems, whereas all children with normal postnatal MRI studies scored within the normal range across all subscales (Table III).
Table III.
Comparison of CBCL and PSI scores in infants with normal postnatal MRI studies and infants with IIVH
| Outcome Measure | Normal Postnatal MRI (n=6); mean ± SD | IIVH (n=13); mean ± SD | P-Value* |
|---|---|---|---|
| Child Behavior Checklist | |||
| Internalizing score | 52.6 ± 7.4 | 61.9 ± 7.6 | 0.01 |
| Externalizing score | 53.5 ± 7.1 | 55.2 ± 7.5 | 0.38 |
| Total score | 51.3 ± 6.7 | 59.8 ± 7.3 | 0.03 |
| Parental Stress Index | |||
| Parental distress score | 68.6 ± 6.9 | 79.4 ± 10.5 | 0.02 |
| Difficult child score | 29.3 ± 6.3 | 30.8 ± 9.6 | 0.29 |
| Parent-child dysfunction | 50.4 ± 7.2 | 60.8 ± 9.2 | 0.03 |
| Total score | 66.3 ± 7.8 | 79.2 ± 10.5 | <0.01 |
Differences between groups are assessed by the Wilcoxon rank-sum test
Fifteen out of the 19 (79%) families indicated that the option of termination of pregnancy was presented at the time of prenatal diagnosis of IIVH. Assessment of impact on the family, as measured by the Parental Stress Index at the time of neurodevelopmental testing, indicated that the mothers of infants with a confirmed diagnosis reported significantly higher overall stress than did mothers of infants with normal postnatal MRI findings (54% versus 33%, p<0.01) although high parental distress was also reported among the latter. High levels of stress were identified on the “parental distress” and “parent-child dysfunction” subscales, whereas scores were within the normal range for the “difficult child” subscale (Table III) (p<0.01).
Comments
In this study, we demonstrate that the diagnostic accuracy of fetal MRI for IIVH in the second trimester is modest when compared to postnatal MRI, with only 68% of prenatal diagnoses confirmed postnatally. We also demonstrate that the development of infants with confirmed postnatal IIVH is associated with an overall good outcome, with only mild developmental delays in a subset of infants. However, overall developmental, behavioral and functional scores tended to be lower among infants with IIVH compared to infants with normal postnatal MRI studies (p<0.05). Finally, we demonstrate that a prenatal diagnosis of IIVH is associated with a persistently elevated level of parental stress in the long term, even in cases where postnatal MRI excluded this diagnosis or where infants with IIVH were doing well.
Embryologically, the cerebellum is one of the first brain structures to arise and one of the last to reach its mature configuration. In fact, cellular proliferation and migration continue in the cerebellum for several months after birth.5 In the ninth gestational week, the cerebellar vermis forms from the midline fusion of the developing cerebellar hemispheres, beginning superiorly and continuing inferiorly, until the entire vermis is closed by the end of the 15th week.15 Bromley et al.16 demonstrated sonographically that the posterior-inferior aspect of the vermis remains open in approximately 4% of fetuses until 17.5 weeks. Therefore, inferior vermian hypoplasia cannot be reliably diagnosed until 18 weeks gestational age, when the development of the cerebellar vermis is complete.17
In our study, all prenatal cases of IIVH were diagnosed after 18 weeks gestational age, when the vermis would be expected to be closed. Moreover, there was no difference in mean gestational age at the time of prenatal diagnosis between those cases confirmed postnatally and those that were not. Our data suggest that the development of the cerebellar vermis may be more variable in some cases than is clinically appreciated at present. Conversely, it is also possible that current fetal MRI techniques lack specificity for the diagnosis of inferior vermian hypoplasia in the second trimester. Serial longitudinal prenatal MRI studies would further our understanding of normal development of the cerebellar vermis and would improve the sensitivity with which this diagnosis is made.
