Abstract
Enteropathogenic Escherichia coli (EPEC) and enterohemorragic E. coli (EHEC) possess a pathogenicity island (PAI), termed the locus of enterocyte effacement (LEE), which confers the capability to cause the characteristic attaching and effacing lesions of the brush border. Due to this common property, these organisms are also termed attaching and effacing E. coli (AEEC). Sequencing of the EHEC O157 genome recently revealed the presence of other putative PAIs in the chromosome of this organism. In this article, we report on the presence of four of those PAIs in a panel of 133 E. coli strains belonging to different pathogroups and serotypes. One of these PAIs, termed O122 in strain EDL 933 and SpLE3 in strain Sakai, was observed in most of the AEEC strains examined but not in the other groups of E. coli. It was also found to contain the virulence-associated gene efa1/lifA. In EHEC O157, PAI O122 is located 0.7 Mb away from the LEE. Conversely, we demonstrated that in many EHEC non-O157 strains and EPEC strains belonging to eight serogroups, PAI O122 and the LEE are physically linked to form a cointegrated structure. This structure can be considered a mosaic PAI that could have been acquired originally by AEEC. In some clones, such as EHEC O157, the LEE-O122 mosaic PAI might have undergone recombinational events, resulting in the insertion of the portion referred to as PAI O122 in a different location.
Certain strains of Escherichia coli are capable of causing diarrheal diseases in human beings and animals by colonizing the intestinal mucosa with a characteristic mechanism known as attaching and effacing (A/E) (28). Colonizing bacteria induce the effacement of epithelial cell microvilli and develop intimate contact with the cell membrane (9, 13). The E. coli strains that show this pathogenic property are referred as attaching and effacing E. coli (AEEC). AEEC can be divided into two main pathogroups: enterohemorragic E. coli (EHEC) and enteropathogenic E. coli (EPEC) (28). EHEC strains produce Shiga toxins (Stx) and cause severe human illnesses, such as hemorrhagic colitis and hemolytic-uremic syndrome (14, 30). The majority of the cases of disease worldwide are caused by strains of serotype O157:H7, but infections caused by EHEC strains belonging to serogroups other than O157, such as O26, O111, O103, and O145, have been increasingly reported (7, 14). EPEC strains do not produce Stx and are not associated with hemolytic-uremic syndrome but represent an important cause of diarrhea in children, in particular in nonindustrialized countries (28), and in young animals of various species (2, 3, 33, 36, 43).
The capability to cause A/E lesions is conferred by the presence of a chromosomal genetic element defined as the locus of enterocyte effacement (LEE) (10, 23). The LEE is constituted by 41 open reading frames (ORFs) organized in five polycistronic operons: LEE1, LEE2, LEE3, tir, and LEE4 (24). The operons LEE1, LEE2, and LEE3 encode the components of a type III secretion system (24), while the LEE4 operon encode proteins which are secreted by the type III secretion machinery (13). The tir operon contains the eae gene, encoding the outer membrane adhesion molecule intimin (10), and the tir gene, encoding the translocated intimin receptor (18). The low GC content of the LEE (10), together with other features, such as the carriage of virulence genes, the large size, and the insertion in chromosomal loci encoding tRNAs (17, 38, 42), indicates that this locus is a pathogenicity island (PAI) (4, 15, 23).
The LEE is not the only genetic element that AEEC strains have acquired by horizontal transfer. In EHEC strains, Stx genes have been transduced by bacteriophages (30), and in some strains, the enterohemolysin (hlyA) and katalase (katP) genes are harbored by a 90-kb plasmid (5, 37).
Acquisition of foreign DNA can generate new bacterial variants with new virulence properties (12, 15), and phylogenetic analyses have suggested that the gain and loss of mobile virulence elements have frequently occurred in separate lineages of pathogenic E. coli (34). Moreover, some pathogenic clones, such as E. coli O157:H7, may be more likely to acquire foreign DNA through recombination as a side effect of a defective mismatch repair system (20).
Recently, the complete DNA sequences of E. coli O157:H7 strains EDL 933 (32) and Sakai (16) were determined. Genetic analyses showed that the EHEC O157 chromosome contains more than 170 genomic islands which are not present in the E. coli K-12 sequence and that 33% of them harbor genes with unknown functions (16, 32). This large amount of foreign DNA comprises the main known virulence-associated genetic elements of this pathogen, such as the LEE and the Stx-converting phages, and could also encode additional virulence factors or other properties involved in colonization of the gastrointestinal tracts of animal reservoirs or in survival through the steps of the food chain.
Some of the exogenous genomic islands described for the EHEC O157 chromosome can be considered PAIs, since they contain putative virulence genes, have a GC content lower than that of the E. coli chromosome, and are inserted in tRNA locus regions.
In particular, a 22-kb PAI designated O122 in strain EDL 933 (32) and SpLE3 in strain Sakai (16) and located in the PheV tRNA locus 0.7 Mb from the LEE contains the 5′ region of the efa1/lifA gene. This AEEC-associated (19) virulence gene is involved in the capability of EHEC to adhere to CHO cells (29) and in the repression of the host lymphocyte activation response by EPEC (19). Recently, a DNA segment containing efa1/lifA was described for the LEE of a rabbit EPEC O15 strain, which was significantly larger (59 kb) than those in human strains (41).
