Abstract
Objective
Dysfunction of neuronal plasticity or remodelling seems to contribute to the pathopysiology of major depression and may cause the well-documented hippocampal changes in depression. We aimed to investigate whether reduced hippocampal volumes correlate with executive dysfunctioning or memory dysfunctioning or with depression severity.
Methods
We recruited 34 inpatients with a previous or current episode of major depression from the department of psychiatry at the Ludwig-Maximilians University of Munich, Germany. We examined the 34 patients and 34 healthy control subjects with structural high resolution MRI. We assessed cognitive functions with the Wisconsin Card Sorting Test (WCST) and the Rey Auditory Verbal Learning Test (RAVLT) and severity of depression with the Hamilton Depression Rating Scale.
Results
Hippocampal volumes and frontal lobe volumes were significantly smaller in patients, compared with healthy control subjects. Furthermore, lower hippocampal volumes were correlated with poorer performance in the WCST. No significant correlations were found between hippocampal volumes and RAVLT performance or severity of depression.
Conclusions
The present findings emphasize that patients with reduced hippocampal volumes show more executive dysfunctions than their counterparts. Thus, the mechanisms resulting in reduced hippocampal volumes seem to be related to the development of major depression.
Medical subject headings: depression, hippocampus, structural MRI, executive memory
Abstract
Objectif
Le dysfonctionnement de la plasticité ou du remodelage des neurones semble contribuer à la pathophysiologie de la dépression majeure et peut causer des changements bien documentés de l'hippocampe dans les cas de dépression. Nous voulions déterminer s'il y a un lien entre la diminution du volume de l'hippocampe et le dysfonctionnement de l'exécution ou de la mémoire, ou la sévérité de la dépression.
Méthodes
Nous avons recruté 34 patients en service interne aux prises avec un épisode antérieur ou en cours de dépression majeure au Département de psychiatrie de l'Université Ludwig-Maximilians à Munich, en Allemagne. Nous avons examiné les 34 patients et 34 sujets témoins en bonne santé au moyen d'une IRM à haute résolution structurelle. Nous avons examiné les fonctions cognitives au moyen du test Wisconsin de classification catégorielle de cartes (WCST) et du test Rey d'apprentissage auditif et verbal (RAVLT) et nous avons évalué la gravité de la dépression au moyen de l'échelle de dépression de Hamilton.
Résultats
Les volumes de l'hippocampe et du lobe frontal étaient beaucoup plus petits chez les patients comparativement à ceux des sujets témoins en bonne santé. De plus, on a établi un lien entre la réduction du volume de l'hippocampe et des résultats plus faibles au WCST. On n'a établi aucune corrélation importante entre le volume de l'hippocampe et les résultats au test RAVLT ou la gravité de la dépression.
Conclusions
Ces constatations mettent l'accent sur le fait que les patients dont le volume de l'hippocampe a diminué montrent plus de dysfonctionnement de l'exécution que les autres. Il semble donc y avoir un lien entre les mécanismes qui entraînent une réduction du volume de l'hippocampe et l'apparition d'une dépression majeure.
Introduction
The hippocampus is a core region in the limbic system and has widespread connections to such diverse cortical areas as the prefrontal cortex, anterior thalamic nuclei, amygdala, basal ganglia and hypothalamus,1 all of which are regions that comprise the neuroanatomical network of mood regulation.2–4 In the past few years, reduced hippocampal volumes have consistently been reported in elderly (aged 60 years and over) 5,6 and also in younger (aged under 60 years) patients with depression.7–9 Two metaanalytic studies confirmed that the hippocampus is consistently reduced in patients with major depression.10,11 Experimental studies support the hypothesis that stress toxicity12 and a lack of neurotrophic factors13 result in the well-known structural abnormalities of the hippocampus. Interestingly, antidepressants have been found to suppress the stress toxicity and to increase hippocampal neurogenesis.14 These mechanisms and other neuroplastic changes, such as those in the synapses,15 may be factors in the neurobiology of depression.
