Abstract
The type 1 sphingosine 1-phosphate (S1P) G protein-coupled receptor (S1P1) transduces signals from S1P that mediate thymocyte emigration, T cell transmigration of lymph nodes, and T cell chemotaxis in tissues. Alterations in expression of functional S1P1 receptors by lymphocytes are the major mechanisms controlling their responses to S1P and were thought to be solely a consequence of the balance between surface down-regulation and insertion. However, results now show that lack of sulfation of tyrosines 19 and 22 of the extracellular N terminus of S1P1 diminishes high-affinity S1P binding and decreases S1P signaling of T cell migration and other functions. Non-sulfatable mutant (Y19,22F)S1P1 endows T cells with lower-affinity binding of [32P]S1P than wild-type S1P1 and transduces lesser effects of S1P on chemotaxis, chemokine-elicited chemotaxis, and T cell receptor-mediated proliferation and cytokine generation. Inhibition of S1P1 tyrosine sulfation or sulfatase removal of S1P1 sulfate in mouse CD4 T cells suppresses immune functional effects of S1P. Tyrosine sulfation of S1P1 may be a major controller of S1P effects on T cell traffic.
Keywords: T cell receptors, lipid mediators, chemotaxis, cytokines
The lysosphingolipid mediator sphingosine 1-phosphate (S1P) affects many functions of different types of cells through a family of five G protein-coupled receptors (GPCRs) designated S1P1 to S1P5 (1, 2). T and B lymphocytes express predominantly S1P1, S1P4, and a much lower level of S1P3, which are down-regulated by immune activation (3, 4). In T cells, only S1P1 is coupled effectively to S1P stimulation of chemotaxis and to S1P inhibition of chemotaxis to chemokines (5). As constitutive concentrations of S1P in most physiological fluids and tissues are sufficiently high to evoke maximal S1P1-dependent effects on normal lymphocytes, it has been assumed that change in the level of expression of functional S1P1 by T cells is the principal mechanism for regional immune tissue control of signal transduction by the S1P-S1P1 axis. A subset of T cells newly isolated from lymphoid organs has greater mean chemotactic responses to S1P than those from blood and lymph. One group has presented evidence that these differences in lymphocyte responses to S1P result from S1P-mediated down-regulation of the level of S1P1 on lymphocytes in blood, lymph, and lymph node compartments with recent immigrants, in contrast to a higher level of S1P1 on lymphocytes departing from lymph nodes in response to S1P (6). However, this is a highly controversial point. It is unclear to what extent S1P-induced translocation of S1P1 receptors from lymphocyte-surface plasma membranes to intracellular lymphocyte compartments, where they are uncoupled from G protein signaling, contributes to down-regulation of effective S1P1 signal transduction. We found only minor differences between the levels of expression of S1P1 by T cells isolated from mouse blood, lymph, and peripheral lymph nodes. Therefore, we have searched for selective posttranslational modifications and alterations in signaling pathways of S1P1, which might account for observed fluctuations in T cell responsiveness to S1P in the absence of major changes in surface membrane levels of S1P1 protein.
Physiological posttranslational tyrosine O-sulfation of proteins is catalyzed by members of a family of membrane-bound tyrosylprotein sulfotransferases (EC 2.8.2.20) in the lumen of the trans-Golgi network (7–9). Although initial observations focused on tyrosine O-sulfation of peptide hormones and other small peptides, the posttranslational sulfation modification has been observed subsequently in many biologically active proteins, including adhesion molecules, connective tissue matrix proteins, immunoglobulins, coagulation factors, diverse other enzymes, and GPCRs for chemokines, the complement-derived peptide inflammatory mediator C5a, and the thyroid- stimulating hormone TSH (10). Two tyrosine-aspartic acid sequences now have been identified as potential targets of tyrosine O-sulfation in S1P1, but this motif is absent from other S1P GPCRs. These sulfo-tyrosines are required for high-affinity S1P1 binding and S1P1-mediated effects on T cell functions.
MATERIALS AND METHODS
Cells and nucleofection techniques
CD4 T cells were isolated from spleens of 6–10 week-old C57BL/6 female mice by immunobead magnetic absorption column chromatography (Miltenyi Biotec, Auburn, CA) as described (11). The purity of the CD4 T cells was at least 93%, as assessed by flow cytometry. Jurkat human leukemic T cells (Dr. Arthur Weiss, UCSF) were cultured at 1 to 5 × 105/ml in RPMI-1640 with 10% heat-treated fetal bovine serum, 100 U/ml of penicillin G, and 100 μg/ml of streptomycin. Jurkat T cells were transfected with two different pcDNA3 expression constructs, one of which contains the sequence encoding full-length human wild-type S1P1 and the other a sequence encoding a human mutant S1P1 in which the bases for tyrosines 19 and 22 both have been replaced with those for phenylalanine (Y19,22F) (QuikChange Site-Directed Mutagenesis Kit, Stratagene, La Jolla, CA). Each construct also contains sequence encoding a c-myc epitope tag at the carboxyl-terminus of S1P1. Nucleofection was performed with the Amaxa Biosystems apparatus (Gaithersburg, MD), their Nucleofector kit V, 3 ug of plasmid DNA per 3 × 106 Jurkat T cells, and program T-14. Viability was at least 90% at 24 h after the procedure.