In this study, we developed a set of molecular tools for investigating the presence of PAI O122 and three other putative PAIs of EHEC O157 in a panel of E. coli strains belonging to various pathogroups and serotypes. We show that PAI O122 is strongly associated with AEEC and that in AEEC other than EHEC O157 it contains the entire efa1/lifA gene. We also show that in many AEEC strains, PAI O122 and the LEE are physically linked to form a larger, mosaic PAI.
MATERIALS AND METHODS
Bacterial strains.
The E. coli strains examined in this study were part of the culture collection of the Istituto Superiore di Sanità and included EPEC, EHEC, enterotoxigenic E. coli (ETEC) (28), enteroaggregative E. coli (EAEC) (25, 28), and cytotoxic necrotizing factor 1-producing E. coli (necrotoxigenic E. coli [NTEC]) (11). Many of these strains were described in previous studies (26, 27, 31). Thirty-one out of 62 EHEC strains and 29 out of 38 EPEC strains were isolated from humans, while the others were isolated from different animal species. The porcine EPEC O45 strain was kindly provided by Josée Harel, Saint Hyacinthe, Quebec, Canada. All of the AEEC strains possessed the intimin-encoding eae gene (31). The EHEC strains produced Stx, as assessed by the Vero cell cytotoxicity assay and PCR amplification of Stx-encoding genes (26). Reference strains E2348/69 (EPEC O127:H6) and EDL 933 (EHEC O157:H7) were also included in this study.
The ETEC strains belonged to serogroups O6, O43, O46, and O147 and included eight strains from humans and two from animal sources. The eight EAEC strains belonged to serogroups O86, O111, O126, and O128 and were isolated from human stools. The eight NTEC strains belonged to serogroups O2, O6, O15, O22, O75, and O83 and were isolated from human urinary tract infections. Five nonpathogenic E. coli strains negative for all of the above-mentioned virulence genes (four isolated from human stools and K-12 strain LE 392) were also included.
Detection of the PAIs of EHEC O157.
The presence of each putative PAI was assessed by dot blot hybridization with three probes corresponding to the left, internal, and right regions of each genomic island. Probes were obtained by PCR amplification of the genomic DNA from EHEC O157 strain EDL 933 with the primer pairs described in Table 1. PCRs were performed with 50-μl reaction mixtures containing 50 ng of template DNA, 0.2 mM deoxynucleoside triphosphates, 1 μM each primer, and 2.5 U of Taq polymerase (Stratagene, Amsterdam, The Netherlands). Genomic DNA was isolated from the bacterial strains by using a NucleoSpin tissue kit (Macherey-Nagel GmbH, Duren, Germany) under the conditions indicated by the manufacturer. Five hundred nanograms of genomic DNA was loaded into each well of a 96-well vacuum manifold and transferred to nylon membranes by applying a vacuum. The filters were washed twice with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and air dried, and DNA was cross-linked by UV exposure. Hybridization, stringent washings, and detection were performed by using an enhanced chemiluminescence direct labeling and detection system under the conditions indicated in the kit manual (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). A PAI was considered present when the strain reacted with at least two of the specific probes. The insertion sites for PAIs O43 and O48 were assessed by PCR amplification of the junctions with the regions shared with E. coli K-12 in the DNA sequence of EHEC O157 strain EDL 933 (Table 1).
TABLE 1.
PCR primers used in this study
| Primer | Target gene | Sequence (5′-3′) | GenBank accession number (nucleotide position) |
|---|---|---|---|
| O7 right probe fwd | Z0262 | TCATGGCACAGACCCGTATTCAGC | AE005199 (1391-1368) |
| O7 right probe rev | TTCATGTCAGGCGCGGTATTTTTG | AE005199 (941-964) | |
| O7 internal probe fwd | Z0260 | CCCAGCCGCCATTACCCACAACAT | AE005198 (10861-10838) |
| O7 internal probe rev | GCTTCAGCGCGTCCGTATCCAGTA | AE005198 (10339-10362) | |
| O7 left probe fwd | Z0245 | AAAAGGTCTGGCATTTGACATTT | AE005197 (2185-2163) |
| O7 left probe rev | GCTGGATATCTCATCTGGATTTTG | AE005197 (1785-1809) | |
