Abstract
Withdrawal anxiety following chronic ethanol is often associated with relapse in recovering alcoholics. It is likely that brain regions regulating anxiety-like behaviors adapt during chronic ethanol to ultimately regulate such behaviors. The central amygdala contains numerous neurotransmitter systems that have been implicated in the regulation of anxiety-like behavior, including corticotropin releasing factor (CRF) and N-methyl-D-Aspartate (NMDA)-type glutamate receptors. Chronic ethanol exposure causes functional adaptations in both CRF and NMDA receptors that are likely to regulate anxiety-like behaviors expressed during withdrawal. However, the molecular mechanisms governing these adaptations remain un-explored. We therefore evaluated these neurotransmitter systems in Sprague-Dawley rats during chronic ingestion of an ethanol-containing liquid diet. Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) demonstrated that pre-proCRF mRNA was significantly up-regulated by chronic ethanol exposure while CRF binding protein mRNA expression did not change. There were also no significant changes observed in any of the NMDA subunit mRNAs, although there was a trend toward greater NR2A mRNA expression during chronic ethanol. Using Western blotting analysis we measured NMDA receptor subunit protein expression. Chronic ethanol exposure did not affect protein levels of the NR1 and NR2B subunits. Like the mRNA measures, chronic ethanol did influence NR2A protein levels but the effects were modest. Our results demonstrate that NMDA receptor subunit mRNA and protein expression are not strongly influenced by exposure to chronic ethanol. This suggests that the functional NMDA receptor adaptations identified by previous studies (Roberto et al., 2004) are likely to be mediated by post-translational events. In contrast, enhanced levels of CRF during/after chronic ethanol are likely to be mediated by increased levels of pre-proCRF mRNA. Together, our findings suggest that adaptations to chronic ethanol by pro-anxiety factors expressed in the central nucleus appear to be mediated by distinct cellular and molecular mechanisms.
Keywords: corticotropin releasing factor, NMDA, central amygdala, real-time RT-PCR, western analysis, chronic ethanol
1. Introduction
The amygdala receives highly processed sensory and cognitive information and projects to regions regulating risk-assessment (e.g. medial prefrontal cortex, bed nucleus of the stria terminalis, nucleus accumbens) as well as regions regulating autonomic responses to emotionally relevant environmental stimuli. This region consequently presents as a nexus for the regulation of affective behaviors like fear and anxiety. Indeed, direct manipulation of amygdala neurotransmitter systems can modulate basal anxiety-like behavior (Moller et al., 1997; Shibata et al., 1989) and disrupt conditioned fear learning (Killcross et al., 1997; Nader et al., 2001). Recently, it has been demonstrated that various amygdala subdivisions modulate drug-seeking behaviors in rodents (Alderson et al., 2000; Di Ciano & Everitt, 2004; Fuchs & See, 2002; McLaughlin & See, 2003), drug cravings in humans (Childress et al., 1999), and the regulation of anxiety-like behaviors during withdrawal from chronic drug exposure (Menzaghi et al., 1994; Rodriguez de Fonseca et al., 1997; Watanabe et al., 2002). These findings illustrate the importance of the amygdala in the regulation of both basal affective behaviors as well as affective behaviors associated with chronic drug exposure.
The central nucleus of the amygdala has received particular attention with regard to anxiety-like behavior during withdrawal from chronic drug exposure. For example, corticotropin releasing factor (CRF) is a neuropeptide synthesized by neurons of the central amygdala (Cassell et al., 1986). CRF is released both locally within the central amygdala and at distant projection sites like the bed nucleus of the stria terminalis and the hypothalamus. Central amygdala CRF is believed to play a central role in the regulation of negative affect associated with drug withdrawal. There is a substantial elevation in extracellular CRF levels in the central amygdala following withdrawal from cocaine self-administration (Richter & Weiss, 1999), antagonist-induced withdrawal from chronic cannabinoid (Rodriguez de Fonseca et al., 1997) and morphine (Heinrichs et al., 1995) exposure, and withdrawal in ethanol-dependent animals (Menzaghi et al., 1994). Injection of CRF receptor antagonists into the central amygdala block anxiety-like responses to withdrawal from chronic ethanol (Rassnick et al., 1993). These findings indicate an intimate association between elevated CRF levels and withdrawal-induced anxiety.