Reduced hippocampal volumes are not specific to major depression11 and are present in psychiatric diseases, including schizophrenia,16 dementia17 and post-traumatic stress disorder,18 as well as in other medical diseases, such as diabetes mellitus.19 The question is whether reduced hippocampal volume impacts the development of major depression or whether it is the result of a disease process that may be related to stress. If neuroplastic mechanisms leading to reduced hippocampal volumes are one of the main neurobiological causes of depression, a correlation between clinical characteristics and hippocampal volumes should be expected. In a previous study, we failed to find significant correlations between hippocampal volumes and depression severity.9 However, other symptoms, such as cognitive dysfunction, should also be considered. The hippocampal formation plays a critical role in the regulation of learning, memory, motivation and emotion.20 Therefore, reduced hippocampal volumes should lead to affective symptoms and cognitive dysfunction (core symptoms in patients with major depression). The cognitive criterion, “impaired ability to think and concentrate,” that is used to diagnose major depression according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV),21 corresponds to deficits in memory, executive functioning and attention.22 The cognitive deficits of major depression have been identified as a significant disabling aspect of the disorder since the concept of so-called “pseudodementia” was introduced in 1952.23 Pseudodementia is commonly regarded as deficits in memory functioning and includes deficits in other neuropsychological domains, as shown by executive dysfunction.24
Loss in hippocampal volume should be related to more particular memory functions, especially the complex memory. Complex memory function can be examined with the Rey Auditory Verbal Learning Test (RAVLT).25 A recently published study found that reduced hippocampal volumes were associated with deficits in visual and verbal memory performance in major depression.26 Such cognitive deficits are often attributed to poor effort or executive dysfunctioning,27 which, in turn, were found to be associated with a longer mean duration of depressive episodes28 and with relapse and recurrence in geriatric depression.29
The Wisconsin Card Sorting Test (WCST) is commonly used to examine executive function in people with psychiatric disorders.30 It seems to be subserved by a frontal-subcortical circuit that originates in the dorsolateral prefrontal cortex and orbitofrontal cortex and then projects through the striatum and thalamus to return to the prefrontal cortex.22 However, the hippocampus may also influence these networks. Wall and Messier31 concluded that the hippocampus–oribitomedial prefrontal circuit can efficiently contribute to the integration of cognition, emotion and behaviour and can thus influence working memory and executive functions. Therefore, the most interesting brain structures seem to be the hippocampus and the frontal cortices.
In the present study, we aimed to examine the hippocampal and frontal lobe volumes of a group of patients with major depression, compared with healthy control subjects. We investigated the association between these brain structures, using the WCST and the RAVLT.
Methods
Thirty-four inpatients with a previous or current episode of major depression were recruited from the department of psychiatry at the Ludwig-Maximilians-University located in Munich, Germany (Table 1). Psychiatric diagnoses were determined by a consensus of at least 2 psychiatrists on the basis of DSM-IV criteria.21 An experienced psychiatrist used the structured clinical interview for DSM-IV (SCID) to examine all of the subjects. Mean illness duration was 6.8 years (standard deviation [SD] 8.8 yr). Fourteen patients had their first depressive episode at the time of assessment, and 20 patients had recurrent depressive episodes. Thirty-one patients were taking antidepressant medications at the time of assessment: 7 were taking mirtazapine, 5 reboxetine, 5 citalopram, 5 amitriptyline, 3 venlafaxine, 2 paroxetine, 2 doxepin, 1 tranylcypromine and 1 fluvoxamine. Four subjects were also taking neuroleptics because of psychotic symptoms. Three patients were taking no medication at the time of MRI scanning.
Table 1
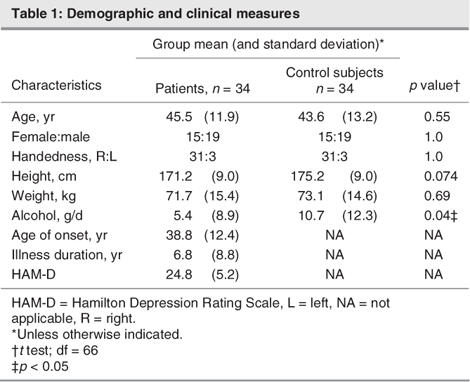
We matched 34 healthy control subjects in a 1-to-1 fashion with respect to age (age range 19–64, mean 43.6, SD 13.2 yr), sex and handedness. Groups did not differ with regard to height or daily alcohol consumption. The widest gap in age difference for all of the pairings was 4 years (2 pairs). Neither the healthy control subjects nor their first-degree relatives had a history of neurological or mental illness. We excluded patients and control subjects if they had a previous head injury, took cortisol or benzodiazepine medication in the last 3 months, had a neurological disease or had comorbidity with other mental illnesses. All patients and control subjects underwent structural MRI scanning. We documented clinical variables for the patients, using the 21-item Hamilton Depression Rating Scale (HAM-D), and we performed the neuropsychological assessment battery, using the WCST30 and the RAVLT.25 Handedness was determined with the Edinburgh inventory.32
We provided patients and subjects with a complete description of the study before we obtained written informed consent. The study design was approved by the local ethics committee and was in accordance with the Declaration of Helsinki.