| O43/48 right probe fwd | Z1214 | CGGTGGGCGCTTATCAGG | AE005276 (12918-12935) |
| O43/48 right probe rev | GGGCCATCGCGTTTCTCA | AE005276 (13931-13914) | |
| O43/48 internal probe fwd | terA | AACCGGCTGAAACCTGATGTC | AE005273 (8830-8850) |
| O43/48 internal probe rev | AATTGCGCCGTTTTCGTTTAC | AE005273 (9675-9655) | |
| O43/48 left probe fwd | Z1128 | TTGCCTACAGGAAAGACACG | AE005270 (7869-7888) |
| O43/48 left probe rev | TGCTACGCCTCAGAATAATACC | AE005270 (8273-8252) | |
| O43 left junction fwd | clpA | CGCCATACCGTCAGCCGTCTTA | AE005269 (9750-9771) |
| O43 left junction rev | Z1120 | CATTTCCCCCGCCCCTTTACT | AE005270 (409-389) |
| O43 right junction fwd | Z1226 | GGGACCGGTGGGGATTTCAT | AE005277 (5971-5990) |
| O43 right junction rev | serW | CCCCCTCACCGCCAGATTAT | AE005277 (6429-6410) |
| O48 left junction fwd | ycdU | GGGGGACCGCCTGAAATAAATCT | AE005306 (1826-1848) |
| O48 left junction rev | Z1559 | ACTCGCCCCGGAATGTCACTG | AE005306 (2988-2968) |
| O48 right junction fwd | Z1664 | CTGCGGCTGCTGGCTGATG | AE005314 (3417-3435) |
| O48 right junction rev | serX | GTGAGGTGTCCGAGTGGCTGAAG | AE005314 (4286-4264) |
| O122 right probe fwd | Z4334 | AGACCCGCCACCCCACGATGTAT | AE005528 (6644-6666) |
| O122 right probe rev | Z4336 | CTGCGGCCCCGGAAAATGAAA | AE005528 (7801-7781) |
| O122 internal probe fwd | Z4326 | TTCAGGAAAACAAGGGGACAAATA | AE005527 (9726-9749) |
| O122 internal probe rev | TGCCAAGTACGCCACAATA | AE005527 (10640-10622) | |
| O122 left probe fwd | ATACGCCAGAGCCGACCAGACCA | AE005527 (3094-3116) | |
| O122 left probe rev | AACCCAGCGCCCCATCGTATTG | AE005527 (4328-4307) | |
| Efa1 fwd | efa1 5′ region | TGGGCAGAACATTTTCACCAGTTG | AJ277443 (46852-46830) |
| Efa1 rev | CTTTCAGGTGGGGAACCATATGGC | AJ277443 (46111-46127) | |
| Efa1 3′ fwd | efa1 3′ region | TGCGCACAATTGACTACAGAGGAA | AJ277443 (38050-38027) |
| Efa1 3′ rev | ATACGACCATCAGGGGAATCAC | AJ277443 (37337-37358) | |
| EspF fwd | espF | CTTCATTTACTCCCTCTCGTCCGGC | AJ277443 (36069-36093) |
Sequence analysis.
Comparative analysis of nucleotide sequences was performed by using advanced BLAST search program 2.0 within the QBLAST system from the National Center for Biotechnology Information. The molecular maps and the positioning of ORFs were determined by using pDRAW32 software version 1.0 (ACA Clone Software).
Molecular analysis of PAI O122.
PCR amplification was used to detect the presence of the efa1/lifA gene and ORF Z4326, encoding, respectively, adherence factor Efa1 (29) and a protein 58% homologous to enterotoxin ShET2 of Shigella flexneri (6). Since the coding sequence of efa1/lifA is about 10 kb long, the 3′ and 5′ regions of the gene were amplified separately.
The primer sequences and locations are listed in Table 1. PCRs were carried out under the conditions described above.
The location of efa1/lifA in PAI O122 was assessed by amplification of the region between this gene and ORF Z4326 by using primers Efa1 rev and O122 internal probe fwd (Table 1 and Fig. 1). The expected PCR product, based on the EHEC O157 strain EDL 933 genomic sequence, was 6.5 kb. The amplicons were obtained by using Herculase Taq polymerase (Stratagene, La Jolla, Calif.) under the conditions indicated by the supplier.
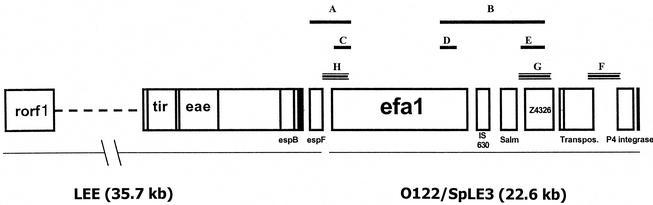
FIG. 1.
Hybridization and PCR strategies used for molecular analysis of the LEE-O122 mosaic PAI in AEEC strains on the basis of the sequence of the LEE region of EHEC O26 strain 413/89-1. Sequences and locations of primers are listed in Table 1. (A) PCR product obtained with primer pair EspF fwd-Efa1 3′ fwd (1.9 kb). (B) PCR product obtained with primer pair O122 internal probe fwd-Efa1 rev (6.5 kb). (C) PCR product obtained with primer pair Efa1 3′ fwd-Efa1 3′ rev (692 bp). (D) PCR product obtained with primer pair Efa1 fwd-Efa1 rev (725 bp). (E) PCR product obtained with primer pair O122 internal probe fwd-O122 internal probe rev (914 bp). (F, G, and H) Hybridization probes corresponding to the left, internal, and right regions of PAIO122, respectively. rorf1, the first ORF in the LEE region of EHEC O26 strain 413/89-1; IS, insertion sequence; Salm, Salmonella sequence; Transpos., transposase.