Quantification of S1P1 expression and [32P]S1P binding
The levels of human wild-type and mutant S1P1 mRNA and protein in Jurkat T cell transfectants and of mouse S1P1 mRNA and protein in isolated mouse splenic T cells before and after treatments and stimulation were determined by real-time PCR and Western blots, respectively. Real-time PCR was performed as described (3). Rabbit anti-S1P1 antibody was generated with a 46-amino acid synthetic peptide representing the N terminus of human S1P1. This antibody detected only one 42–48 kDa band in Western blots of 10 ug of cellular proteins extracted from S1P GPCR null HTC4 rat hepatoma cells transfected with S1P1, but not of those transfected only with other S1P GPCRs. Rabbit anti-S1P1 antibody also detected one band of the same size in Western blots of 5 ug of selectively extracted membrane proteins from freshly isolated mouse splenic T cells and human blood T cells (Mem-PER membrane protein extraction kit and PAGE prep Advance kit, Pierce Biotechnology, Inc., Rockford, IL). The membrane S1P1 bands were markedly diminished after 24 h of incubation with adherent anti-CD3 plus anti-CD28 antibodies due to down-regulation and loss of surface membrane S1P1, as described (3, 12). Western blots of extracts of Jurkat T cell transfectants also were developed with mouse anti-c-myc (EQKLISEEDL) IgG1, kappa monoclonal antibody (clone 9E10, Roche Diagnostics Corp., Indianapolis, IN).
[32P]S1P was prepared and binding assays were performed as described (13), with minor modifications. HEK293 human embryonic kidney cells were transiently transfected separately with c-myc epitope tagged constructs of human wild-type and Y19,22F mutant S1P1 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Two days later, transfectants were washed twice in Tris-buffered saline (pH 7.4, TBS) and pre-incubated for 10 min on ice in TBS containing 4 mg/ml of fatty acid-free BSA with and without 10 μM unlabeled S1P. [32P]S1P was then added at 10 to 600 nM, and transfectants were incubated for 30 min on ice, washed twice in TBS containing 0.4 mg/ml fatty acid-free BSA, and lysed in 0.5% SDS for quantitation of binding by scintillation counting.
Determination of sulfation of S1P1
Suspensions of 3 to 5 × 106 Jurkat T cell-transfectants or mouse splenic CD4 T cells per milliliter of phosphate-free RPMI-1640 with 10% charcoal- and dextran-absorbed (S1P-free) fetal bovine serum, 1 mM MgCl2, 100 U/ml of penicillin, and 100 ug/ml of streptomycin were preincubated for 1 h without and with 10 mM sodium chlorate or 2 h without and with 1 U of arylsulfatase (from Aerobacter aerogenes, Sigma-Aldrich, St. Louis, MO) prior to addition of 0.5–1 mCi of 35S-sulfate (MP Biomedicals, Irvine, CA) and further incubation for an additional 24–48 h. The T cells then were harvested, washed twice, and re-suspended in 0.1 ml of 0.1 M sodium acetate (pH 4.5) containing HALT broadly specific mixture of protease inhibitors (Pierce Biotechnology, Rockford, IL) in 1.5 ml Eppendorf tubes, and incubated for 30 min at 37°C. These T cell suspensions received 1% (v:v) NP-40 detergent (Sigma-Aldrich), were incubated with rocking for 30 min at 4°C, homogenized for 30 s with an electric motor-driven plastic pestle (Kontes Glass Co., Vineland, NJ), re-incubated for 30 min at 4°C, homogenized again for 30 s, and centrifuged at 2,000 × g for 5 min at 4°C. Of each 2000 × g supernate, 15% was removed, pH was adjusted to 7.5 with 1 M Tris-HCl (pH=9.5), and chondroitinase ABC (0.5 U/sample, affinity-purified from Proteus vulgaris, Sigma-Aldrich) was added before incubation for 60 min at 37°C. The remaining 85% of each sample was incubated with 5 μl of rabbit anti-S1P1 serum and/or 5 ug of mouse anti-c-myc antibody for 30 min at 37°C and 16 h at 4°C, and then 50 ul of suspension of agarose-coupled protein G (Pierce Biotechnology) for 1 h at 37°C and 4 h at 4°C. Each suspension of agarose-protein G was sedimented at 1000 × g and washed twice with 1 ml of 0.1 M sodium acetate (pH 6.0) prior to quantification of 35S04 by liquid scintillation counting. The chondroitinase ABC-treated 2000 × g supernates of homogenates of each sample were boiled in Laemmli’s solution (4:1, v:v) and electrophoresed in 12% polyacrylamide-SDS gels (Invitrogen Life Technology), which were analyzed for 35S by phosphor-imaging after drying and application of Fluoro-hance (RPI Corp., Mt. Prospect, IL).