Over the past decade, technological advances such as faster scan times and higher resolution have led to the increasing use of fetal MRI to supplement ultrasound.18, 19 Nevertheless, fetal MRI remains a relatively new neuroimaging technique, and its diagnostic accuracy depends on image quality as well as the expertise of the interpreter.18 The accuracy with which CNS anomalies are diagnosed using fetal MRI increases with gestational age due to improved imaging quality and the change in appearance of anomalies over time. The few studies examining the diagnostic accuracy of fetal MRI compared with early postnatal MRI for CNS malformations have suggested that fetal MRI can replace postnatal MRI for infants with CNS anomalies.18, 20
Fetal MRI has become a valuable tool but as yet lacks the diagnostic accuracy of postnatal studies. In a recent report, Scher et al.21 demonstrated that only 64% of fetal CNS diagnoses were confirmed postnatally by fetal MRI. These limitations are particularly evident for the in utero diagnosis of posterior fossa malformations because of the relatively small size of the structures as well as technical limitations such as low spatial resolution, limited pulse sequence options, and low signal-to-noise due to the distance from the imaging coil.19 Although artifact from fetal motion is minimized with fast MR techniques, fetal motion may still affect image quality, especially in the second trimester. Further potential limitations of fetal MRI are variations in the imaging technique and lack of standardized neuroimaging criteria for the diagnosis of IIVH. Given these current limitations, it is difficult to advocate for the substitution of postnatal MRI with fetal MRI.
The small number of studies reporting the outcome of IIVH have been limited largely to heterogeneous samples and informal documentation of neurodevelopmental progress from chart reviews or pediatrician reports.4, 9 Some of these studies have reported a high incidence of neurologic abnormalities, motor dysfunction, and mental retardation, leading some investigators to conclude that the prognosis for normal intellectual development is poor.4, 22 Conversely, others have suggested a somewhat better prognosis, reporting mild hypotonia and motor incoordination with normal intelligence in 30–75% of their cohort.9, 23
Our data indicate that infants with IIVH are free of major neurodevelopmental impairments and functional disabilities. However, overall developmental, functional and behavioral profiles in infants with IIVH are shifted one standard deviation below the normative mean of 100, compared with infants with normal postnatal MRI studies. Approximately one-quarter of our children with IIVH demonstrated developmental delays in gross and fine motor skills and expressive language abilities, which translated into concomitant functional difficulties in motor, social and communication skills.
In recent years, the traditional view of cerebellar function as a pure motor center has been challenged.24, 25 More recent studies in older children and adults have extended the role of the cerebellum to non-motor functions such as language, socialization and cognition.24, 25 Our data support this notion, with 23% of infants with IIVH demonstrating deficits not only in motor function but in the domains of expressive language, behavior and cognition.
A prenatal diagnosis of a fetal brain abnormality is a major parental stressor, not only because of the diagnostic uncertainty in some cases but also because the long-term outcome is often unclear. Surprisingly, despite the fact that a diagnosis of IIVH has been considered a relatively minor and/or normal variant in previous studies,23, 26 the option to terminate pregnancy had been discussed with 79% of our families by caretakers outside our center. Moreover, a prenatal diagnosis of IIVH, including cases of false positive diagnosis, was associated with high levels of parental stress. Elevated parental stress was reported not only among parents of children with identified developmental delay, but was reported also by parents whose children were functioning within normal limits. It is important to note that although 15% of the normal population can report high levels of stress14, 33% (more than twice as many) of parents in our study reported high levels of stress despite normal postnatal MRI studies. Our findings corroborate those of others where foreknowledge of a prenatal diagnosis is associated with increased parental anxiety and impaired coping.2, 27
This study has several potential limitations. First, the fetal and postnatal MRI studies were not re-read in a blinded fashion; rather, we used the clinical diagnosis of IIVH made by our pediatric neuroradiologists at the time of the prenatal and postnatal MRI studies. We elected to design our research question in this manner to examine the current diagnostic accuracy of fetal MRI in a major center for IIVH compared with diagnostic accuracy of postnatal MRI. Secondly, the time frame of our study covered a 5-year period during which the fetal MRI imaging technique and the accuracy of its clinical interpretation may have improved with increased experience. However, in cases diagnosed in 2001–2003 we did examine the accuracy of the fetal MRI diagnosis and found no statistically significant inter-year differences, suggesting that an early lack of expertise did not play a role in our findings. Moreover, the two fetal cases identified in 1999–2000 were both confirmed with postnatal imaging. The increase in the number of fetal cases of IIVH is most likely a reflection of the overall increase in patients referred to our center over this period. Finally, it is difficult to ascertain whether the fetal diagnosis of IIVH may have changed prior to birth because no follow-up fetal MRI studies were performed between the initial fetal MRI diagnosis and the postnatal MRI study.