The primer pair EspF fwd-Efa1 3′ fwd (Table 1 and Fig. 1) was used to assess whether the efa1/lifA gene was adjacent to the espF gene, which corresponds to the last ORF of the LEE. Since the amplicon was expected to be 1.9 kb, PCRs were performed by using the Herculase enzyme.
RESULTS
Besides PAI O122, putative PAIs O7, O43, and O48 present in the sequences of EHEC O157 strain EDL 933 (32) and EHEC O157 strain Sakai (16) were chosen for investigation. PAIs O43 and O48 are two identical copies of the same 88-kb DNA fragment inserted in the serW and serX tRNA loci, respectively (16, 32). PAIs O7 (35.4 kb) and O122 are inserted in the aspV and pheV tRNA loci, respectively (16, 32).
Distribution of the PAIs of EHEC O157 in AEEC strains.
The presence of the PAIs in 133 E. coli strains belonging to different pathogroups and serogroups was investigated. The AEEC strains included 23 EHEC O157, 40 EHEC non-O157, and 39 EPEC strains. Thirty-one E. coli strains belonging to different pathogroups (ETEC, EAEC, and NTEC) or exhibiting none of the considered virulence factors were examined for comparison. For each PAI, genomic DNA was hybridized with three probes specific for the left, internal, and right regions of the island. To distinguish between PAIs O43 and O48, all of the positive isolates were subjected to amplification of the junction regions to identify the insertion sites. The results are shown in Table 2. As expected, PAI O122 was found in all of the EHEC O157 strains investigated and was also very common in both EHEC non-O157 strains (87.5%) and EPEC strains (89.7%). It appeared to be unique to AEEC, since it was not detected in any of the strains belonging to the other pathogroups.
TABLE 2.
Distribution of EHEC O157 putative PAIs in E. coli strains
| E. coli | Serogroup | No. of strains
|
|||||
|---|---|---|---|---|---|---|---|
| Examined | Positive for:
|
||||||
| O#7 | O#43 | O#48 | O#43 + O#48 | O#122 | |||
| EHEC | O18 | 2 | 0 | 0 | 0 | 0 | 0 |
| O26 | 12 | 1 | 8 | 2 | 2 | 12 | |
| O45 | 3 | 3 | 0 | 0 | 0 | 2 | |
| O86 | 1 | 0 | 1 | 0 | 0 | 1 | |
| O103 | 3 | 3 | 2 | 0 | 0 | 3 | |
| O111 | 8 | 8 | 1 | 0 | 7 | 8 | |
| O118 | 3 | 0 | 1 | 1 | 1 | 3 | |
| O121 | 1 | 1 | 1 | 0 | 0 | 1 | |
| O123 | 1 | 0 | 0 | 0 | 1 | 1 | |
| O128 | 1 | 1 | 0 | 0 | 0 | 0 | |
| O145 | 4 | 0 | 4 | 0 | 0 | 4 | |
| O152 | 1 | 1 | 0 | 0 | 0 | 0 | |
| O157 | 23 | 23a | 0 | 6 | 17a | 23 | |
| EPEC | O26 | 9 | 2 | 2 | 1 | 4 | 9 |
| O45 | 1 | 1 | 0 | 0 | 0 | 1 | |
| O55 | 4 | 3 | 1 | 0 | 0 | 4 | |
| O86 | 2 | 0 | 1 | 0 | 0 | 0 | |
| O103 | 2 | 2 | 0 | 0 | 0 | 2 | |
| O111 | 3 | 3 | 1 | 1 | 1 | 3 | |
| O114 | 1 | 1 | 0 | 0 | 0 | 1 | |
| O125 | 3 | 0 | 2 | 0 | 0 | 2 | |
| O127 | 5 | 0 | 1 | 0 | 0 | 5b | |
| O128 | 6 | 3 | 3 | 1 | 0 | 5 | |
| NDc | 3 | 1 | 1 | 1 | 1 | 3 | |
| EAEC | 8 | 4 | 0 | 0 | 0 | 0 | |
| ETEC | 10 | 5 | 1 | 0 | 0 | 0 | |
| NTEC | 8 | 0 | 3 | 0 | 0 | 0 | |
| Nonpathogenicd | 5 | 0 | 0 | 0 | 0 | 0 | |
Including reference strain EDL 933.
Including reference strain E2348/69.
ND, not determined.
Strains showing no virulence genes associated with the other pathogroups.
PAI O7 was found in all of the EHEC O157 strains and in about half of the EHEC non-O157, EPEC, EAEC, and ETEC strains examined but not in NTEC and nonpathogenic E. coli strains. PAIs O43 and O48, in combination or alone, were observed in all of the EHEC O157 strains, in 80.0% of the EHEC non-O157 strains, and in 56.4% of the EPEC strains examined. These PAIs were also present in ETEC strains (10.0%) and NTEC strains (37.5%) but not in EAEC and nonpathogenic E. coli strains.
Molecular analysis of PAI O122 in various AEEC strains.
