Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic of the halogenated dioxins and one of the most poisonous substances known to man. The major toxic effects of TCDD on reproduction are decreased fertility and diminished ability to maintain a pregnancy. Granulosa cells obtained from hormonally stimulated women participating in an in-vitro fertilization program were cultured with 3.1 femtomolar, 3.1 picomolar and 3.1 nanomolar TCDD. While inhibin B production was not altered, inhibin A production increased significantly after four hours of exposure to both nanomolar and micromolar TCDD concentrations. By eight hours of exposure to these concentrations of dioxin, human luteinizing granulosa cells exhibited a pronounced increase in inhibin A, nearly quadrupling secretion from unexposed control cells. TCDD continued to increase inhibin A secretion at the picomolar concentration at 24 and 36 hours. It is conceivable that TCDD may act at the ovary to augment inhibin A secretion, thereby reducing FSH-stimulable estrogen secretion by granulosa cells.
Keywords: dioxin, inhibin, human, granulosa cells
Introduction
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic environmental pollutant of the halogenated aromatic hydrocarbon (HAH) class [1]. This toxicant is produced from combustion in the presence of chlorine and as an indirect by-product in the manufacture of certain herbicides, defoliants, insecticides, and disinfectants. Waste incineration contributes most significantly to anthropogenic point-source production of dioxins while forest fires and volcanic eruptions comprise the greatest natural sources of TCDD. Because it is lipophilic, TCDD readily concentrates up the food chain. Traces of HAHs are found in all elements of the ecosystem, including fish, wildlife, and humans (in serum, adipose tissue, and milk) [2]. The animal products in our diet account for 90% of our TCDD exposure [3]. Its persistence and ubiquity in the environment make it difficult to ascertain the impact of TCDD on human health, although the toxic effects of TCDD certainly include a decrease in reproductive fitness in many animals [4,5].
TCDD is known to alter steroidogenesis in the female reproductive system. Estradiol secretion by human luteinized granulosa cells (hLGC) was decreased when cultured with TCDD for 8, 12, and 24 hours [6]. This effect was time dependent as estradiol secretion increased above controls after 36 and 48 hours. Adding the aromatizable precursor substrate androstenedione abolished the initial inhibition by TCDD of estradiol secretion. Therefore, TCDD during relatively brief exposures likely affects steroidogenesis at or before the conversion of androstenedione to estrone or testosterone. The addition of dehydroepiandrosterone (DHEA), a CYP17/17,20-lyase product, also prevented TCDD’s decrease in estrogen secretion [7]. When either 17 α hydroxypregnenolone or 17 α hydroxyprogesterone, precursors of androgens, was added to the hGLC culture, TCDD was not hindered in its ability to decrease estrogen secretion [7]. This suggests that aromatase activity remains intact during TCDD treatment. Collectively, these data suggest that a likely target of TCDD in human cells is CYP17/17,20-lyase activity [8]. TCDD thereby exacts its effects on the enzymes of the ovarian steroidogenic pathway. However, TCDD’s effects on the overall regulation of steroid production and release by altering the reproductive axis generally [9,10] further complicate this scenario. Removing higher regulatory controls (hypothalamus and pituitary) by using an in-vitro paradigm, allows us to evaluate dioxin’s effects directly at the human ovarian cell level. A potential site for this effect is, thereby, inhibin production by granulosa cells, which can then inhibit FSH secretion centrally, reducing estrogen peripherally.
Inhibins play an indirect role in estradiol secretion. Both inhibin A and B inhibit follicle-stimulating hormone (FSH) beta subunit mRNA [11] and secretion from the anterior pituitary, and decreased FSH concentrations can then lead to decreased estradiol secretion. Inhibins belong to the transforming growth factor β (TGF β) superfamily. Structurally, inhibin A and B are composed of the same alpha subunit while they differ in their beta subunit. Inhibin B is primarily released from preantral follicles under the stimulation of FSH and insulin-like growth factor 1 (IGF-1) [12]. Antral follicles and the corpus luteum are the major sources of inhibin A [12]. Circulating concentrations of both forms of inhibin vary with the menstrual cycle, with fluctuations affecting estradiol secretion; and inhibin A expression has been shown to be modulated by TCDD in the GC of rat follicles [13]. Inhibin B rises in the follicular phase, while inhibin A peaks in the luteal phase [14] in human, while the two inhibins are differentially expressed in other species also [15].