Along with CRF, glutamate signaling in the central amygdala is likely to play a significant regulatory role in this region’s modulation of affective responses to drug withdrawal. Chronic ethanol exposure enhances glutamate release and has been associated with adaptations in subunit-specific contributions to NMDA receptor-mediated synaptic responses (Roberto et al., 2004). Similar findings have been reported in the adjacent lateral/basolateral nuclei (Floyd et al., 2003). In addition, NMDA receptor antagonists injected directly into the central amygdala block the robust anxiety-like behavior precipitated by naloxone-induced withdrawal from chronic morphine (Watanabe et al., 2002). NMDA receptor activation can facilitate CRF-release by central amygdala neurons (Cratty & Birkle, 1999); and, enhanced glutamate signaling in the central amygdala can increase CRF release at distinct sites (Gabr et al., 1995). These findings together suggest that adaptations by central amygdala neurotransmitter systems, particularly NMDA receptors, are likely to play a significant role in the behavioral responses to withdrawal from chronic drug exposure, including chronic ethanol.
Despite substantial evidence of functional adaptations in CRF- and NMDA-mediated signaling during or following chronic ethanol, the molecular events governing such alterations remain unclear. A detailed understanding of these mechanisms would offer a clearer understanding of the potential therapies that address the long-term consequences of alcohol abuse and withdrawal. We have therefore utilized molecular and cellular approaches to specifically examine the impact of chronic ethanol exposure on central amygdala NMDA and CRF systems.
2. Materials & Methods
2.1 Animal Procedures
All animal procedures were performed in accordance with a WFUSM Animal Care and Use Committee-approved protocol that was consistent with the NIH animal care and use policy. Male Sprague Dawley rats (~120g; Harlan, Indianapolis IN) were singly housed and placed on an 8hr-on/16hr-off light/dark cycle. After four days acclimation to the housing facility/conditions, rats were introduced to a commercially available Lieber-DeCarli (Lieber & DeCarli, 1989) liquid diet (Bio-Serv, Frenchtown NJ). The next day, all standard chow was removed; and rats were given only the control liquid diet. Individuals were then divided into two groups: ‘control’ animals that continued to receive the standard liquid diet over the entire experiment (n=18), and; ‘chronic ethanol’ animals (n=19) that received an iso-caloric, ethanol-containing liquid diet. These latter rats were introduced to the ethanol diet by increasing the percent ethanol in the diet over 2–3 days. Ethanol-exposed animals received 4–6% ethanol over a total of 10–12 days. Levels of the ethanol-containing liquid diet were titrated to each individual such that total consumption of ethanol was maintained or escalated over the entire exposure period. During the chronic ethanol exposure, animals ingested 11.6±0.3g/kg/day ethanol from the liquid diet (n=19). Using a commercially available NAD/alcohol dehydrogenase assay (Diagnostic Chemicals Ltd., Oxford CT), blood-alcohol levels were measured at the time of sacrifice in ‘sentinel’ animals and were 153±13mg/dl (n=9). These values are almost identical to our previously published reports (Floyd et al., 2003; McCool et al., 2003).
On the final day of exposure, intoxicated animals were anesthetized with halothane and decapitated. 400μm-thick coronal brain slices were prepared on a vibratome using ice-cold modified Ringer’s solution (180mM sucrose, 30mM NaCl, 4.5mM KCl, 1mM MgCl2, 26mM NaHCO3, 1.2mM NaH2PO4, 10mM D-glucose; bubbled constantly with 95% O2 /5% CO2) and placed in a tissue holder containing room-temperature standard Ringer’s solution (94mM NaCl replaces the 180mM sucrose for a final [NaCl] of 124mM) until use (~10min). The central amygdala was immediately dissected, frozen rapidly on dry ice, and kept at −80°C until use. Different sets of animals were used to measure the effects of chronic ethanol ingestion on mRNA (n=8 controls and 9 ethanol-exposed) and protein expression (n=10 control and 10 ethanol-exposed).