The sequence data available for EHEC O157 strain EDL 933 indicate that, beside the 5′ region of efa1/lifA, PAI O122 contains another putative virulence gene, ORF Z4326, coding for a protein 38% homologous to enterotoxin ShET2, encoded by the senA gene of S. flexneri (6). Therefore, a PCR approach was developed to investigate the presence of efa1/lifA and ORF Z4326 or portions of their sequences in AEEC strains. Since the entire coding sequence of the efa1/lifA gene was about 10 kb (29), two primer pairs able to amplify the 5′ and 3′ regions were used to assess its presence (Table 1). All 40 PAI O122-negative strains were negative in both the efa1/lifA- and the ORF Z4326-specific PCR assays. The results obtained with the 93 PAI O122-positive strains are shown in Table 3. ORF Z4326 was detected in all of the strains examined. As expected from the strain EDL 933 sequence, all of the EHEC O157 strains lacked the 3′ end of the efa1/lifA gene. The same result was obtained for two of the four EHEC O145 strains examined. Conversely, the complete sequence of the efa1/lifA gene was found in most (58 strains) of the EHEC non-O157 and EPEC strains. Strains of EPEC O125 (two strains examined), EPEC O127 (three out of five strains) EPEC O128 (three out of five strains), and EHEC O86 (one strain) represented a few exceptions.
TABLE 3.
PCR analysis of PAI O#122 in AEEC strains
| E. coli | Serogroup (intimin type) | No. of strains with the indicated PCR profile | PCR amplification (+) or no amplification (−) of:
|
||||
|---|---|---|---|---|---|---|---|
| ORF Z4326a | Efa1-5′b | Efa1-3′c | Efa1- Z4326d | EspF- Efa1e | |||
| EHEC | O26 (β) | 1 | + | − | − | NDf | − |
| O26 (β) | 1 | + | + | + | − | − | |
| O26 (β) | 4 | + | + | + | + | + | |
| O26 (β) | 6 | + | + | + | − | + | |
| O45 (β) | 1 | + | + | + | + | − | |
| O45 (ɛ) | 1 | + | + | + | + | − | |
| O86 (γ) | 1 | + | − | − | ND | − | |
| O103 (ɛ) | 3 | + | + | + | + | − | |
| O111 (γ2) | 1 | + | + | + | − | − | |
| O111 (γ2) | 1 | + | + | + | − | − | |
| O111 (γ2) | 5 | + | + | + | + | − | |
| O111 (γ2) | 1 | + | + | + | + | + | |
| O118 (β) | 2 | + | + | + | + | − | |
| O118 (β) | 1 | + | + | + | + | + | |
| O121 (ɛ) | 1 | + | + | + | + | − | |
| O123 (β) | 1 | + | + | + | − | + | |
| O145 (γ1) | 2 | + | + | − | + | − | |
| O145 (γ1) | 2 | + | + | + | + | +g | |
| O157 (γ1) | 23h | + | + | − | + | − | |
| EPEC | O26 (β) | 4 | + | + | + | + | + |
| O26 (β) | 2 | + | + | + | + | − | |
| O26 (β) | 2 | + | + | + | − | + | |
| O26 (β) | 1 | + | + | + | − | − | |
| O45 (β) | 1 | + | + | + | + | − | |
| O55 (γ) | 2 | + | + | + | + | − | |
| O55 (γ) | 2 | + | + | + | − | − | |
| O55 (α) | 2 | + | + | + | − | − | |
| O103 (β) | 2 | + | + | + | + | − | |
| O111 (β) | 2 | + | + | + | + | − | |
| O111 (β) | 1 | + | + | + | + | + | |
| O114 (β) | 1 | + | + | + | + | + | |
| O125 (α) | 2 | + | − | − | ND | − | |
| O127 (γ) | 3 | + | − | − | ND | − | |
| O127 (α) | 1 | + | + | + | − | − | |
| O127 (α) | 1i | + | + | + | + | + | |
| O128 (β) | 1 | + | − | − | ND | − | |
| O128 (γ) | 2 | + | − | − | ND | − | |
| O128 (β) | 1 | + | + | + | + | − | |
| O128 (β) | 1 | + | + | + | − | + | |
| ND (β) | 1 | + | + | + | + | + | |
| ND (α) | 1 | + | + | + | + | + | |
| ND (β) | 1 | + | + | + | + | − | |
E in Fig. 1.
5′ Region of efa1/lifA (D in Fig. 1).
3′ Region of efa1/lifA (C in Fig. 1).
Linking region between the 5′ region of efa1/lifA and ORF Z4326 (B in Fig. 1).
Linking region between the LEE and PAI O#122 (A in Fig. 1).
ND, not determined.
The amplicon size was 4.5 kb.
Including reference strain EDL 933.
Reference strain E2348/69.
efa1/lifA is located in PAI O122 in most AEEC strains.