The present study examines for the first time the effects of TCDD at environmentally relevant doses on the production of inhibin by cultured hLGC. Our results suggest that this is one mechanism of TCDD’s estrogen-modulatory effects.
Materials and Methods
Chemicals
TCDD (>99% purity) was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). The stock solution was 1 mg TCDD/ml p-dioxane vehicle (Sigma, St. Louis, MO). Culture medium consisted of DMEM/F12 (Gibco BRL, Grand Island, NY) with 5% FBS (Gibco BRL, Grand Island, NY) and 50ug/ml gentamycin sulfate (Sigma, St. Louis, MO).
Human Granulosa Cell Isolation, Purification, and Culture
Human luteinizing granulosa cells (hLGC) were obtained from women undergoing ovarian stimulation in an in-vitro fertilization program at the Waukesha Memorial Hospital In-Vitro Fertilization Laboratory, Waukesha, Wisconsin. Appropriate protocols were approved by the Human Subjects Institutional Review Board. Cells were collected from groups of 2 and 3 women on two different occasions. Follicular fluid (FF) was collected in 15-ml centrifuge tubes approximately 36 hours after a 10,000 IU human chorionic gonadotropin (hCG) injection to simulate the midcycle LH surge. The FF was centrifuged at 300 × g for 5 minutes followed by 5 minutes at 600 × g to isolate the LGC. A firm layer of LGC then lay on top of the red blood cell (RBC) pellet. This layer was transferred to a new 15-ml centrifuge tube with a Pasteur pipet. Up to 1 ml of culture medium was added to the cell suspension. HLGC aggregates were mechanically dispersed with a 1-ml pipet tip. One ml of the cell suspension was layered over 5 ml 45% Percoll (Sigma, St. Louis, MO) and centrifuged at 300 × g for 30 minutes. The hLGC layer was aspirated from the top of the Percoll. HLGC were pooled into one 15-ml centrifuge tube and mixed. This pool was diluted with up to 10 ml of culture medium and washed twice by centrifugation, discarding the supernatant each time. The final volume was brought up to 1 ml. The purified hLGC were counted in a hemacytometer and adjusted to a concentration of 1 × 106 cells/ml. The viability was determined by exclusion of 0.2% trypan blue dye. The cells were placed on ice for less than 60 minutes for travel. Upon arrival at the University of Wisconsin at Milwaukee, the hLGC viability and count were again determined. Cell viability was not affected significantly. The concentration of cells was adjusted to 1 × 105/ml and 500ul was loaded per well of Permanox-coated Lab-Tek slides (Nunc, Naperville, IL). Cells were incubated at 37°C, 5% CO2, and humidified air (>98% humidity) overnight in culture medium. Medium was aspirated and replaced with medium containing either 0.1% p-dioxane (control), 3.1 fM TCDD, 3.1 pM TCDD, 3.1 nM TCDD or 3.1 μM TCDD. Medium was collected for immunoassay (RIA) at 0, 4, 8, 12, 24, 36, and 48 hours. Medium was snap frozen in 100% ethanol/dry ice and stored at −20°C until time of assay.
ELISA
Inhibin A and inhibin B ELISAs were performed using ultrasensitive assays (Serotec Ltd, Oxford, Oxon, UK) as described previously [16]. Standard controls used were recombinant human inhibin A and inhibin B. The inhibin A assay had a detection limit of 3.9 pg/ml while the inhibin B assay had one of 15.6 pg/ml. Samples were analyzed in two assays. The intraassay coefficients of variation for the two inhibin A assays were 2.0% and 1.6%. The interassay coefficient of variation for inhibin A was 3.4%.