Measurement of chemotaxis, proliferation, and gamma-interferon (IFN-gamma) generation
Chemotaxis of Jurkat T cell transfectants to S1P (Sigma-Aldrich) and CXCL-12 (Peprotech, Rocky Hill, NJ) and of mouse splenic CD4 T cells to S1P, CCL-21, and CCL-5 (Peprotech) without and after pretreatment in 10 mM sodium chlorate for 24 h or 1 U/ml of arylsulfatase for 4 h, as for studies of sulfation, was quantified as described (5). The medium was RPMI-1640 with 10% charcoal- and dextran-extracted fetal bovine serum, Transwell chemotactic chambers had 5 um pore filters that had been coated with 100 ug/ml of collagen, and the number of T cells migrating through filters in 4 h are expressed as a percentage of those added initially to the upper compartment. Effects of S1P on proliferation of Jurkat T cell transfectants and mouse splenic CD4 T cells without and after the same pretreatments with sodium chlorate or arylsulfatase were determined by uptake of 3H-thymidine (ICN Pharmaceuticals, Inc., Costa Mesa, CA), as described (14). Replicate suspensions of 2 × 105 T cells in 0.4 ml of RPMI-1640 with 10% charcoal- and dextran-extracted fetal bovine serum, 100 U/ml of penicillin, and 50 ug/ml of streptomycin were incubated in 48-well plates without or with 10−9 to 10−6 M S1P and without or with stimulation by 0.5 ug per well of adherent anti-CD3 antibody plus 10 ng/ml of phorbol myristate acetate for Jurkat T cell transfectants and 0.5 ug each per well of adherent anti-CD3 and anti-CD28 antibodies for mouse CD4 T cells. After 24 h of incubation, each well received 1 uCi of 3H- thymidine and was incubated for an additional 24 h before harvesting cells for quantification of radioactivity (14). Aliquots of supernatant medium (50 μl) were removed from each well of the cultures of mouse CD4 T cells after 24 h for ELISA measurements of IFN-gamma, as described (14).
RESULTS
The amino-terminal amino acid sequences of human and mouse S1P1 contain two tyrosine residues (19 and 22) flanked by aspartic acid (Fig. 1). S1P2 has one tyrosine with a single adjacent glutamic acid, which has not been a site for sulfation; none of the other S1P GPCRs has a tyrosine with a neighboring aspartic acid or glutamic acid. Similar tyrosine motifs with flanking aspartic acid residues also have been observed in four of the chemokine GPCRs (Fig. 1), some glycoprotein hormone GPCRs and other diverse biologically active proteins (15–17). As sulfation of these tyrosines in chemokine and glycoprotein hormone GPCRs is required for their recognition of ligands and signal transduction, the dependence of S1P1 functions on tyrosine sulfation was examined in T cell systems.
Figure 1.

Tyrosine sulfation sites in the amino-terminal sequences of S1P GPCRs and some chemokine GPCRs. For S1P1, h = human and m = mouse. The CCR8 sequence is mouse and all of the other chemokine GPCRs are human. Tyrosine (Y) residues near one or more acidic amino acids (D or E) are underlined.
Introduction of c-myc-tagged wild-type and mutant (Y19,22F)S1P1 into Jurkat T cells by the nucleofection modification of electroporation (Amaxa) resulted in similar levels of total expression of both types of S1P1, as assessed by real-time PCR quantification of mRNA encoding S1P1 (Fig. 2A) and Western blot analyses of extracted S1P1 protein-cmyc tag (Fig. 2B). S1P1 mRNA content was maximal in the first 24 h after completion of transfection and declined progressively in the next 48 h, whereas S1P1 protein was at similar plateau levels for at least 48 h in both the wild-type and Y19,22F mutant S1P1 transfectants. The levels of Jurkat T cell surface S1P1 also were similar in the wild-type and mutant S1P1 transfectants, as assessed by Western blots of cell membrane protein-selective extracts (not shown) and flow cytometric detection of surface S1P1 protein (Fig. 2C). Thus biochemical and functional differences between the wild-type and mutant (Y19,22F)S1P1 Jurkat T cell transfectants observed at 24 and 48 h are not attributable to dissimilar levels of expression of the respective S1P1 GPCRs.
Figure 2.