To confirm that efa1/lifA is located in PAI O122 in AEEC strains other than EHEC O157 strains, we designed a PCR assay able to amplify the sequence between the 5′ region of efa1 and ORF Z4326 of strain EDL 933. The expected 6.5-kb PCR product was obtained from 66 out of the 83 efa1/lifA-positive strains (Table 3), indicating that the gene is located in PAI O122 regardless of the AEEC pathogroup or serotype.
PAI O122 and LEE are contiguous in many EHEC and EPEC strains.
A recent report described a 15-kb DNA region flanking the LEE in a rabbit EPEC O15:H− strain (41). That region contained both efa1/lifA and ORF Z4326 (senA). Moreover, two identical genes are located in a DNA fragment contiguous to the LEE in EHEC O26:H− strain 413/89-1 (GenBank accession no. AJ277443).
These observations prompted us to develop a PCR strategy to investigate the existence of a similar LEE-O122 PAI in other AEEC clones. Based on the sequence of EHEC O26 strain 413/89-1, a primer pair able to amplify the 1.9-kb region between the espF in the LEE and the 3′ region of efa/lifA in PAI O122 (Fig. 1A) was used to analyze the 58 AEEC strains possessing the entire efa1/lifA gene. The link between the LEE and PAI O122 was shown in 15 EHEC non-O157 strains belonging to five serogroups and 10 EPEC strains belonging to five serogroups (Table 3). The PCR product obtained was of the expected size (1.9 kb) in 23 strains, but a larger product (4.5 kb) was obtained in two EHEC O145 strains.
DISCUSSION
Genomic plasticity has a primary role in the positive selection of organisms, which have to compete for the colonization of ecological niches and/or for the exploitation of limited resources. In bacteria, genomic plasticity is largely enhanced by horizontal gene transfer, a powerful tool in microbial evolution (1). Large DNA fragments containing virulence-associated genes, referred to as PAIs (4, 15), can be exchanged between different bacterial species, and their acquisition can generate new pathogenic variants. PAIs often carry genes derived from plasmids and phages, considered PAIs precursors, which have been assembled together as cointegrated structures and then stabilized by selective pressure through the inactivation of mobility and ricombination genes (22). Several authors have suggested that this patchwork model has been the basis for the emergence of new pathogenic clones (8, 21, 35). EHEC O157 can be considered a good example of such a model of evolution. The analysis of its genome sequence (16, 32) has shown that almost 20% of the chromosome contains foreign DNA absent from the chromosome of E. coli K-12 and that this foreign DNA probably has been acquired from other bacterial species through horizontal gene transfer (34). Like the LEE, other regions of this foreign DNA can be considered putative PAIs, since they carry virulence-associated genes and are located within tRNA loci (16, 32).
In this report, we have shown that some of the putative PAIs of EHEC O157 are present in other AEEC clones and in E. coli strains belonging to other pathogroups. In particular, our results indicated that PAIs O7, O43, and O48 are quite common among pathogenic E. coli, since they have been detected in strains belonging to EHEC, EPEC, EAEC, ETEC, and NTEC pathogroups.
Conversely, the presence of PAI O122 seems to be a peculiar feature of AEEC strains, since it was detected in most of the EHEC and EPEC strains but not in the other groups of E. coli examined. PAI O122 of EHEC O157 strains (16, 32) contains the 5′ region of the virulence-associated gene efa1/lifA (19, 29). In this report, we show that PAI O122 carried by AEEC strains other than EHEC O157 contains the entire gene. efa1/lifA was recently the object of several studies. Nicholls et al. (29) showed that it confers a sevenfold increase in the ability of EHEC O111:H− strain E45035 to adhere to CHO cell monolayers compared to the ability of a defective efa1 mutant of the same strain. A role in the adherence properties of EHEC O157 strain Sakai was also hypothesized for the 5′ region of efa1/lifA (40). Accordingly, the presence efa1/lifA in non-O157 EHEC strains has been associated with the capability of colonizing the intestinal tract of cattle and of inducing diarrhea in young calves (39). Finally, Klapproth et al. (19) showed that efa1/lifA is involved in the repression of host interleukins by EPEC strain E2648/69. Beside efa1/lifA, the presence in PAI O122 of at least another putative virulence gene, such as ORF Z4326 (senA), and its conserved structure in the vast majority of AEEC strains support the possible role of this PAI in the pathogenesis of AEEC infections.
The finding that PAI O122 is strongly associated with AEEC is in good agreement with a previous report from Klapproth et al. (19), who described the presence of efa1/lifA in AEEC but not in other E. coli pathogroups. This strong association with AEEC strains could be due to a physical link between PAI O122 and LEE that is recognizable in the chromosomes of EHEC and EPEC strains belonging to many serogroups. Evidence suggesting such a link was already present in the literature. A DNA sequence containing both the efa1/lifA gene and ORF Z4326 (senA) has been described for the rabbit EPEC O15 strain 83/39 LEE, which is significantly larger than those previously described (41). Moreover, a DNA sequence from EHEC O26:H− strain 413/89-1 in which the LEE is contiguous to a region similar to PAI O122 has been released in GenBank under accession number AJ277443. However, none of these DNA regions had been recognized as PAI O122 of EHEC O157 strains. Using a PCR strategy based on the amplification of the region spanning espF in the LEE and efa/lifA in PAI O122, we showed that the presence of the LEE-O122 mosaic PAI is not restricted to EHEC O26 and EPEC O15 strains, since it was identified in another 25 EHEC non-O157 and EPEC strains belonging to eight different serogroups. The strains found negative in this PCR assay may have the two islands inserted in different chromosomal sites, as in EHEC O157 strains EDL 933 (32) and Sakai (16), or may simply have differences in the sequences of the primer targets. We cannot exclude the possibility that in some strains, the region spanning espF and efa1/lifA was too large to be amplified, since variability in the size of this region was observed.