2.2 Preparation of RNA and Real-time RT-PCR
Total RNA was isolated from frozen central amygdala according to published procedures (Floyd et al., 2003). Genomic DNA was removed by digestion with DNase I; and, RNA was quantified using spectrophotometry. Total RNA from the entire forebrain of single chow-fed Sprague-Dawley rat was used to standardize expression levels of our target genes. Reverse transcription of total RNA was performed using random hexanucleotides; RNA was removed from the resulting cDNA by digestion with RNaseH. For real-time PCR, two-to-five ‘ng equivalents’ of the cDNA mixture were combined with 9μM primers, 0.25μM probe, and 1X Universal PCR Master Mix (Applied Biosystems, Foster City CA). The PCR reaction consisted of initial incubations at 50°C for 2 min followed by 95°C for 10 min; steps were 40 cycles of 95°C for 15 s followed by 60°C for 1 min. The “relative standard curve” method (Johnson et al., 2000) was used to compare expression levels of mRNAs between the control and chronic ethanol samples as previously described (Floyd et al., 2004; Floyd et al., 2003). The expression level of each subunit was normalized to glyceraldehyde phosphate dehydrogenase (GAPDH) in the same sample.
Primers and probes for the various rat NMDA receptors subunits and glyceraldehyde phosphate dehydrogenase (GAPDH) have been described elsewhere (Floyd et al., 2003). Using public sequences from GenBank, primer/probe sets were similarly designed from corticotropin releasing factor (CRF; accession# M54987) and CRF binding protein (CRFBP; accession# X58023) using PrimerExpress software (version 3.0, Applied Biosystems). These sets were: for CRF, 5’ primer – CCA GGG CAG AGC AGT TAG CT (nucleotide# 672–691), 3’ primer – GCA ACA TTT CAT TTC CCG ATA ATC (nucleotide# 724–747), probe – CAA GCT CAC AGC AAC AGG AAA CTG ATG G (nucleotide#695–722); for CRFBP, 5’ primer – CAG CCA TGT CAC CGA ACT TCA AAC (nt#113–136), 3’ primer – AAA GCA GGA AAG GGT CGT AGA CTG (nt#212–235), and probe – ATG CCA CTT CAC TCT GAT CCT CCT GA (nt#141–166). All probes were 5’-labeled with 6-FAM, 3’-labeled with BHQ1, and purified with HPLC by the manufacturer (Integrated DNA Technologies, Coralville IA).
2.3 Western Blot Analysis
Lysis buffer (10mM Tris pH 7.5, 2% SDS, 2mM DTT) was added to isolated central amygdala at 10μl/mg tissue; and tissue was disrupted by brief sonication. Protein yield was quantified using the BCA assay (Pierce Chemical, Rockford IL). 10–20μg total protein was loaded onto 4–15% SDS-polyacrylamide pre-cast gels (Bio-Rad, Hercules CA), separated, and transferred to a nitrocellulose membrane (Hybond N; Amersham, Piscataway NJ). The membrane was blocked with PBS-T (150mM NaCl, 5.2mM Na2HPO4, 1.7mM KH2PO4, 0.1% Tween-20) containing 5% non-fat dry milk. Blots were then incubated overnight at 4°C in PBS-T/1% milk containing primary antibody that recognizes all NR1 subunits (‘NR1-pan’ antibody, 0.5μg/ml; Chemicon, Temecula CA), NR1 subunits containing the NR1-C1 splice cassette (1μg/ml; BD Pharmingen, San Jose CA), NR2A subunit proteins (1:4000; Chemicon), or NR2B subunit proteins (1:4000; Chemicon). The next morning, blots were washed extensively with PBS-T and exposed to goat anti-rabbit or goat anti-mouse 2° antibodies labeled with peroxidase (1:3000; SIGMA, St. Louis MO) for several hours at room temperature. Detection of bound 2° antibody was performed using enhanced chemiluminescence (Pierce, Rockford IL). To normalize expression between experiments, blots were also probed with mouse monoclonal antibody directed against β-actin (1:10,000; Chemicon) followed by HRP-labeled goat anti-mouse secondary antibody (1:3,000 dilution; Sigma). Band intensity was quantified from digital images of x-ray film using standard procedures (Jiang et al., 2002).
2.4 Data analysis and Statistics
For both the mRNA and protein studies, mean expression levels were determined from two-three independent experiments for each sample. Average values from these experiments are reported as mean±SEM. Statistical comparisons between each treatment group were performed with the standard Student’s t-test (Motulsky, 1995).