Statistical Analysis
Data were initially analyzed using two-way analysis of variance (ANOVA), split-plot for time (i.e., a repeated-measures design). As there was no interaction between dose and time, we subsequently analyzed each time point separately by one-way ANOVA. Multiple-comparison testing was accomplished using the conservative Scheffe’s post-hoc test (P < 0.05).
Results
Inhibin A secretion from hLGC increased significantly with TCDD treatment (Figure 1). After four hours of treatment with nM and uM TCDD, inhibin a secretion was amplified one-and-a-half fold. After eight hours, TCDD at pM, nM and μM concentrations caused inhibin a secretion to nearly quadruple. HLGC treated with nM TCDD exhibited a significant sustained increase in inhibin A through 36 hours of culture. Because of a rise in secretion of inhibin in control cultures at 12 hours, there were no significant differences at this time (P= 0.051), although a similar pattern was observed. By 48 hours there was a plateau in inhibin A secretion across treatment groups.
Figure 1.
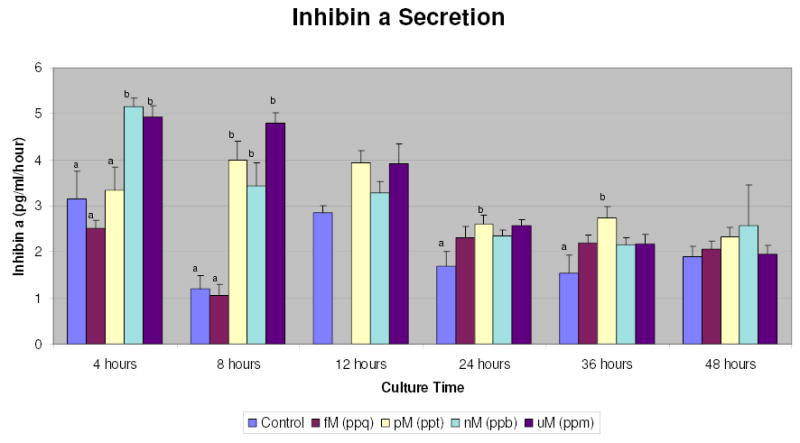
Modulation of inhibin A secretion by TCDD-treated human luteinized granulosa cells in vitro. fM, femtomolar, ppq, parts per quadrillion; pM, picomolar, ppt, parts per trillion; nM, nanomolar, ppb, parts per billion; μM, micromolar, ppm, parts per million a,b Different letters above bars denote significant differences.
Measurements of inhibin B showed no significant differences from control cultures at any time points or concentrations of TCDD (data not shown). Most samples were, in fact, below detectable limits of the assay.
Discussion
At a dose relevant to human body burden and dietary exposure (3.1 pM) [6], TCDD significantly increased inhibin A levels. This is the first study confirming TCDD action on inhibin A production by hLGC. This increase in inhibin A appears to be inversely correlated with other hormonal changes observed in previous studies; e.g., . TCDD decreased the levels of estrogen secretion in hLGC culture [6]. Preliminarily, we have also observed in a subset of hLGC a statistically significant (p<0.05) diminution by at last 50% in estradiol-17β secretion at eight hours of incubation with all four concentrations of TCDD (fM, pM, nM, and μM); and by approximately 30% (p<0.05) in progesterone secretion at four and eight hours at nM and μM concentrations of TCDD only (not shown; there was no effect on androstenedione or testosterone secretion). Further, in rats treated in utero and lactationally (IUL) with TCDD, there was a decrease in circulating estradiol and other estradiol-induced effects [17].
We did not observe high concentrations of inhibin B in culture. In fact many of our values were below detectable limits. Since we cultured luteinized granulosa cells after hormonal stimulation with an artificially and pharmacologically induced “LH surge”, it is not surprising that so little inhibin B was present. Small developing follicles primarily secrete inhibin B [12], and all of our aspirated follicles were of preovulatory size. Also, there is a rise in inhibin B during the follicular phase followed by a fall during the luteal phase [18], and aspirated hGC luteinize in culture, mimicking a luteal phase. In contrast, inhibin A rises during the luteal phase [19].