MRI procedures
MRI images were obtained with a coronal T2- and proton density–weighted Dual-Echo-Sequence (TR 3710 msec/TE 22/90 msec; total acquisition time 9 min, number of acquisitions 1; FOV 230 mm; matrix 240 × 256, slice thickness 3 mm) and a 3D-MPRAGE sequence (TR/TE 11.6 msec/4.9 msec; total acquisition time 9 min, number of acquisitions 1; FOV 230 mm; matrix 512 × 512, slice thickness 1.5 mm). We used the commercial software package Analyze for further image processing, with size reduction from 16 to 8 bit and transformation to a uniform matrix of 256 × 256 on 192 slices of 1.5 mm slice thickness. All data sets were realigned and resampled 3 dimensionally in the anterior commissure-posterior commissure (AC-PC) line, according to Tailairach coordinates, with the software program BRAINS (Brain Research: Analysis of Images, Networks and Systems).33 Regions of interest (ROI) were marked with the aid of an interactive cursor-guided system on a computer display of coronal MRI images. BRAINS allowed the ROI to be controlled on sagittal and transverse sections simultaneously and on the segmentation to enable us to calculate the intracranial content, the frontal lobe of each side, and the grey and white matter volume (mL3) within the defined ROI.
We used Niemann and colleagues'34 definition of the hippocampus and Convit and others'35 description of the amygdala to detect the hippocampal–amygdala border. One rater, trained in MRI and blinded to the diagnosis of the data sets, measured the ROI. A short description is given in Figure 1, and measurements are described in detail elsewhere.9 On each scan, we began with the most posterior coronal slice, where the hippocampus was clearly detectable. Both the fimbria and the subiculum were included in the measurement. The uncal sulcus aided in the delineation of the medial border. More anteriorly, the shape of the hippocampus is comparable to a rabbit with the head directed vertically. Now the uncal sulcus separates the uncus from the underlying parahippocampal gyrus and forms the basal border of the hippocampus. The anterior part of the hippocampus ends where the cornu inferius of the lateral ventricle becomes vertically oriented. The amygdala-hippocampal boundaries were defined in the sagittal plane and then projected to the coronal plane.
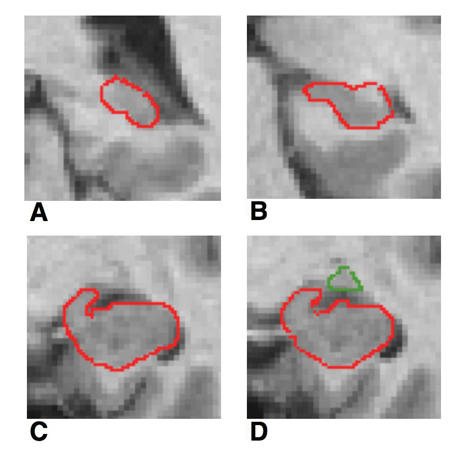
Fig. 1: Coronal MRI slices that run in occipito-rostral direction: (A) most posterior coronal slice where the hippocampus was clearly detectable, (B) hippocampal body; (C) the shape of the hippocampus may be compared with a rabbit with the head vertically directed, (D) amygdala-hippocampal transition area (HATA).
To determine interrater reliability, 10 brains were randomly selected and ROI was determined by 2 raters independently. The intraclass correlation for the interrater reliability of hippocampal volume (rICC = 0.95) was high. For the intrarater reliability, one rater selected 10 brains (hippocampal volume: rICC = 0.92).
Neuropsychological assessment
The neuropsychological assessment was carried out 2 days before or after the MRI scanning, during the first week of treatment in hospital.
The WCST was used to assess executive functioning, cognitive flexibility, maintenance of a cognitive set and working memory.30 A computer version of the test was used, in which subjects were asked to match a series of 128 cards to a set of 4 target stimuli. The dimensions of similarity refer to colour, form and number of stimuli on the target cards and individual item. Subjects were provided with feedback on each item individually, after they sorted each of the item cards, and the categories changed during the assessment.
We used the German version of the RAVLT.36 This test measures verbal learning and memory. It includes immediate recall (reflecting concentration and memory), cumulative learning with exposure and practice, interference, long-delay recall and recognition discriminability. In the RAVLT, subjects are instructed to recall as many words of a 15-item list as possible, after each of 5 separate learning trials. A new list is presented once, requiring immediate recall (interference) after long-delay (20 min) free recall of the first list. At the end, a list of words are read to the subject, who is asked to recognize words from the first list (recognition discriminability).