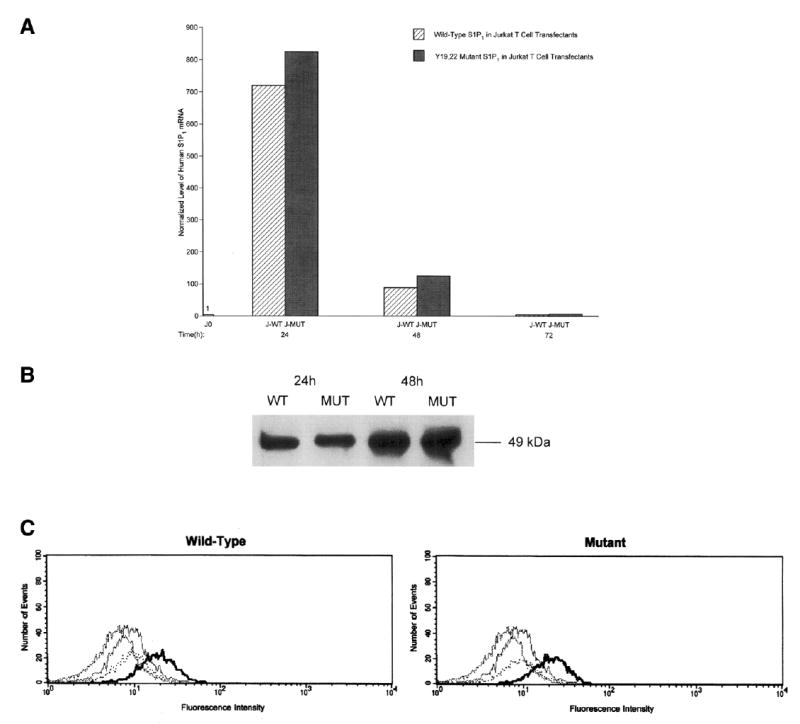
Demonstration of equal expression of human wild-type and Y19,22F mutant S1P1 GPCRs in Jurkat T cell transfectants. A) Levels of human S1P1 mRNA in Jurkat transfectants were determined by real-time PCR by using expression of hypoxanthine phosphoribosyl transferase (HPRT) for normalization of each value. J0 = untransfected Jurkat T cell; J-WT = Jurkat T cell transfected with wild-type S1P1; J-MUT = Jurkat T cell transfected with Y19,22F mutant S1P1. B) Western blot analysis of c-myc in S1P1 protein of Jurkat transfectants and other cell lines. Each lane received 10 ug of total cellular proteins extracted from Jurkat T cell transfectants at 24 and 48 h after nucleofection. Lanes from left to right: J-WT at 24 h; J-MUT at 24 h; J-WT at 48 h; and J-MUT at 48 h. C) Flow cytometric quantification of expression of wild-type (left-hand frame) and Y19,22F mutant (right-hand frame) S1P1 receptors in Jurkat T cell transfectants at 48 h after nucleofection. The dark-line tracing in each frame depicts results from incubation of transfectant with rabbit anti-S1P1 antibody plus Alexa-Fluor 488-labeled goat anti-rabbit IgG (Molecular Probes Inc., Eugene, OR), and the other lighter-line tracings are from untransfected Jurkat T cells incubated with both antibodies (second largest rightward shift), and J-WT and J-MUT transfectants incubated with only Alexa-Fluor 488-labeled second antibody.
Sulfation of Y19 and Y22 in wild-type S1P1 of Jurkat S1P1 transfectants and mouse splenic CD4 T cells was demonstrated by uptake of 35SO4 specifically into a 42–48 kDa protein that was absent in Jurkat Y19,22F mutant S1P1 transfectants and less intensely labeled in Jurkat wild-type S1P1 transfectants and in mouse CD4 T cells, which had been preincubated with the tyrosine sulfation inhibitor sodium chlorate (Fig. 3). The level of S1P1 total mRNA and protein, and of surface S1P1 protein, expressed in mouse CD4 T cells was not changed significantly by treatment with sodium chlorate. 35SO4-tyrosine-labeled S1P1 protein then was precipitated separately by anti-S1P1 polyclonal antibody and anti-c-myc monoclonal antibody, which yielded significantly lower levels of 35S from Y19,22F mutant (MUT) S1P1 and sodium chlorate-treated wild-type (WT) S1P1 Jurkat T cell transfectants than untreated WT S1P1 Jurkat T cell transfectants and from sodium chlorate-treated mouse splenic CD4 T cells than untreated mouse splenic CD4 T cells (Table 1).
Figure 3.

Sulfation of S1P1 receptors of Jurkat T cell transfectants and mouse splenic CD4 T cells. The five lanes in order from left to right are CD4 T cells treated with sodium chlorate (SC); untreated CD4 T cells; untreated J-MUT; untreated J-WT; and J-WT treated with SC.
Table 1.
Immunoprecipitation of 35SO4 tyrosine-labeled S1P1 Rs in transfectants
| J-WT | J-MUT | J-WT+SC | Mouse CD4 T cells | Mouse CD4 T cells+SC | |
|---|---|---|---|---|---|
| A-S1P1 | 2106-3482 | 325-604* | 784-1123+ | 2205-3640 | 703-991* |
| A-c-myc | 2418-3644 | 459-913* | 927-1405+ | 2108-4113 | 694-1322* |
Each number is the range of cpm detected in three different studies. J-WT, Jurkat T cells expressing the human wild-type S1P1 receptor; J-MUT, Jurkat T cells expressing the human Y19,22F mutant S1P1 receptor; SC = sodium chlorate. Statistical significance of differences from WT/untreated levels was calculated by a paired t test;
P < 0.05 and
P < 0.01.