The marked increase in inhibin A at 4 and 8 hours was consistent with earlier data showing a decrease in estrogen secretion with TCDD exposure in rats [4]. A decrease in serum estrogen was also observed in vivo with TCDD exposure [13]. This response could in part be due to an increase in inhibin A, which acts to decrease the release of FSH from the anterior pituitary. A fall in FSH will contribute to a decrease in the amount of estrogen secreted. Therefore, one of the estrogen-modulating mechanisms of TCDD may be to stimulate inhibin A release from granulosa cells. We have shown that TCDD can also act directly on the anterior pituitary, as FSH β mRNA was inhibited with IUL TCDD exposure in the rat, although immunoassayable heterodimeric FSH did not decrease significantly in peripheral blood [11].
TCDD acts as an estrogen-modulating agent in many paradigms. In rats treated with TCDD there was a decrease in all 17 β-estradiol-induced uterine responses, uterine weight, peroxidase activity, estrogen receptor, and progesterone receptor [20,21]. TCDD likewise can affect the activity of steroidogenic enzymes. TCDD-treated JEG-3 (human carcinoma) cells exhibited a decrease in aromatase activity [22]. But this action is not likely in hLGC. TCDD caused a decrease in estradiol secretion in hLGC, but with the addition of androstenedione, an aromatase substrate, this effect was abolished [6], indicating normal aromatase activity. An enzyme located earlier in the steroidogenic pathway is therefore implicated. When dehydroepiandrosterone (DHEA), a CYP17/17,20-lyase product was added along with TCDD, TCDD’s action on estradiol secretion was attenuated [7]. However, as TCDD also caused a decrease in progesterone production [7,23, our preliminary results], TCDD may act on more than one enzyme in the ovarian steroidogenic pathway. In rat granulosa cells, TCDD diminished activity of basal and FSH-stimulated P450 side chain cleavage and aromatase activities with no effect on 3 β hydroxysteroid dehydrogenase (HSD) [24]. These differences are presumably attributable to differences in the models and species, making comparison among studies difficult. It should be noted that our cultures did not include gonadotropin (human chorionic gonadotropin) as was shown for other studies [ 7,8]. It is therefore conceivable that an interaction occurred between declining hormone support and TCDD.
Along with changes in steroid production, TCDD also alters follicular development. Although the number of primordial follicles was not changed with TCDD exposure [25], there was a reduction in specific size classes of preantral and antral follicles [26]. Follicular atresia occurs via granulosa cell apoptosis, and atresia occurs throughout ovarian development and maturation. We have also demonstrated that TCDD induced apoptosis in cultured hLGC [6]. These aforementioned effects of TCDD on the different follicle types correlate well with the findings of the present study. Follicle types that produce inhibin A were more affected than follicles that produce inhibin B. Changes in inhibin A might also therefore exert a direct effect within the developing follicle.
In conclusion, our results indicate that TCDD stimulated inhibin A but not inhibin B production from cultured human luteinized granulosa cells at doses as low as pM TCDD. This suggests that an environmentally relevant concentration of TCDD can exert a direct action on the granulosa cells to stimulate inhibin release. This inhibin release likely plays a role in the observed decrease in FSH β subunit mRNA in rat pituitary and subsequent diminution in estradiol secretion by TCDD.
Acknowledgments
This work was supported in part by NIH ES08342, NIH ES011569 and the Office of Research on Women’s Health. This work would not have been possible without the cooperation and assistance of embryologists Mark Roesler and Amy Granlund of the Medical College of Wisconsin.