3. Results
Chronic Ethanol Ingestion Up-regulates mRNA Levels for Some Pro-anxiety Factors
To assess whether neurotransmitter systems in brain regions regulating anxiety-like behavior adapt during chronic ethanol exposure, we measured the mRNA expression of several ‘pro-anxiety’ factors in the central amygdala derived from control and chronic ethanol-exposed animals. Corticotropin-releasing factor expressed in the central amygdala has been extensively characterized as a central regulatory component controlling anxiety-like behavior (Rassnick et al., 1993; Richter et al., 2000; Rodriguez de Fonseca et al., 1997; Sajdyk et al., 1999). Importantly, the expression of pre-proCRF mRNA was significantly increased following chronic ethanol. Normalized relative expression was 6.38±0.22 in central amygdala from control animals (n=7) and 7.99±0.66 in ethanol-exposed central amygdala (Fig. 1B; P<0.05, t-test). This corresponds to a 25% increase during the chronic ethanol exposure. Since the biological activity of CRF is tightly controlled by CRF binding-protein (Behan et al., 1995; Ungless et al., 2003), we also examined the impact of chronic ethanol on this gene product. The mRNA expression levels for CRF binding protein were not significantly altered by chronic ethanol with relative levels being 1.3±0.1 in control amygdala tissue and 1.4±0.2 in ethanol-treated animals (Fig. 1C; P>0.05, t-test). Importantly, chronic ethanol exposure also did not significantly influence GAPDH mRNA levels – 2.4±0.4 in control samples and 2.2±0.5 in central amygdala following chronic ethanol (Fig. 1D). These latter findings demonstrate that normalization of target gene levels to GAPDH did not produce any treatment-specific bias.
Figure 1.

Chronic ethanol ingestion differentially regulates components of the central amygdala/CRF system. (A) Example of real-time PCR reactions using primers/probe for pre-proCRF (□, ▪) and the ubiquitous gene glyceraldehyde phosphate dehydrogenase (GAPDH; ○, ^) and total RNA from central amygdala (‘CeN’, open symbols) and total forebrain (‘Fb’, closed symbols). The threshold cycle, CT, for each product is defined as the cycle where log[fluorescence] increases above an arbitrary level (dashed line). CT values for each gene product in each sample are shown in parentheses. (B) The expression of pre-proCRF mRNA is significantly increased during chronic ethanol exposure. Relative expression units were determined by dividing the apparent levels of pre-proCRF mRNA by the apparent levels of GAPDH expression in each sample (see Methods section). * – P<0.05, two-sided Student’s t-test. (C) The expression of CRF binding protein mRNA was not significantly affected by chronic ethanol exposure. (D) Chronic ethanol ingestion did not influence the expression of GAPDH in these experiments. Relative expression levels were therefore not significantly influenced by the normalization of target gene expression to GAPDH in each sample.
Due to functional adaptations by central amygdala NMDA receptors during chronic ethanol exposure (Roberto et al., 2004), we also examined the influence of chronic ethanol ingestion on the expression of NMDA receptor subunit mRNAs (Table 1). Unlike pre-proCRF, this treatment did not significantly affect mRNA levels for the NMDA receptor subunits. In fact, levels of most subunit mRNAs changed less than 10%. However, the expression of some subunit mRNAs was characterized by substantial animal-to-animal variance. Indeed, although there was an apparent 20% increase in NR2A expression during chronic ethanol, this variance prevented detection of a significant effect in this set of animals. These findings suggest that mRNAs for different pro-anxiety neurotransmitter systems in the central amygdala are uniquely modulated by chronic ethanol ingestion.
Table 1.
Chronic Ethanol Ingestion and NMDA Subunit mRNA Expression
| Treatment | ||||
|---|---|---|---|---|
| Subunit | Control LDa | EtOH LDa | CeN Threshold Cycleb | Relative to Forebrain Expressionc |
| NR1 | 2.39±0.21 | 2.22±0.20 | 24.4±0.5 | 0.80±0.05 |
| NR2A | 1.99±0.32 | 2.39±0.31 | 25.8±0.3 | 0.48±0.04 |
| NR2B | 1.91±0.13 | 1.86±0.12 | 23.7±0.3 | 0.74±0.06 |
| NR2C | 1.74±0.04 | 1.73±0.05 | 28.3±0.2 | 0.59±0.04 |
| NR2D | 2.58±0.43 | 2.38±0.25 | 30.6±0.3 | 1.42±0.15 |
| NR3A | 1.77±0.20 | 1.97±0.23 | 28.0±0.3 | 0.53±0.08 |
| NR3B | 2.32±0.18 | 2.53±0.26 | 29.1±0.2 | 0.85±0.03 |
– values are normalized to GAPDH levels determined for each sample (See Methods section) and are not representative of absolute expression levels.