The specific binding of [32P]S1P by the mutant S1P1 transfectant was significantly less than that by the wild-type S1P1 transfectant at 30, 60, 100, 300 and 600 nM S1P (Fig. 4). This difference is attributable predominately to a lower-affinity of the same number of S1P1 receptors, as revealed by computer-assisted analyses of Scatchard plots. The respective mean Bmax values (n=3) are 3.82 pmol for wild-type S1P1 and 3.64 pmol for mutant S1P1, which confirms their expression of nearly the same total number of cellular S1P1 receptors. The different binding affinities of wild-type and Y19,22F mutant S1P1 receptors were reflected in their respective Kd values of 18 and 52 nM (n=3). The mean affinity of wild-type S1P1 for [32P]S1P, therefore, was determined to be approximately threefold higher than that of Y19,22F mutant S1P1.
Figure 4.
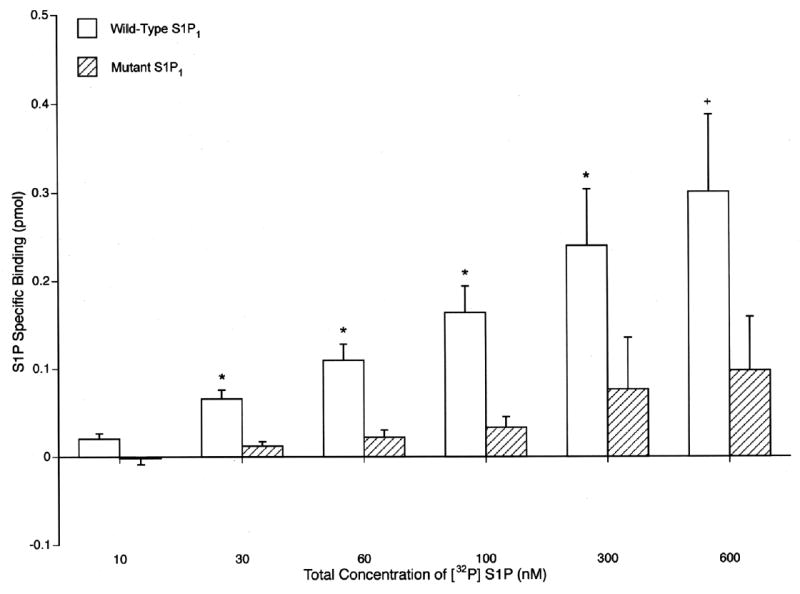
Lower affinity of binding of [32P]-S1P by Y19,22F mutant S1P1 than wild-type S1P1 in HEK293 cell transfectants. Each column and bar shows the mean ± SD of the results of triplicate determinations. The significance of differences between binding by wild-type and mutant S1P1 was calculated by a two-sample ttest and depicted by +P < 0.05 and *P < 0.025.
The dependence of T cell functional responses to S1P on sulfation of Y19 and Y22 was investigated initially in terms of both direct S1P-elicited chemotaxis and S1P inhibition of chemokine-induced chemotaxis of Jurkat S1P1 transfectants. At 10−8 and 10−7 M, S1P elicited significant chemotaxis of Jurkat wild-type S1P1 transfectants, but not of Jurkat mutant S1P1 transfectants (Fig. 5A). In two of these studies, mean net chemotactic responses to 3 × 10−8 M and 3 × 10−7 M S1P were 10.8 and 6.5% of initial Jurkat wild-type S1P1 transfectants, respectively, but only 2.9 and 2.3% of initial Jurkat mutant S1P1 transfectants. When added to the T cell compartment of chemotactic chambers at 3 × 10−7 and 3 × 10−6 M, S1P significantly inhibited chemotaxis of Jurkat wild-type S1P1 transfectants to the chemokine CXCL-12 (SDF-1α) without altering the responses of Jurkat mutant S1P1 transfectants (Fig. 5B). When mouse CD4 T cells, in which S1P1 is the predominant S1P receptor, were incubated with 10 mM sodium chlorate under conditions that reduced tyrosine sulfation by ~65% (Fig. 3), chemotaxis to 10−8 and 10−7 M S1P was suppressed by respective means of 70 and 62% (Fig. 5C). In contrast, sodium chlorate treatment was much less inhibitory for responses to optimal concentrations of the chemokines CCL-21 and CCL-5. Preincubation of mouse CD4 T cells in a concentration of arylsulfatase that rapidly removed more than 85% of sulfate from the tyrosine residues (data not shown) suppressed chemotaxis to S1P by means of over 80 to 95% (Fig. 5C). Arylsulfatase also distinguished between the responses to the two chemokines, as there was greater inhibition of chemotaxis elicited by CCL-5 than by CCL-21 (Fig. 5C). Arylsulfatase inhibition of chemotaxis to CCL-5 was a mean of 42 to over 50%, whereas there was only marginal reduction in chemotaxis to the lower concentration of CCL-21. As discussed, this is attributable to functionally significant tyrosine sulfation of the relevant chemokine GPCRs.
Figure 5.