References
- 1.Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicology. Ann Rev Toxicol. 1982;22:517–54. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 2.Safe S, Astroff B, Harris M, Azcharewski T, Dickerson R, Romkes M, Biegel L. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds as antioestrogens: characterization and mechanisms of action. Pharmacol Toxicol. 1991;69:400–9. doi: 10.1111/j.1600-0773.1991.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 3.Baars AJ, Bakker MI, Baumann RA, Boon PE, Freijer JI, Hoogenboom LAP, Hoogerbrugge R, van Klaveren JD, Liem AKD, Traag WA, de Vries J. Dioxins, dioxin-like PCBs and non-dioxin-like PCBs in foodstuffs: occurrence and dietary intake in The Netherlands. Toxicol Letters. 2004;151:51–61. doi: 10.1016/j.toxlet.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- 5.Hutz RJ. Reproductive endocrine disruption by environmental xenobiotics that modulate the estrogen-signaling pathway, particularly tetrachlorodibenzo-p-dioxin (TCDD) J Reprod Develop. 1999;45:1–12. [Google Scholar]
- 6.Heimler I, Rawlins RG, Owen H, Hutz RJ. Dioxin perturbs, in a dose- and time-dependent fashion, steroid secretion, and induces apoptosis of human luteinized granulosa cells. Endocrinology. 1998;139:4373–4379. doi: 10.1210/endo.139.10.6264. [DOI] [PubMed] [Google Scholar]
- 7.Moran FM, Lohstroh P, VandeVoort CA, Chen J, Overstreet JW, Conley AJ, Lasley BL. Exogenous steroid substrate modifies the effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on estradiol production of human luteinized granulosa cells in vitro. Biol Reprod. 2003;68:244–251. doi: 10.1095/biolreprod.102.007161. [DOI] [PubMed] [Google Scholar]
- 8.Moran FM, VandeVoort CA, Overstreet JW, Lasley BL, Conley AJ. Molecular target of endocrine disruption in human luteinizing granulosa cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin: inhibition of estradiol secretion due to decreased 17alpha- hydroxylase/17,20-lyase cytochrome P450 expression. Endocrinology. 2003;144:467– 473. doi: 10.1210/en.2002-220813. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Johnson DC, Rozman KK. Reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female rats: ovulation, hormonal regulation, and possible mechanisms. Toxicol Appl Pharmacol. 1995;133:321–327. doi: 10.1006/taap.1995.1157. [DOI] [PubMed] [Google Scholar]
- 10.Petroff BK, Croutch CR, Hunter DM, Wierman ME, Gao X. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) stimulates gonadotropin secretion in the immature female Sprague-Dawley rat through a pentobarbital- and estradiol-sensitive mechanism but does not alter gonadotropin-releasing hormone (GnRH) secretion by immortalized GnRH neurons in vitro. Biol Reprod. 2003;68:2100–2106. doi: 10.1095/biolreprod.102.010439. [DOI] [PubMed] [Google Scholar]
- 11.Chaffin CL, Trewin AL, Watanabe G, Taya G, Hutz RJ. Disruption of the pituitary gonadal axis due to in-utero and lactational exposure to dioxin in the peripubertal female rat. Biol Reprod. 1997;56:1498–1502. doi: 10.1095/biolreprod56.6.1498. [DOI] [PubMed] [Google Scholar]
- 12.Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med. 2002;227:724–752. doi: 10.1177/153537020222700905. [DOI] [PubMed] [Google Scholar]
- 13.Petroff BK, Gao X, Oshima K, Shi FX, Son DS, Roby KF, Rozman KK, Watanabe G, Taya K, Terranova PF. Effects of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) on serum inhibin concentrations and inhibin immnostaining during follicular development in female Sprague-Dawley rats. Reprod Toxicol. 2002;16:97–105. doi: 10.1016/s0890-6238(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 14.Pezzani I, Reis FM, Di Leonardo C, Luisi S, Santuz M, Driul L, Cobellis L, Petraglia F. Influence of non-gonadotrophic hormones on gonadal function. Mol Cell Endocrinol. 2000;16:37–42. doi: 10.1016/s0303-7207(99)00221-x. [DOI] [PubMed] [Google Scholar]