– Threshold cycles (Ct) for central amygdala samples were collapsed across treatments. Assuming equivalent efficiencies for cDNA and PCR product formation, ‘1’ Ct unit would be equivalent to a 2-fold difference in expression.
– values = 2(−ΔCt) where ΔCt is the difference between the threshold cycles for equivalent masses of total forebrain RNA and our samples (collapsed across treatments). Values close to ‘1’ would indicate expression levels equivalent to total forebrain.
Chronic Ethanol Ingestion Differentially Regulates NMDA Receptor Subunit Proteins
Because changes in NR2A contribution to the functional NMDA receptor might have meaningful implications for glutamate signaling in the central amygdala, we chose to examine the expression of NMDA receptor subunit proteins. For the NR1-pan, NR1-C1, NR2A, and NR2B subunit proteins, we normalized apparent expression levels to β-actin expression in each sample to reduce the influence of sample-to-sample differences in quality and to control for errors in quantification or sample loading. Importantly, chronic ethanol exposure did not significantly influence β-actin-like immunoreactivity across all our experiments. Across all experiments, levels of β-actin were 334±38 OD units in control samples (n=10) and 337±35 OD units in ethanol-exposed samples (n=10). For the NMDA subunits, chronic ethanol ingestion did not significantly alter protein levels. NR1 subunits containing the C1 C-terminal splice cassette were unaffected by chronic ethanol, with normalized expression being 4.26±0.18 in control central amygdala and 4.44±0.18 in ethanol exposed tissue (Fig. 2B; P>0.05, t-test; n=10 in each treatment group). In a subset of animals, normalized levels of total NR1 subunit protein (Fig. 2A) were 2.21±0.07 in control CeN (n=6) and 2.08±0.18 in CeN from ethanol-exposed animals (n=5; P>0.05, Student’s t-test). Levels of NR2A subunit protein tended to be increased by chronic ethanol exposure, from 2.21±0.07 normalized units in control samples to 2.50±0.13 units during ethanol (13% increase, Fig. 2C; P~0.06, t-test). Levels of NR2B-like immunoreactivity were not significantly influenced by chronic ethanol ingestion – 1.32±0.09 normalized units in controls vs. 1.38±0.11 in ethanol-exposed central amygdala samples (Fig. 2D; P>0.05, test). These findings suggest that different NMDA receptor subunit proteins may be uniquely regulated by chronic alcohol. Furthermore, the modest up-regulation of NR2A-like immunoreactivity during this exposure is consistent with the apparent changes in mRNA expression for this subunit.
Figure 2.
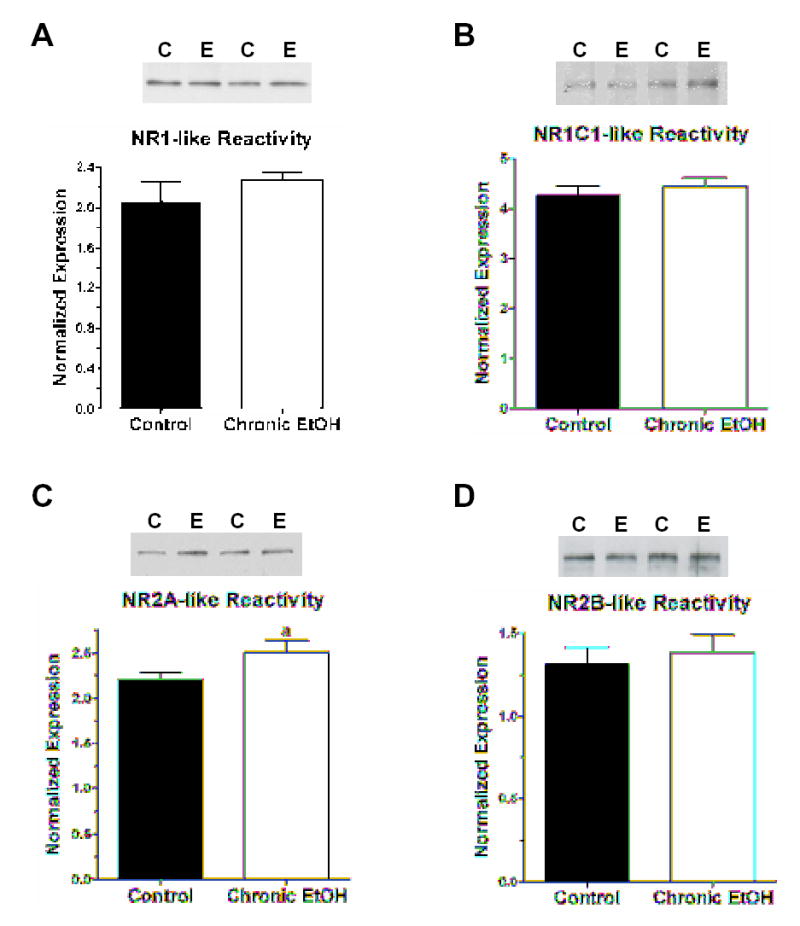
Chronic ethanol ingestion differentially influences the apparent expression of some NMDA receptor subunit proteins. (A) Chronic ethanol exposure does not influence levels of total NR1 subunit protein or those subunits containing the C1 splice cassette (B). Examples of ‘typical’ western blots are shown at the top of each figure to illustrate the alternative loading of samples from control (‘C’) and chronic ethanol (‘E’) central amygdala. The summary of data obtained for each subunit is shown at the bottom of each figure. NMDA receptor subunit protein expression was normalized to the apparent levels of β-actin in each sample (see Methods). β-actin expression was not significantly affected by chronic ethanol exposure (see text). (C) Chronic ethanol ingestion modestly increases NR2A-like immunoreactivity in central nucleus samples. NR2A subunit protein was increased by ~20% compared to control central amygdala. ‘a’ – P~0.06, two-sided Student’s t-test. (D) Chronic ethanol exposure did not significantly influence the expression of NR2B-like immunoreactivity in the central amygdala.