Requirement for S1P1 tyrosine sulfation in S1P chemotactic effects on Jurkat T cell transfectants and mouse CD4 T cells. A) Direct stimulation of chemotaxis by S1P. Each column and bar depicts the mean ± SD of the results of four studies conducted in duplicate. B) Suppression of CXCL-12-elicited chemotaxis by S1P in the T cell (upper) compartment. CXCL-12 was at 100 nM. Significant differences between migration evoked by S1P and that of unstimulated Jurkat cells and significant suppression by S1P of chemotaxis induced by CXCL-12 were calculated by a two-sample t-test and are depicted by +P < 0.05 and *P < 0.01. C) Suppression of mouse CD4 T cell chemotaxis to S1P by sodium chlorate (SC) inhibition of sulfation and by arylsulfatase cleavage of tyrosine sulfates. Each column and bar depicts the mean ± SD of the results of three studies conducted in duplicate. The meanings of statistical symbols are the same as in (A and B) and depict significance of suppression.
The requirement for S1P1 tyrosine sulfation in migration of T cells also was observed in studies of other immune functions. T cell receptor-driven proliferation of Jurkat T cells expressing wild-type S1P1 receptors was decreased significantly by S1P with maximal mean suppression of 35% at 10−7 M S1P, whereas proliferation of Jurkat T cells expressing Y19,22F mutant S1P1 receptors was not affected by any concentration of S1P (Fig. 6A). In related analyses of mouse spleen CD4 T cells, S1P suppressed T cell receptor-driven proliferation significantly in a concentration-dependent relationship up to a mean of 32% at 10−7 M S1P (Fig. 6B). Pretreatment of the mouse T cells with sodium chlorate or arylsulfatase, under conditions that reduced tyrosine sulfation of S1P1 receptors, eliminated S1P suppression of proliferation and elicited slight stimulation of proliferation by the lowest concentration of S1P tested (Fig. 6B). Quantification of IFN-gamma in culture fluids from the mouse CD4 T cells, revealed significant S1P concentration-dependent inhibition with more than 50% suppression at 10−8 M and nearly 90% suppression at 10−6 M S1P (Fig. 7). As for S1P signaling of T cell migration and proliferation, similar preincubation of mouse T cells with sodium chlorate or arylsulfatase eliminated any effect of the same concentrations of S1P on T cell receptor-driven production of IFN-γ (Fig. 7).
Figure 6.
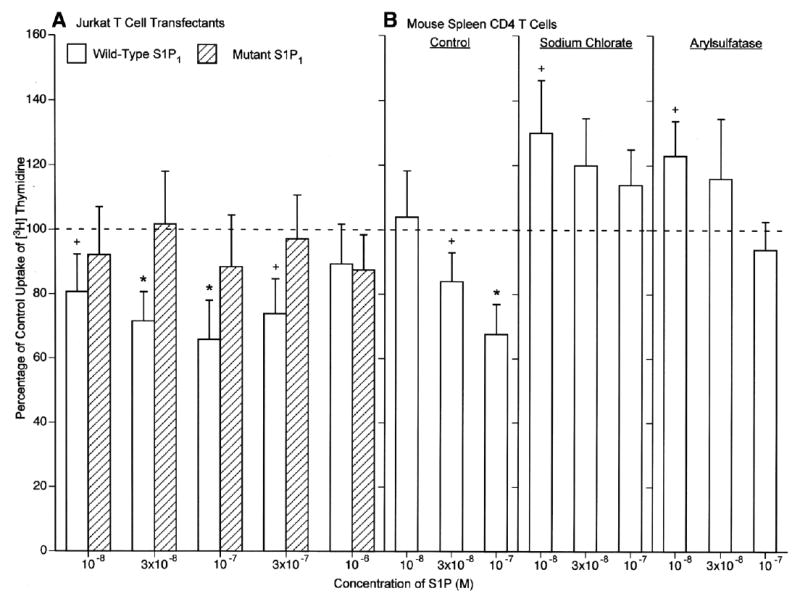
Requirement for S1P1 sulfation in S1P suppression of T cell proliferation. A) Jurkat T cells nucleofected with either the wild-type or Y19,22F mutant S1P1 receptor were incubated without (control=100%) or with S1P and stimulated with anti-CD3 antibody and phorbol myristate acetate. B) Mouse spleen CD4 T cells were preincubated without (control) and with sodium chlorate and arylsulfatase as in Fig. 5, incubated without (100%) or with S1P, and stimulated with anti-CD3 and anti-CD28 antibodies. A, B) each column and bar is the mean ± SD of three sets of results. Statistical significance was calculated and depicted as in Fig. 5.
Figure 7.