As far as the origin of the LEE-O122 mosaic PAI is concerned, it is interesting that in both of the EHEC O157 strains that have been sequenced so far (16, 32), the 3′ region of PAI O122 contains a duplication of about 2,000 bp of the 3′ region of the LEE of the same strains. Together with our results, this observation suggests that the LEE and PAI O122 may have been originally acquired as a unique large PAI and that in some clones, such as EHEC O157, they separated later on, following events of chromosomal rearrangement. This hypothesis is also supported by the presence of the LEE-O122 mosaic PAI in strains belonging to all of the AEEC clonal lineages other than EHEC O157: EPEC1, EHEC 2, and EPEC 2 (42). However, we cannot exclude the possibility that the two PAIs had separate origins and that recombination events elicited their fusion in some AEEC clones.
The existence of large PAIs containing the 34-kb core region of the LEE sufficient to induce the A/E phenotype has been described already for EHEC O103 (17) and O26 (GenBank accession number AJ277443) strains and for two rabbit EPEC O103 and O15 strains (41). This report identifies PAI O122 of EHEC O157 strains as a tessera of at least two of those PAIs and describes the presence of the LEE-#O122 mosaic PAI in strains belonging to several AEEC clones. In some other clones, the mosaic PAI may have undergone recombination events, resulting in the insertion of parts of the mosaic in different tRNA loci. Further studies are needed to clarify the structures of the mosaic LEE PAIs and their contributions to the pathogenesis of AEEC infections.
Acknowledgments
This work was supported by a grant from the European Union (E.U. project number QLK2-2000-00600).
The authors are solely responsible for the work described in this article, and their opinions are not necessarily those of the European Union.
Editor: V. J. DiRita
REFERENCES
- 1.Arber, W. 1993. Evolution of prokaryotic genomes. Gene 135:49-56. [DOI] [PubMed] [Google Scholar]
- 2.Beutin, L. 1999. Escherichia coli as a pathogen in dogs and cats. Vet. Res. 30:285-298. [PubMed] [Google Scholar]
- 3.Blanco, J. E., M. Blanco, J. Blanco, A. Mora, L. Balaguer, M. Mourino, A. Juarez, and W. H. Jansen. 1996. O serogroups, biotypes, and eae genes in Escherichia coli strains isolated from diarrheic and healthy rabbits. J. Clin. Microbiol. 34:3101-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum, G., M. Ott, A. Lischewski, A. Ritter, H. Imrich, H. Tschape, and J. Hacker. 1994. Excision of large DNA regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect. Immun. 62:606-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunder, W., H. Schmidt, and H. Karch. 1996. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:3305-3315. [DOI] [PubMed] [Google Scholar]
- 6.Buchrieser, C., P. Glaser, C. Rusniok, H. Nedjari, H. D'Hauteville, F. Kunst, P. Sansonetti, and C. Parsot. 2000. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38:760-771. [DOI] [PubMed] [Google Scholar]
- 7.Caprioli, A., A. E. Tozzi, G. Rizzoni, and H. Karch. 1997. Non-O157 Shiga-toxin-producing Escherichia coli infections in Europe. Emerg. Infect. Dis. 3:578-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De la Cruz, F., and J. Davies. 2000. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 8:128-133. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg, M. S., J. B. Kaper, and B. B. Finlay. 1997. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 5:109-114. [DOI] [PubMed] [Google Scholar]
- 10.Elliott, S., L. A. Wainwright, T. McDaniel, B. MacNamara, M. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 11.Falbo, V., T. Pace, L. Picci, E. Pizzi, and A. Caprioli. 1993. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect. Immun. 61:4909-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 14.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 15.Hacker, J., G. Blum-Oehler, I. Muhldorfer, and H. Tschape. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 23:1089-1097. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 17.Jores, J., L. Rumer, S. Kiessling, J. B. Kaper, and L. H. Wieler. 2001. A novel locus of enterocyte effacement (LEE) pathogenicity island inserted at pheV in bovine Shiga toxin-producing Escherichia coli strain O103:H2. FEMS Microbiol. Lett. 204:75-79. [DOI] [PubMed] [Google Scholar]
- 18.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 19.Klapproth, J. M., I. C. Scaletsky, B. P. McNamara, L. C. Lai, C. Malstrom, S. P. James, and M. S. Donnenberg. 2000. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect. Immun. 68:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeClerc, J. E., B. Li, W. L. Payne, and T. A. Cebula. 1996. High mutation frequencies among Escherichia coli and Salmonella pathogens. Science 274:1208-1211. [DOI] [PubMed] [Google Scholar]