In summary, we have demonstrated that IIVH in the second trimester may be overdiagnosed by fetal MRI and therefore warrants postnatal MRI confirmation. Whether these false positive diagnoses reflect current limitations of fetal MRI or are due to inherent variations in vermian development remains to be determined. Our study suggests that although infants with IIVH are developing well overall, their rate of milestone acquisition appears to be slower than the population norm. Long-term follow up is needed to ascertain whether this mild developmental lag is transient or whether these children may be at greater risk for long-term developmental difficulties. The diagnosis of a fetal brain anomaly is a major stressor for pregnant parents and effective counseling depends on accurate prenatal diagnosis and expertise in predicting longterm outcome. The data presented here should contribute to a better understanding of the current diagnostic limitations and prognostic implications of IIVH.
Statistics Usage
Continuous perinatal data were summarized using the median, mean, and standard deviation, and categorical factors were summarized using proportions. Performance on developmental outcome testing was also described using means and standard deviations for continuous data, and proportions for categorical data. Group differences on clinical, neuroimaging, and developmental outcome variables were compared with the T-test or Wilcoxon rank sum test for continuous variables, or the Fisher’s exact test for dichotomous data.
Acknowledgments
The authors thank the Clinical Research Program of Children’s Hospital Boston for statistical consultation. The authors also thank Shaye Moore and Shelly Robinson for help with manuscript preparation.
Footnotes
Reprints: Not avaliable
Condensation: Fetal inferior vermian hypoplasia is associated with mild developmental delays in a small subset of children, and may be overdiagnosed by fetal MRI.
Sources of Financial Support: Catherine Limperopoulos is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research. The authors acknowledge support from the Hearst Fund, LifeBridge Fund and NIH grant NINDS 37945. This project was funded in part by grant MO1-RR02172 from the National Center for Research Resources, National Institutes of Health, to the Children’s Hospital Boston General Clinical Research Center.
References
- 1.Adamsbaum C, Moutard ML, Andre C, et al. MRI of the fetal posterior fossa. Pediatr Radiol. 2005;35:124–40. doi: 10.1007/s00247-004-1316-3. [DOI] [PubMed] [Google Scholar]
- 2.Sjogren B. Psychological indications for prenatal diagnosis. Prenat Diagn. 1996;16:449–54. doi: 10.1002/(SICI)1097-0223(199605)16:5<449::AID-PD893>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Domingo Z, Peter J. Midline developmental abnormalities of the posterior fossa: correlation of classification with outcome. Pediatr Neurosurg. 1996;24:111–8. doi: 10.1159/000121026. [DOI] [PubMed] [Google Scholar]
- 4.Estroff JA, Scott MR, Benacerraf BR. Dandy-Walker variant: prenatal sonographic features and clinical outcome. Radiology. 1992;185:755–8. doi: 10.1148/radiology.185.3.1438757. [DOI] [PubMed] [Google Scholar]
- 5.Patel S, Barkovich AJ. Analysis and classification of cerebellar malformations. AJNR Am J Neuroradiol. 2002;23:1074–87. [PMC free article] [PubMed] [Google Scholar]
- 6.Keogan MT, DeAtkine AB, Hertzberg BS. Cerebellar vermian defects: antenatal sonographic appearance and clinical significance. J Ultrasound Med. 1994;13:607–11. doi: 10.7863/jum.1994.13.8.607. [DOI] [PubMed] [Google Scholar]
- 7.Carroll SG, Porter H, Abdel-Fattah S, Kyle PM, Soothill PW. Correlation of prenatal ultrasound diagnosis and pathologic findings in fetal brain abnormalities. Ultrasound Obstet Gynecol. 2000;16:149–53. doi: 10.1046/j.1469-0705.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsao K, Chuang NA, Filly RA, Barkovich AJ, Goldstein RB. Entrapped fourth ventricle: another pitfall in the prenatal diagnosis of Dandy-Walker malformations. J Ultrasound Med. 2002;21:91–6. doi: 10.7863/jum.2002.21.1.91. [DOI] [PubMed] [Google Scholar]
- 9.Chang MC, Russell SA, Callen PW, Filly RA, Goldstein RB. Sonographic detection of inferior vermian agenesis in Dandy-Walker malformations: prognostic implications. Radiology. 1994;193:765–70. doi: 10.1148/radiology.193.3.7972821. [DOI] [PubMed] [Google Scholar]
- 10.Ecker JL, Shipp TD, Bromley B, Benacerraf B. The sonographic diagnosis of Dandy-Walker and Dandy-Walker variant: associated findings and outcomes. Prenat Diagn. 2000;20:328–32. [PubMed] [Google Scholar]
- 11.M ullen E. The Mullen Scales of Early Learning. American Guidance Service 1995.