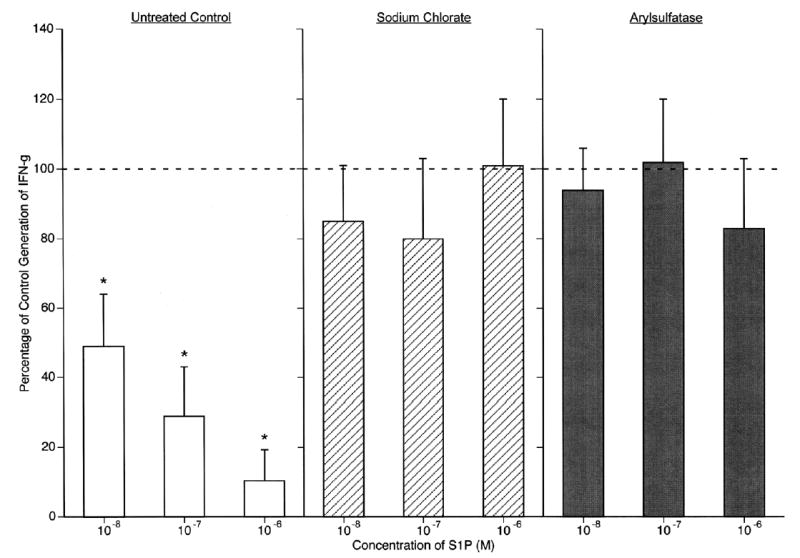
Requirement for S1P1 sulfation in S1P suppression of mouse spleen CD4 T cell generation of IFN-gamma. Mouse CD4 T cells were preincubated with sodium chlorate and arylsulfatase as in Fig. 5, incubated without (100% control) or with S1P, and stimulated with anti-CD3 and anti-CD28 antibodies. Supernates were harvested after 24 h for ELISA assays. Each column and bar is the mean ± SD of three sets of results, and statistical significance was calculated and depicted as in Fig. 5. Mean control (100%) levels of IFN-gamma were 6.6, 4.8, and 5.9 ng/ml, respectively, for control, sodium chlorate-treated, and arylsulfatase-treated T cells.
DISCUSSION
The present limited knowledge of mechanisms regulating cellular expression of S1P1 receptors suggests differences from those regulating some other GPCRs, which may possibly be attributable to the usual persistent exposure of S1P receptors to high nanomolar to micromolar levels of its ligand S1P in vivo. Re-introduction of S1P into suspensions of lymphocytes that have been deprived of S1P results in nearly complete down-regulation of S1P1 receptors, full return of their expression within hours, and sustained expression of normal levels of S1P1 receptors despite maintenance of normal blood concentrations of S1P (11). Thus the level of expression of S1P1 receptors by lymphocytes is very insensitive to steady-state concentrations of S1P. The other relevant recent finding is a poor relationship between responsiveness of lymphocyte migration to S1P and their level of expression of S1P1 receptor surface-membrane protein. Our search for other mechanisms regulating lymphocyte expression of fully functional S1P1 receptors led to the discovery of sulfation of amino-terminal tyrosines, which is required for high-affinity binding of S1P and consequent signal transduction.
Expression of wild-type S1P1 and a mutant (Y19,22F)S1P1, which is not susceptible to amino-terminal sulfation, in a model T cell system revealed no differences in their level of S1P1 mRNA, total protein or cell-surface protein expression (Fig. 2). The lack of sulfation of the mutant (Y19,22F) S1P1, however, diminished the affinity of binding of S1P and S1P effects on migration and proliferation (Table 1, Figs. 3–6). That total binding of [32P]S1P by model T cells expressing wild-type S1P1 receptors was the same as those bearing Y19,22F mutant S1P1 receptors, whereas the affinity of the former was half an order-of-magnitude-higher confirms the prediction that specific characteristics of the N terminus are principally determinants of binding affinity (Fig. 4) (18). Findings were extended to native S1P1 receptors and a wider range of immune functions by studies of mouse splenic CD4 T cells. Inhibition of sulfation and cleavage of sulfates of native S1P1 receptors (Fig. 3), under conditions that did not alter the level of S1P1 protein expression in T cell membranes, significantly reduced S1P effects on T cell migration, proliferation and cytokine generation (Figs. 5–7).
Confirmation of the role of tyrosine O-sulfation in functions of other lymphocyte GPCRs for migration-regulating factors came indirectly from demonstration of differential effects of de-sulfation on chemokine GPCR activities. Arylsulfatase distinguished between the T cell chemotactic responses to the two chemokines examined, as there was greater inhibition of chemotaxis elicited by CCL-5 than by CCL-21 (Fig. 5C). These results are not unexpected because the CCR7 GPCR for CCL-21 has no sulfation-susceptible tyrosine residues in its amino-terminal sequence and thus would not be affected by sodium chlorate and arylsulfatase treatments. The partial susceptibility of chemotactic responses to CCL-5 to arylsulfatase inhibition appears to be attributable to the dependence of ligand recognition by one of its GPCRs, namely CCR5, on sulfation of amino-terminal tyrosine residues (Fig. 1), whereas its other two alternative GPCRs CCR1 and CCR3 have no sulfatable tyrosine residues.