Statistical analyses
Morphometric data were tested with the Kolmogorov–Smirnov test and were subjected to a repeated-measurement analysis of covariance (ANCOVA) to assess the main and interaction effects of the within-subjects factors hemisphere (left, right) and the between-subjects factors diagnosis (depression, healthy), using total cranial volume as the covariate. Significant interactions were resolved by univariate ANCOVA on the hippocampal volumes for each region. For the diagnostic group, we used total cranial volume as the covariate. We performed partial correlation coefficients for correlations between the WCST and RAVLT and the hippocampal and frontal volumes, controlling for age. We used Spearman correlation coefficients to examine the relation between hippocampal volumes and HAM-D scores.
Results
Morphometric data
Morphometric data were normally distributed. Patients with major depression and healthy control subjects did not differ in age, sex, years of education, height or weight (Table 1). Control subjects consumed significantly more daily alcohol. No significant difference in total brain volumes between patients and control subjects occurred.
The brain volumetric data are shown in Table 2. A significant main effect on the hippocampal grey matter was found for diagnosis, with significantly smaller hippocampal volumes in patients than in control subjects (F65 = 12.1, p = 0.001). The hemisphere effect was significant (F65 = 6.4, p = 0.014), whereas the interaction of diagnosis and hemisphere was not significant (F65 = 0.68, p = 0.41). This indicated larger right than left hippocampal volumes in patients and in control subjects. Moreover, patients with major depression exhibited significantly smaller hippocampal white matter volumes than did control subjects (F65 = 25.0, p < 0.001). We found a significant interaction of hemisphere and diagnosis, with the hippocampal white matter reduction most pronounced at the left side; hippocampal white matter was also significant at the right side (F65 = 6.4, p = 0.014). The main hemisphere effect was not significant (F65 = 0.001, p = 0.97).
Table 2
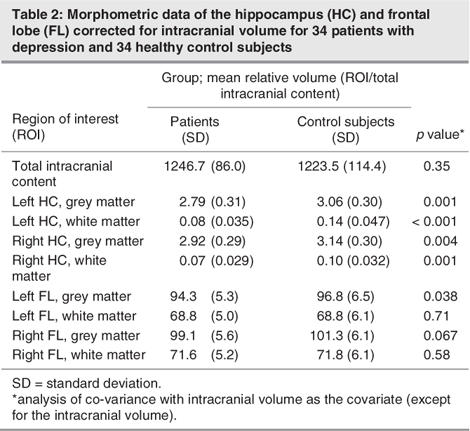
The volumes of the left and right frontal grey matter were significantly smaller in patients, compared with control subjects (F65 = 4.6, p = 0.036), when total intracranial content was controlled for. No significant differences were found for the white matter of the frontal lobe (F65 = 0.23, p = 0.63). Uncorrected volumes were significantly smaller for the left frontal lobe grey matter and showed a trend toward significantly smaller right frontal lobe grey matter volumes (Table 2).
Correlations between brain structures and cognitive functions
Hippocampal volumes were significantly correlated with the number of perseveration errors (Fig. 2) and perseveration responses in the WCST (Table 3), when we controlled for age. Correlations between hippocampal volumes and measurements of the RAVLT were not significant, and frontal lobe volumes did not show significant correlations to the neuropsychological tests.
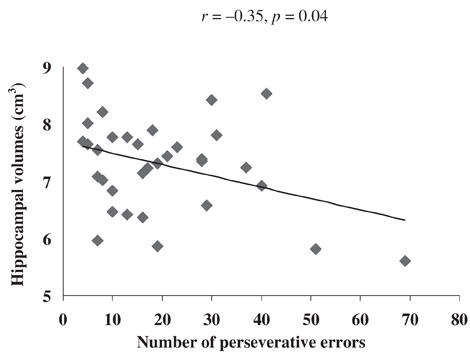
Fig. 2: Scattergramm for the left hippocampal grey matter and perseveration errors of the Wisconsin Card Sorting Test for 34 patients with major depression.
Table 3
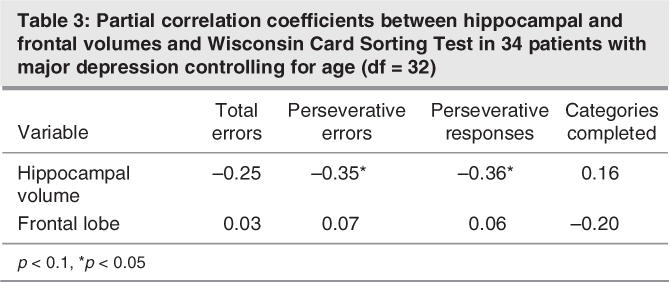
Pearson correlations between age and hippocampal or frontal volumes were not significant for either healthy control subjects or patients with depression. Further, age of onset, illness duration and depression severity was not significantly correlated with hippocampal or frontal volumes.