- 15.Kishi H, Ohshima K, Itoh M, Arai KY, Nakano S, Watanabe G, Taya K. Changes in expression of inhibin subunits in the cyclic golden hamster (Mesocricetus auratus) and the regulation of inhibin alpha subunit production by luteinizing hormone. Zoolog Sci. 2002;19:225–32. doi: 10.2108/zsj.19.225. [DOI] [PubMed] [Google Scholar]
- 16.Kojima C, Kondo M, Jin W, Shimizu K, Itoh M, Watanabe G, Groome NP, Taya K. Secretion of inhibin a and inhibin b during pregnancy and early postpartum period in Japanese monkeys. Endocrine. 2002;18:21–5. doi: 10.1385/ENDO:18:1:21. [DOI] [PubMed] [Google Scholar]
- 17.Chaffin CL, Peterson RE, Hutz RJ. In utero and lactational exposure of female holtzman rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: modulation of the estrogen signal. Biol Reprod. 1996;55:62–67. doi: 10.1095/biolreprod55.1.62. [DOI] [PubMed] [Google Scholar]
- 18.Groome NP, Illingsworth PJ, Obrien M, Pai R, Rodger FE, Mather JP, McNeilly AS. Measurement of dimeric inhibin B throughout the human menstrual cycle . J Clin Endocrinol Metab. 1996;81:1401–1405. doi: 10.1210/jcem.81.4.8636341. [DOI] [PubMed] [Google Scholar]
- 19.Groome NP, Illingsworth PJ, Obrien M, Cooke I, Ganesan TS, Baird DT, McNeilly AS. Detection of dimeric inhibin throughout the human menstrual cycle by two-site enzyme immunoassay. Clin Endocrinol. 1994;40:717–723. doi: 10.1111/j.1365-2265.1994.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 20.Astroff B, Safe S. 2,3,7,8-Tetrachlorobenzo-p-dioxin as an antiestrogen: effect on rat uterine peroxidase activity. Biochem Pharmacol. 1990;39:485–8. doi: 10.1016/0006-2952(90)90054-o. [DOI] [PubMed] [Google Scholar]
- 21.Romkes M, Safe S. Comparative activities of 2,3,7,8-Tetrachlorobenzo-p-dioxin and progesterone on antiestrogens in the female rat uterus. Toxicol Appl Pharmacol. 1988;92:368–80. doi: 10.1016/0041-008x(88)90177-9. [DOI] [PubMed] [Google Scholar]
- 22.Drenth HJ, Bouwman DA, Seinen W, Van den Berg M. Effects of some persistent halogenated environmental contaminants on aromatase (cyp19) activity in the human choriocarcimona cell line JEG-3. Toxicol Appl Pharmacol. 1998;148:50–55. doi: 10.1006/taap.1997.8307. [DOI] [PubMed] [Google Scholar]
- 23.Enan E, Moran F, VanderVoort CA, Stewart DR, Overstreet JW, Lasley BL. Mechanism of toxic action of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in cultured human luteinized granulosa cells. Reprod Toxicol. 1996;10:497–483. doi: 10.1016/s0890-6238(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 24.Dasmahapatra AK, Wimpee BAB, Trewin AL, Wimpee CF, Ghorai JK, Hutz RJ. Demonstation of 2,3,7,8-tetrachlorodibenzo-p-dioxin attenuation of P450 steroidogenic enzyme mRNAs in rat granulosa cell in vitro by competitive reverse transcriptase-polymerase chain reaction assay. Mol Cell Endocrnol. 2000;164:5–18. doi: 10.1016/s0303-7207(00)00245-8. [DOI] [PubMed] [Google Scholar]
- 25.Flaws JA, Sommer RJ, Silbergeld EK, Peterson RE, Hirshfield AN. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces genital dysmorphogenesis in the female rat. Toxicol Appl Pharmacol. 1997;147:351–62. doi: 10.1006/taap.1997.8295. [DOI] [PubMed] [Google Scholar]
- 26.Heimler I, Trewin AL, Chaffin CL, Rawlins RG, Hutz RJ. Modulation of ovarian follicle maturation and effects on apoptotic cell death in Holtzman rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in utero and lactationally. Reprod Toxicol. 1998;12:69–73. doi: 10.1016/s0890-6238(97)00101-9. [DOI] [PubMed] [Google Scholar]