4. Discussion
One of the central findings of this report is the chronic ethanol-induced up-regulation of pre-proCRF mRNA. Chronic ethanol enhances both basal levels of CRF in central amygdala dialysates during the exposure as well as during acute withdrawal (<24hr) (Merlo Pich et al., 1995). In addition, increased amygdala CRF tissue content is maintained several weeks after withdrawal (Zorrilla et al., 2001). The up-regulated pre-proCRF mRNA reported here could clearly provide the mechanistic basis for increased CRF release during chronic ethanol and acute withdrawal, although the contribution of this mechanism to longer lasting alterations in amygdala CRF is less clear. Similar effects have been noted in the bed nucleus of the stria terminalis (Olive et al., 2002), a primary output area for CRF-containing neurons in the central amygdala. Enhanced pre-proCRF expression in the central amygdala could contribute significantly to chronic ethanol-induced alterations of CRF peptide in numerous brain regions.
It is also noteworthy that CRF and CRF binding protein were differentially regulated by chronic ethanol. CRF-BP is believed to be a negative regulator of adrenocorticotropic hormone (ACTH) secretion by clearing ‘free’ CRF (Behan et al., 1995) and preventing CRF’s positive influence on ACTH. However, some reports indicate that the CRF/CRF-BP complex may itself be the active ligand for CRF-R2 receptors (Ungless et al., 2003). This requirement for a CRF/CRF-BP interaction suggests that chronic ethanol-induced increases in CRF expression, but not CRF-BP, would enhance physiological processes mediated by free CRF, presumably mediated by high affinity CRF-R1 receptors (Myers et al., 1998). This hypothesis is supported by alcohol-induced up-regulation of CRF-R1 expression in the hypothalamus (Lee & Rivier, 1997), a structure that also receives significant CRF-innervation from the central amygdala. Finally, CRF-R2 agonists can attenuate the pronounced increase in anxiety-like behavior following withdrawal from chronic ethanol (Valdez et al., 2004). Our CRF findings lend support to the hypothesis that chronic ethanol may disrupt the balance between CRF-R1- and CRF-R2-mediated events which could in turn contribute to the dis-regulation of anxiety-like behaviors during withdrawal.
The mechanisms governing ethanol-induced up-regulation of pre-proCRF mRNA are not clear at present. In fact, very little is known regarding the transcriptional regulation of this gene in general. Glucocorticoids mediate robust negative regulation of hypothalamic CRF expression (Ma et al., 2001). These interactions might suggest that stress hormones could regulate the response of pre-proCRF to chronic ethanol. However, there is conflicting evidence regarding the effects of chronic ethanol on resting levels of corticosterone (Guaza et al., 1983; Rasmussen et al., 2000). Despite these contrasting reports, chronic exposure can blunt both circadian cycling of corticosterone (Sipp et al., 1993) as well as the responsiveness of the HPA axis to external stressors (Ogilvie et al., 1998). These findings suggest that chronic ethanol’s interaction with the HPA axis may be relatively subtle. Subsequently, the role played by circulating glucocorticoids in the regulation of pre-proCRF mRNA expression, particularly in response to chronic drug exposure, remains unclear.