Age was significantly related to the number of perseveration errors (r33 = 0.41, p = 0.015) and perseveration responses (r33 = 0.40, p = 0.02) on the WCST and with immediate recall on the RAVLT (r33 = –0.60, p = 0.001). Partial correlations controlling for age did not reveal significant correlations between cognitive tests and age of onset, illness duration or depression severity.
Correlations between brain structures and depression severity
Spearman correlation coefficients for the relation between hippocampal volumes and HAM-D scores were not significant (r33 = 0.08, p = 0.67). Also, frontal lobe volumes did not show significant correlations with HAM-D scores (r = –0.11, df = 33, p = 0.54).
Discussion
Our data are in line with previous studies that demonstrated significantly smaller hippocampal volumes in patients with major depression, compared with healthy control subjects.5,9 In contrast to other studies, we did not find a significant correlation between illness duration and hippocampal volume. However, we did not assess the cumulative time that patients had depression, so this negative finding must be interpreted with caution. Because Sheline and colleagues5 reported a negative correlation between total time with depression (in a lifetime) and hippocampal volume in women, it has been suggested that the reduced hippocampal volume may be a consequence of repeated periods of major depression. Recently these findings have been confirmed by other studies in men and women.37,38 The duration of untreated depressive episodes was correlated with reduced hippocampal volumes.39 However, in a follow-up investigation over 1 year, no significant changes of hippocampal volumes were observed, and patients with a small hippocampal volume had a more chronic clinical course or had more relapses.40 This may be the reason for the correlation between small hippocampal volume and longer time spent with depression.
We found a significant reduction of frontal lobe volumes for patients with major depression, compared with healthy control subjects. Previous examinations of the frontal lobe volumes have not provided consistent results. In contrast to our study, no significant alterations were observed in the whole frontal cortical volumes in late-onset depression.41 Interestingly, for subregions of the frontal lobe, reductions of the prefrontal cortical volume in late-onset depression,42 the orbitofrontal cortex in remitted major depression,43 and the subgenual prefrontal gyrus in familial depression and adolescent onset depression44 have been demonstrated. Current reviews have summarized the evidence in support of the idea that both prefrontal and temporolimbic regions are involved in depression.
Association between structural brain abnormalities and cognitive functions
Because the hippocampus was found to be crucial for declarative (that is, explicit) memory,45 we expected there to be a relation between verbal learning and hippocampal volumes. However, we did not see any significant association between reduced hippocampal or frontal lobe volumes and poorer performance on the RAVLT. The patients did well on the test, thus a lack of bad performance may account for this negative result, although the memory scores were spread enough within the range of good performance.
Shah and others compared 20 patients with treatment-resistant depression with 20 healthy control subjects and 20 remitted patients with voxel-based analysis and found that reduced grey matter density in the left hippocampus was related to poorer performance on the verbal memory test.46 Moreover, reduced hippocampal volume was also found to be associated with visual and verbal memory impairment, as measured with the RAVLT.26 However, a metaanalysis from 33 studies on the relation between hippocampal volume and memory ability in healthy subjects did not support the relation. Studies with children, adolescents and young adults show a significantly negative relation between hippocampal volume and memory. In people aged 60 years and over, such a relation is more complex, and the evidence for a positive relation between hippocampal size and episodic memory ability was surprisingly weak.47
Positive and negative correlations were observed in other psychiatric disorders. Significant correlations were detected between larger volumes of the right hippocampus and poorer performance in verbal working memory in patients with bipolar disorder,4,8 and reduced medial temporal lobe structures have been associated with verbal memory impairments in temporal lobe epilepsy.48 Whereas postmortem and MRI investigations of patients with temporal lobe epilepsy indicate a relation between the structural hippocampal changes and verbal memory, this association is not yet clear in patients with affective disorders.
The reduction in hippocampal volumes may be of considerable functional significance because of their possible relation to cognitive dysfunctioning. Such functional deficits are often attributed to poor information encoding, poor effort or difficulties with executive functioning.27 Notably, we found an association between reduced hippocampal volumes and executive dysfunctioning.