- 12.S parrow S, Balla DA, Cicchette DV. Vineland Adaptive Behavior Scales, Interview Edition, Survey Form Manual: A Revision of the Vineland Social Maturity Scale. Circle Pines, 1984.
- 13.A chenbach TM. Manual for Child Behavior Checklist/4–18 and 1991 Profile. Burlington, 1991.
- 14.A bidin RR. Parenting Stress Index/Short Form. 1995.
- 15.L emire RJ, Loeser JD, Leech RW, Alvord EC. Normal and abnormal development of the human nervous system. Hagerstown MD: Harper & Row, 1975.
- 16.Bromley B, Nadel AS, Pauker S, Estroff JA, Benacerraf BR. Closure of the cerebellar vermis: evaluation with second trimester US. Radiology. 1994;193:761–3. doi: 10.1148/radiology.193.3.7972820. [DOI] [PubMed] [Google Scholar]
- 17.Laing FC, Frates MC, Brown DL, Benson CB, Di Salvo DN, Doubilet PM. Sonography of the fetal posterior fossa: false appearance of mega-cisterna magna and Dandy-Walker variant. Radiology. 1994;192:247–51. doi: 10.1148/radiology.192.1.8208946. [DOI] [PubMed] [Google Scholar]
- 18.Blaicher W, Bernaschek G, Deutinger J, Messerschmidt A, Schindler E, Prayer D. Fetal and early postnatal magnetic resonance imaging--is there a difference? J Perinat Med. 2004;32:53–7. doi: 10.1515/JPM.2004.009. [DOI] [PubMed] [Google Scholar]
- 19.Golja AM, Estroff JA, Robertson RL. Fetal imaging of central nervous system abnormalities. Neuroimaging Clin N Am. 2004;14:293–306. doi: 10.1016/j.nic.2004.03.006. viii. [DOI] [PubMed] [Google Scholar]
- 20.Patel TR, Bannister CM, Thorne J. A study of prenatal ultrasound and postnatal magnetic imaging in the diagnosis of central nervous system abnormalities. Eur J Pediatr Surg. 2003;13 (Suppl 1):S18–22. doi: 10.1055/s-2003-44752. [DOI] [PubMed] [Google Scholar]
- 21.Scher MS, Kidder BM, Shah D, Bangert BA, Judge NE. Pediatric neurology participation in a fetal diagnostic service. Pediatr Neurol. 2004;30:338–44. doi: 10.1016/j.pediatrneurol.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Golden JA, Rorke LB, Bruce DA. Dandy-Walker syndrome and associated anomalies. Pediatr Neurosci. 1987;13:38–44. doi: 10.1159/000120299. [DOI] [PubMed] [Google Scholar]
- 23.Sawaya R, McLaurin RL. Dandy-Walker syndrome. Clinical analysis of 23 cases. J Neurosurg. 1981;55:89–98. doi: 10.3171/jns.1981.55.1.0089. [DOI] [PubMed] [Google Scholar]
- 24.Schmahmann JD, Sherman JC. Cerebellar cognitive affective syndrome. Int Rev Neurobiol. 1997;41:433–40. doi: 10.1016/s0074-7742(08)60363-3. [DOI] [PubMed] [Google Scholar]
- 25.Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16:444–7. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- 26.Pilu G, Visentin A, Valeri B. The Dandy-Walker complex and fetal sonography. Ultrasound Obstet Gynecol. 2000;16:115–7. doi: 10.1046/j.1469-0705.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- 27.Hunfeld JA, Tempels A, Passchier J, Hazebroek FW, Tibboel D. Brief report:parental burden and grief one year after the birth of a child with a congenital anomaly. J Pediatr Psychol. 1999;24:515–20. doi: 10.1093/jpepsy/24.6.515. [DOI] [PubMed] [Google Scholar]