Our findings also provide the first measures of NMDA subunit distribution in the central amygdala. NR1, NR2A, and NR2B mRNAs were readily detected in total RNA prepared from this brain region. In addition, if one assumes approximately equivalent efficiencies for the various subunit PCR reactions, the CT values for each subunit (Table 1) would suggest that the relative abundance of NR subunit mRNAs in the central amygdala would be NR1 = NR2B >NR2A >> NR2C = NR3A > NR3B > NR2D. This is consistent with recent reports suggesting the NR2B-containing receptors contribute significantly to adult central amygdala NMDA receptor-mediated synaptic responses (Sah & Lopez De Armentia, 2003). The differences between subunit CT values in the central amygdala samples (Table 1) would further suggest that NR2A, NR2C, and NR3A subunit mRNAs are relatively less abundant in the central amygdala compared to their expression in the forebrain. Conversely, despite its low expression in comparison to the other NMDA receptor subunits, the NR2D subunit mRNA is relatively more abundant in the central amygdala compared to forebrain. The distribution of the various NMDA receptor subunits across the distinct central amygdala subdivisions and within phenotypically unique central amygdala neuronal populations remains to be determined.
Although we do not yet know if the adaptations reported here are directly associated with functional alterations in the NMDA receptor, this is likely to be the case. Roberto et al. (Roberto et al., 2004) recently reported that chronic exposure to ethanol vapor increases ifenprodil and ethanol inhibition of NMDA-mediated EPSCs in central amygdala preparations, consistent with functional alterations in synaptic receptors containing the NR2B subunit. It is possible that the apparent up-regulation in NR2A expression following chronic ingestion of an ethanol-containing liquid diet reported here is particular to this exposure paradigm. Indeed, more robust ethanol exposures may accentuate the effects on NR2A reported here and could potentially influence additional subunits as well. Despite this, it would be difficult to characterize functional adaptations in this subunit. NR2A-specific ligands are difficult to use in slice recordings; and, the distinct biophysical characteristics bestowed by this subunit are likely to be masked by the expression of multiple NR2 subunits in this brain region. Although we did not find any evidence of adaptations in NR2B subunit mRNA or protein expression, chronic ethanol exposure of hippocampal neurons in vitro alters the localization of NR2B-containing NMDA receptors away from somatic compartments to synaptic sites (Carpenter-Hyland et al., 2004). Along with increased ifenprodil-sensitivity of NMDA-mediated synaptic responses following chronic ethanol inhalation (Roberto et al., 2004), these findings together suggest that the mechanisms governing NMDA receptor adaptations to chronic ethanol exposure may be distinct for each subunit and can include alterations in both gene transcription/translation as well as receptor localization. When considered in conjunction with apparent brain region-specific adaptations, the effects of chronic ethanol on the NMDA receptor are likely to be complex.
The central amygdala joins an expanding list of brain regions that respond to chronic ethanol exposure by up-regulating NMDA receptor function and/or expression. Chronic ethanol also increases MK801 binding in related brain regions like the striatum (Gulya et al., 1991). And, we have recently demonstrated that increased NR1 mRNA expression is associated with functional increases in the apparent density of NMDA receptors expressed by acutely isolated neurons from the neighboring lateral/basolateral amygdala (Floyd et al., 2003). Together with a recent demonstration of increased glutamate release in the central amygdala following chronic ethanol (Roberto et al., 2004), these findings suggest increased glutamate signaling throughout the major amygdaloid subdivisions. Given the well-established role of the amygdala in the learning of and expression of fear-like or anxiety-like behaviors, we believe these NMDA receptor adaptations may be directly related to the complex and important relationship between withdrawal-induced anxiety and alcohol abuse. In support of this, NMDA receptor antagonists injected directly into the central amygdala (CeN) can block the acquisition of learned fear (Goosens & Maren, 2003) and also block aversive behaviors associated with withdrawal from chronic opiate exposure (Watanabe et al., 2002). Co-incident adaptations in CRF expression and glutamate signaling are likely to play a significant role in such behaviors, including ethanol withdrawal-induced anxiety.