The demonstration that lack of sulfation of critical tyrosines in the N terminus of S1P1 impairs both high-affinity binding of S1P (Fig. 4) and S1P1 mediation of effects of S1P on T cell functions (Figs. 5–7) does not prove that defective binding of S1P alone is the cause of diminished functional responses to S1P. The data argue against any tyrosine sulfation requirement for production or cell-surface expression of S1P1 in lymphocytes, as neither genetic deletion nor biochemical reductions in sulfation altered the level of detectable cell-surface S1P1 protein (Fig. 2C). However, it remains to be determined whether tyrosine sulfates separately influence S1P1 signaling, S1P-evoked down-regulation and intracellular traffic of S1P1, or regulation of expression of S1P1 by immune stimuli. One of the major deficiencies in our understanding of how the S1P-S1P1 axis regulates lymphocyte traffic is the lack of knowledge of lymphocyte mechanisms for controlling tissue site-specific differences in their level of expression of functional S1P1 receptors. A recent report suggests that rapid fluctuation in lymphocyte-surface expression of S1P1 protein is the principal means of controlling functional responses of lymphocytes to S1P (6), but the true role of this one mechanism has not been established definitively. The possible contributions of altered states of tyrosine sulfation, differences in other posttranslational modifications, and variations in coupling to signaling pathways now are being delineated in several systems. The involvement of any one mechanism may differ with the stimulus and specific immune tissue site.
Acknowledgments
The authors are grateful to Yvonne Kong for expert technical assistance and to Robert Chan for highly skilled assistance with graphics and manuscript preparation. This research was supported by grants PO-HL68738 and RO1-HL-31809 from the National Institutes of Health.
References
- 1.Chun J, Goetzl E, Hla T, Ingarashi Y, Lynch K, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Tigyi G, Goetzl EJ. Lysolipid mediators in cell signaling and disease. Biochim Biophys Acta. 2002;1582:VII. [Google Scholar]
- 3.Graeler M, Goetzl E. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 4.Goetzl EJ, Rosen H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J Clin Invest. 2004;114:1531–1537. doi: 10.1172/JCI23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graeler M, Shankar G, Goetzl E. Cutting edge: suppression of T cell chemotaxis by sphingosine 1-phosphate. J Immunol. 2002;169:4084–4087. doi: 10.4049/jimmunol.169.8.4084. [DOI] [PubMed] [Google Scholar]
- 6.Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RW, Huttner WB. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosylprotein sulfotransferase. J Biol Chem. 1983;258:11,326–11,334. [PubMed] [Google Scholar]
- 8.Lee RW, Huttner WB. (Glu62, Ala30, Tyr8)n serves as high-affinity substrate for tyrosylprotein sulfotransferase: a Golgi enzyme. Proc Natl Acad Sci USA. 1985;82:6143–6147. doi: 10.1073/pnas.82.18.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang Y, Lane WS, Moore KL. Tyrosylprotein sulfotransferase: purification and molecular cloning of an enzyme that catalyzes tyrosine O-sulfation, a common posttranslational modification of eukaryotic proteins. Proc Natl Acad Sci USA. 1998;95:2896–2901. doi: 10.1073/pnas.95.6.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore KL. The biology and enzymology of protein tyrosine O-sulfation. J Biol Chem 278. 2003;24:243–24246. doi: 10.1074/jbc.R300008200. [DOI] [PubMed] [Google Scholar]
- 11.Graeler MH, Kong Y, Karliner JS, Goetzl EJ. Protein kinase C epsilon dependence of the recovery from down-regulation of S1P1 G protein-coupled receptors of T lymphocytes. J Biol Chem. 2003;278:27,737–27,741. doi: 10.1074/jbc.C300147200. [DOI] [PubMed] [Google Scholar]
- 12.Graler MH, Huang MC, Watson S, Goetzl EJ. Immunological effects of transgenic constitutive expression of the type 1 sphingosine 1-phosphate receptor by mouse lymphocytes. J Immunol. 2005;174:1997–2003. doi: 10.4049/jimmunol.174.4.1997. [DOI] [PubMed] [Google Scholar]
- 13.Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorsam G, Graeler MH, Seroogy C, Kong Y, Voice JK, Goetzl EJ. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J Immunol. 2003;171:3500–3507. doi: 10.4049/jimmunol.171.7.3500. [DOI] [PubMed] [Google Scholar]
- 15.Bannert N, Craig S, Farzan M, Sogah D, Santo NV, Choe H, Sodroski J. Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines. J Exp Med. 2001;194:1661–1673. doi: 10.1084/jem.194.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farzan M, Babcock GJ, Vasilieva N, Wright PL, Kiprilov E, Mirzabekov T, Choe H. The role of post-translational modifications of the CXCR4 amino terminus in stromal-derived factor 1 alpha association and HIV-1 entry. J Biol Chem. 2002277:29,484–29,489. doi: 10.1074/jbc.M203361200. [DOI] [PubMed] [Google Scholar]
- 17.Costagliola S, Panneels V, Bonomi M, Koch J, Many MC, Smits G, Vassart G. Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J. 2002;21:504–513. doi: 10.1093/emboj/21.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sardar VM, Bautista DL, Fischer DJ, Yokoyama K, Nusser N, Virag T, Wang D, Baker DL, Tigyi G, Parrill AL. Molecular basis for the lysophosphatidic acid receptor agonist selectivity. Biochem Biophys Acta. 2002;1582:309–317. doi: 10.1016/s1388-1981(02)00185-3. [DOI] [PubMed] [Google Scholar]