The WCST is one of the most widely used tests to assess executive functioning such as problem-solving, decision making, inhibitory control and working memory.30 WCST performance was consistently found to be lower in patients with schizophrenia49; However, it does not seem to be specific to that disorder, because schizophrenia is characterized by a broad base of cognitive impairment, with varying degrees of deficit in all ability domains, as measured by standard clinical tests.49 Since the WCST assesses various functions, it is difficult to differentiate its part in working memory from problem-solving capacity or other executive functions. Therefore, it is not surprising that patients with unipolar depression performed poorly on the WCST.22 Fourteen of 15 studies demonstrated impairments of executive functioning in major depression.22 Several brain regions in addition to the prefrontal cortex were shown to affect performance on the WCST.22 The disturbances in prefrontal areas that were demonstrated may be a necessary but not sufficient condition for a poor WCST performance.
We detected a higher amount of perseveration errors in the WCST to be related to smaller hippocampal volumes. Although the explained variance was small (at 10%), these results support the view that the hippocampus plays a role in executive dysfunction, at least in major depression. One limitation may be the low significance, which was not corrected for an alpha error. The number of correlations was also low because the total hippocampal volume and frontal lobe volume were each correlated with the WCST and the RAVLT, so that only 4 comparisons were carried out. Interestingly, a relation between WCST performance and hippocampal alterations was also demonstrated in temporal lobe epilepsy patients with hippocampal sclerosis. We believe the poor test performance in these patients was caused by hippocampal pathology.50 Other studies found that the presence of temporal lobe structural abnormalities did not significantly affect executive function as measured by the WCST.51 Further, Berman and colleagues used oxygen-15-water PET scans to demonstrate a relation between WCST performance and prefrontal and hippocampal areas in 40 healthy control subjects with regional cerebral bloodflow measures. In this study, performance on the WCST resulted in a prefrontal blood flow activation associated with a decreased hippocampal blood flow.52
We did not find a significant association between frontal lobe volumes and WCST in the patients with depression. Two other structural MRI studies investigated the relation between frontal lobe subregions and WCST performance in patients with schizophrenia. Szeszko and colleagues53 found a significant correlation between executive dysfunction and the anterior cingulate gyrus in men with a first episode of schizophrenia (measured with the WCST). In patients with schizophrenia, dorsolateral prefrontal volumes were associated with executive dysfunction.54
We must emphasize that the present study investigated the whole frontal lobe. The frontal lobe regions are very complex, and examinations of anatomic subregions of the frontal lobe, such as the dorsolateral prefrontal cortex or the orbitofrontal cortex, might have shown different results. Two examples demonstrate this complexity. The orbitofrontal cortex, as a part of the prefrontal cortex, is implicated in executive function. It is uniquely positioned to use associative information to project into the future and to use the value of perceived or expected outcomes to guide decisions.55 The dorsolateral prefrontal cortex has a crucial role in cognitive control of motor behaviour. Physiological studies based on data obtained from monkeys performing various cognitive tasks report region-specific neuronal activity within the dorsolateral prefrontal cortex.56 Future studies should take this complexity into account (i.e., by using voxel-based morphometry).
Association between structural brain abnormalities and depression severity
There was no evidence for a correlation between volume reduction in the frontal lobe or hippocampus and depression severity. Although some studies showed a correlation between reduced hippocampal volumes and a more severe depressive symptomatology,57,58 most structural studies that investigated the relation to depression severity did not find a significant correlation between ratings of depressive symptomatology and hippocampal reduction, which supports our negative findings.9,24,37,40,59,60
Conclusions
The significant relation found between reduced hippocampal volumes and executive dysfunctioning suggests that these structural alterations (due to neuroplastic changes) might be relevant for the development of the disease or indicative of a more broad disturbance of cerebral function in depression.
Patients with smaller hippocampal volumes show more cognitive deficits; therefore, smaller hippocampal volume could signal a type of depression that has more executive dysfunctioning. A previous study provided evidence that patients with a small hippocampal volume have a more severe clinical outcome than do patients with normal hippocampal volumes.40
Moreover, a reduced left hippocampal grey matter density was found to be related to a chronic course of depression.46 Poorer performance on executive tasks, in turn, is supposed to predict poorer outcome in subjects with depression. More perseveration errors on the modified WCST were associated with a longer mean duration of depressive episodes26 and with relapse and recurrence in older patients with depression.27 Therefore, executive dysfunctioning in depression, hippocampal volume loss and poor outcome might be related and perhaps might be linked to neuroplastic changes. It is difficult to examine the consequences of neuroplastic changes in vivo because no ligand for neuroplastic changes is available. It would be interesting to see whether patients with other mental illnesses (e.g., schizophrenia) also show a correlation between WCST performance and the hippocampus or whether such a relation is more specific to major depression.