Acknowledgments
This work supported by AA14445 (BAM) and by the “Multi-Disciplinary Training in the Biology of Alcoholism” program (T32 AA007565, KAL). We are also grateful of Dr. Dustin Dubois for his insightful comments on this manuscript.
References
- Alderson HL, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the basolateral amygdala on the acquisition of heroin-seeking behavior in rats. Psychopharmacology (Berl) 2000;153:111–9. doi: 10.1007/s002130000527. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–82. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–68. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. Journal of Comparative Neurology. 1986;246:478–99. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cratty MS, Birkle DL. N-methyl-D-aspartate (NMDA)-mediated corticotropin-releasing factor (CRF) release in cultured rat amygdala neurons. Peptides. 1999;20:93–100. doi: 10.1016/s0196-9781(98)00147-8. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–73. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-Term Ethanol Self-Administration by Cynomolgus Macaques Alters the Pharmacology and Expression of GABAA Receptors in Basolateral Amygdala. J Pharmacol Exp Ther. 2004;311:1071–9. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–9. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–33. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Gabr RW, Birkle DL, Azzaro AJ. Stimulation of the amygdala by glutamate facilitates corticotropin-releasing factor release from the median eminence and activation of the hypothalamic-pituitary-adrenal axis in stressed rats. Neuroendocrinology. 1995;62:333–9. doi: 10.1159/000127022. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci. 2003;117:738–50. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Guaza C, Torrellas A, Borrell S. Adrenocortical response to acute and chronic ethanol administration in rats. Psychopharmacology (Berl) 1983;79:173–6. doi: 10.1007/BF00427806. [DOI] [PubMed] [Google Scholar]
- Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res. 1991;547:129–34. [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- Jiang J, McCool BA, Parrish AR. Cadmium- and mercury-induced intercellular adhesion molecule-1 expression in immortalized proximal tubule cells: evidence for a role of decreased transforming growth factor-beta1. Toxicol Appl Pharmacol. 2002;179:13–20. doi: 10.1006/taap.2001.9345. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Wang K, Smith JB, Heslin MJ, Diasio RB. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochem. 2000;278:175–84. doi: 10.1006/abio.1999.4461. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–80. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Alcohol increases the expression of type 1, but not type 2 alpha corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. Brain Res Mol Brain Res. 1997;52:78–89. doi: 10.1016/s0169-328x(97)00226-x. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197–211. [PubMed] [Google Scholar]
- Ma XM, Camacho C, Aguilera G. Regulation of corticotropin-releasing hormone (CRH) transcription and CRH mRNA stability by glucocorticoids. Cell Mol Neurobiol. 2001;21:465–75. doi: 10.1023/a:1013863205647. [DOI] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963:165–77. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, Koob GF. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann N Y Acad Sci. 1994;739:176–84. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–47. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Motulsky, H. (1995). Intuitive biostatistics New York: Oxford University Press.
- Myers DA, Trinh JV, Myers TR. Structure and function of the ovine type 1 corticotropin releasing factor receptor (CRF1) and a carboxyl-terminal variant. Mol Cell Endocrinol. 1998;144:21–35. doi: 10.1016/s0303-7207(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem. 2001;8:156–63. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res. 1998;22:243S–247S. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–20. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–49. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–61. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Richter RM, Zorrilla EP, Basso AM, Koob GF, Weiss F. Altered amygdalar CRF release and increased anxiety-like behavior in Sardinian alcohol-preferring rats: a microdialysis and behavioral study. Alcohol Clin Exp Res. 2000;24:1765–72. [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–4. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Sah P, Lopez De Armentia M. Excitatory synaptic transmission in the lateral and central amygdala. Ann N Y Acad Sci. 2003;985:67–77. doi: 10.1111/j.1749-6632.2003.tb07072.x. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–15. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Shibata S, Yamashita K, Yamamoto E, Ozaki T, Ueki S. Effects of benzodiazepine and GABA antagonists on anticonflict effects of antianxiety drugs injected into the rat amygdala in a water-lick suppression test. Psychopharmacology (Berl) 1989;98:38–44. doi: 10.1007/BF00442003. [DOI] [PubMed] [Google Scholar]
- Sipp TL, Blank SE, Lee EG, Meadows GG. Plasma corticosterone response to chronic ethanol consumption and exercise stress. Proc Soc Exp Biol Med. 1993;204:184–90. doi: 10.3181/00379727-204-43650. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–7. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–72. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–81. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]