- 21.Lederberg, J. 1997. Infectious disease as an evolutionary paradigm. Emerg. Infect. Dis. 3:417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, C. A. 1996. Pathogenicity islands and the evolution of bacterial pathogens. Infect. Agents Dis. 5:1-7. [PubMed] [Google Scholar]
- 23.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellies, J. L., S. J. Elliott, V. Sperandio, M. S. Donnenberg, and J. B. Kaper. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 25.Morabito, S., H. Karch, P. Mariani-Kurkdjian, H. Schmidt, F. Minelli, E. Binghen, and A. Caprioli. 1998. Enteroaggregative, Shiga toxin-producing Escherichia coli O111:H2 associated with an outbreak of hemolytic-uremic syndrome. J. Clin. Microbiol. 36:840-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morabito, S., H. Karch, H. Schmidt, F. Minelli, P. Mariani-Kurkdjian, F. Allerberger, K. A. Bettelheim, and A. Caprioli. 1999. Molecular characterization of verocytotoxin-producing Escherichia coli of serogroup O111 from different countries. J. Med. Microbiol. 48:891-896. [DOI] [PubMed] [Google Scholar]
- 27.Morabito, S., R. Tozzoli, A. Caprioli, H. Karch, and A. Carattoli. 2002. Detection and characterization of class 1 integrons in enterohemorrhagic Escherichia coli. Microb. Drug Resist. 8:85-91. [DOI] [PubMed] [Google Scholar]
- 28.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien, A. D., J. W. Newland, S. F. Miller, R. K. Holmes, H. W. Smith, and S. B. Formal. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694-696. [DOI] [PubMed] [Google Scholar]
- 31.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marches, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 33.Pohl, P. H., J. E. Peeters, E. R. Jaquemin, P. F. Lintermans, and J. G. Mainil. 1993. Identification of eae sequences in enteropathogenic Escherichia coli strains from rabbits. Infect. Immun. 61:2203-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid, S. D., C. J. Herbelin, A. C. Bumbaugh, R. K. Selander, and T. S. Whittam. 2000. Parallel evolution of virulence in pathogenic Escherichia coli. Nature 406:64-67. [DOI] [PubMed] [Google Scholar]
- 35.Sandner, L., L. E. Eguiarte, A. Navarro, A. Cravioto, and V. Souza. 2001. The elements of the locus of enterocyte effacement in human and wild mammal isolates of Escherichia coli: evolution by assemblage or disruption? Microbiology 147:3149-3158. [DOI] [PubMed] [Google Scholar]
- 36.Saridakis, H. O., S. A. El Gared, M. C. Vidotto, and B. E. C. Guth. 1997. Virulence properties of Escherichia coli strains belonging to enteropathogenic (EPEC) serogroups isolated from calves with diarrhea. Vet. Microbiol. 54:145-153. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperandio, V., J. B. Kaper, M. R. Bortolini, B. C. Neves, R. Keller, and L. R. Trabulsi. 1998. Characterization of the locus of enterocyte effacement (LEE) in different enteropathogenic Escherichia coli (EPEC) and Shiga-toxin producing Escherichia coli (STEC) serotypes. FEMS Microbiol. Lett. 164:133-139. [DOI] [PubMed] [Google Scholar]
- 39.Stevens, M. P., P. M. van Diemen, G. Frankel, A. D. Phillips, and T. S. Wallis. 2002. Efa1 influences colonization of the bovine intestine by Shiga-toxin producing Escherichia coli serotypes O5 and O111. Infect. Immun. 70:5158-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatsuno, I., H. Kimura, A. Okutani, K. Kanamaru, H. Abe, S. Nagai, K. Makino, H. Shinagawa, M. Yoshida, K. Sato, J. Nakamoto, T. Tobe, and C. Sasakawa. 2000. Isolation and characterization of mini-Tn5Km2 insertion mutants of enterohemorragic Escherichia coli O157:H7 deficient in adherence to Caco-2 cells. Infect. Immun. 68:5943-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tauschek, M., A. R. Strugnell, and R. M. Robins-Browne. 2002. Characterization and evidence of mobilization of the LEE pathogenicity island of rabbit-specific strains of enteropathogenic Escherichia coli. Mol. Microbiol. 44:1533-1550. [DOI] [PubMed] [Google Scholar]
- 42.Wieler, L. H., T. K. McDaniel, T. S. Whittam, and J. B. Kaper. 1997. Insertion site of the locus of enterocyte effacement in enteropathogenic and enterohemorrhagic Escherichia coli differs in relation to the clonal phylogeny of the strains. FEMS Microbiol. Lett. 156:49-53. [DOI] [PubMed] [Google Scholar]
- 43.Zhu, C., J. Harel, M. Jacques, C. Desautels, M. S. Donnenberg, M. Beaudry, and J. M. Fairbrother. 1994. Virulence properties and attaching-effacing activity of Escherichia coli O45 from swine postweaning diarrhea. Infect. Immun. 62:4153-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]