Hippocampal alterations seem to have negative effects on cognitive functioning and on the clinical outcome of patients with major depression, and animal models have shown that stress-related hippocampal changes may be reversible by antidepressant treatment.12 Therefore, future studies should investigate whether effective therapeutic strategies have a positive effect both on executive functioning and on preventing hippocampal volume loss.
Acknowledgments
This study was supported by the German Federal Research Ministry within the support of German Research Networks in Medicine as part of the project German Research Network on Depression.
Footnotes
Contributors: Drs. Frodl, Reiser, Möller and Meisenzahl designed the study. Drs. Frodl, Schaub, Banac, Leinsinger, Jäger and Bottlender and Mss. Kümmler, Born and Charypar acquired the data; Drs. Frodl and Zetzsche analyzed the data. Dr. Frodl wrote the article and all other authors critically reviewed it. All authors gave final approval for publication.
Competing interests: None declared.
Correspondence to: Dr. Thomas Frodl, Department of Psychiatry, Nußbaumstr. 7, 80336 Munich, Germany; fax 0049-89-5160-5343; Thomas.Frodl@med.uni-muenchen.de
References
- 1.Rosene DL, Van Hoesen GW. The hippocampal formation of the primate brain: a review of some comparative aspects of cytoarchitecture and connections. In: Jones EG, Peters A, editors. Cerebral cortex. Vol. 6. New York: Plenum; 1987. p. 345-456.
- 2.Salloway S, Cummings J. Subcortical disease and neuropsychiatric illness. J Neuropsychiatry Clin Neurosci 1994;6:93-9. [DOI] [PubMed]
- 3.Soares JC, Mann JJ. The anatomy of mood disorders: review of structural neuroimaging studies. Biol Psychiatry 1997;41:86-106. [DOI] [PubMed]
- 4.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 2000;126:413-31. [DOI] [PubMed]
- 5.Sheline YI, Sanghavi M, Mintun MA, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19:5034-43. [DOI] [PMC free article] [PubMed]
- 6.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry 2000;48:301-9. [DOI] [PubMed]
- 7.Mervaala E, Fohr J, Kononen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000;30:117-25. [DOI] [PubMed]
- 8.Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry 2000;157:115-8. [DOI] [PubMed]
- 9.Frodl T, Meisenzahl EM, Zetzsche T, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 2002;159:1112-8. [DOI] [PubMed]
- 10.Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 2004;161:598-607. [DOI] [PubMed]
- 11.Videbech P, Ravnikilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 2004;161:1957-66. [DOI] [PubMed]
- 12.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35. [DOI] [PubMed]
- 13.Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron 2002;34:13-25. [DOI] [PubMed]
- 14.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301:805-9. [DOI] [PubMed]
- 15.Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry 2002;17:306-10. [DOI] [PubMed]
- 16.Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 2001;11:520-8. [DOI] [PubMed]
- 17.Kantarci K, Jack CR Jr. Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin N Am 2003;13:197-209. [DOI] [PubMed]
- 18.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus 2005;15:798-807. [DOI] [PubMed]
- 19.den Heijer T, Vermeer SE, van Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003;46:1604-10. [DOI] [PubMed]
- 20.Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampale lesions: a review. Neurosci Biobehav Rev 1983;7:119-88. [DOI] [PubMed]
- 21.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. text revision (DSM-IV-TR). Washington: American Psychiatric Association; 2000.
- 22.Ottowitz WE, Dougherty DD, Savage CR. The neural network for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry 2002;10:86-99. [DOI] [PubMed]
- 23.Madden JJ, Luhan JA, Kaplan LA, et al. Nondementing psychosis in older persons. JAMA 1952;150:1567-72. [DOI] [PubMed]
- 24.Cassens G, Wolfe L, Zola M. The neuropsychology of depression. J Neuropsychiatry Clin Neurosci 1990;2:202-13. [DOI] [PubMed]
- 25.Rey A. Ĺexamen clinique en psychologie. Paris: Press Universitaire de France; 1958.
- 26.Hickie I, Naismith S, Ward PB, et al. Reduced hippocampal volumes and memory loss in patients with early-and late-onset depression. Br J Psychiatry 2005;186:197-202. [DOI] [PubMed]
- 27.Elliot R. The neuropsychological profile in unipolar depression. Trends Cogn Sci 1998;2:447-54. [DOI] [PubMed]
- 28.Fossati P, Ergis AM, Allilaire JF. Problem-solving abilities in unipolar depressed patients: comparison of performance on the modified version of the Wisconsin and the California sorting tests. Psychiatry Res 2001;104:145-56. [DOI] [PubMed]
- 29.Alexopoulos GS, Meyers BS, Young RC, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry 2000;57:285-90. [DOI] [PubMed]
- 30.Milner B. Effects of different brain lesions on card sorting. Arch Neurol 1963;9:90-100.
- 31.Wall PM, Messier C. The hippocampal formation – orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res 2001;127:99-117. [DOI] [PubMed]
- 32.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97-113. [DOI] [PubMed]
- 33.Andreasen NC, Cohen G, Harris G, et al. Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992;4:125-33. [DOI] [PubMed]
- 34.Niemann K, Hammers A, Coenen VA, et al. Evidence of a smaller left hippocampus and left temporal horn in both patients with first episode schizophrenia and normal control subjects. Psychiatry Res 2000;99:93-110. [DOI] [PubMed]
- 35.Convit A, McHugh P, Wolf OT, et al. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res 1999;90:113-23. [DOI] [PubMed]
- 36.Helmstaedter C, Durwen HF. In: VLMT – Verbaler Lern-und Merkfähigkeitstest nach Rey. Göttingen: Hogrefe; 2001. [PubMed]
- 37.MacQueen GM, Campbell S, McEwen BS, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A 2003;100:1387-92. [DOI] [PMC free article] [PubMed]
- 38.Bell-McGinty S, Butters MA, Meltzer CC, et al. Brain morphometry in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry 2002;159:1424-7. [DOI] [PubMed]
- 39.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry 2003;160:1516-8. [DOI] [PubMed]
- 40.Frodl T, Meisenzahl EM, Zetzsche T, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry 2004;65:492-9. [DOI] [PubMed]
- 41.Pantel J, Schroder J, Essig M, et al. Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord 1997;42:69-83. [DOI] [PubMed]
- 42.Kumar A, Jin Z, Bilker W, et al. Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci U S A 1998;95:7654-8. [DOI] [PMC free article] [PubMed]
- 43.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry 2002;51:273-9. [DOI] [PubMed]
- 44.Botteron KN, Raichle ME, Drevets WC, et al. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry 2002;51:342-4. [DOI] [PubMed]
- 45.Zola-Morgan SM, Squire LR. The primate hippocampal formation: evidence for a time limited role in memory storage. Science 1990;250:288-300. [DOI] [PubMed]
- 46.Shah PJ, Ebmeier KP, Glabus MF, et al. Cortical gray matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry 1998;172:527-32. [DOI] [PubMed]
- 47.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: a review and meta-analysis. Neuropsychologia 2004;42:1394-413. [DOI] [PubMed]
- 48.Ali SO, Denicoff KD, Altshuler LL, et al. A preliminary study of the relation of neuropsychological performance to neuroanatomic structures in bipolar disorder. Neuropsychiatry Neuropsychol Behav Neurol 2000;13:20-8. [PubMed]
- 49.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998;12:426-45. [DOI] [PubMed]
- 50.Corcoran R, Upton D. A role for the hippocampus in card sorting? Cortex 1993;29:293-304. [DOI] [PubMed]
- 51.Martin RC, Sawrie SM, Gilliam FG, et al. Wisconsin Card Sorting performance in patients with temporal lobe epilepsy: clinical and neuroanatomical correlates. Epilepsia 2000;41:1626-32. [DOI] [PubMed]
- 52.Berman KF, Ostrem JL, Randolph C, et al. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography. Neuropsychologia 1995; 33:1027-46. [DOI] [PubMed]
- 53.Szeszko PR, Bilder RM, Lencz T, et al. Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res 2000;43:97-108. [DOI] [PubMed]
- 54.Seidman LJ, Yurgelun-Todd D, Kremen WS, et al. Relationship of prefrontal and temporal lobe MRI measures to neuropsychological performance in chronic schizophrenia. Biol Psychiatry 1994;35:235-46. [DOI] [PubMed]
- 55.Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci 2006;29:116-24. [DOI] [PMC free article] [PubMed]
- 56.Hoshi E. Functional specialization within the dorsolateral prefrontal cortex: a review of anatomical and physiological studies of non-human primates. Neurosci Res 2006;54:73-84. [DOI] [PubMed]
- 57.Vakili K, Pillay SS, Lafer B, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry 2000;47:1087-90. [DOI] [PubMed]
- 58.Saylam C, Ucerler H, Kitis O, et al. Reduced hippocampal volume in drug-free depressed patients. Surg Radiol Anat 2006;28:82-7. [DOI] [PubMed]
- 59.Caetano SC, Hatch JP, Brambilla P, et al. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res 2004;132:141-7. [DOI] [PubMed]
- 60.Rosso IM, Cintron CM, Steingard RJ, et al. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry 2005;57:21-6. [DOI] [PubMed]


