Abstract
The explosive, destructive course of Bacillus endophthalmitis has been attributed to the production of toxins during infection. In this study we analyzed the contribution of toxins controlled by the global regulator plcR to the pathogenesis of experimental Bacillus endophthalmitis. Isogenic plcR-deficient mutants of Bacillus cereus and Bacillus thuringiensis were constructed by insertional inactivation of plcR by the kanamycin resistance cassette, aphA3. Rabbit eyes were injected intravitreally with approximately 100 CFU of wild-type B. cereus or B. thuringiensis or a plcR-deficient mutant. The evolution of endophthalmitis resulting from each plcR-deficient mutant was considerably slower than that caused by each wild-type strain. Retinal function was not eliminated until 42 h postinfection in rabbits with endophthalmitis caused by the plcR-deficient mutants, whereas wild-type infections resulted in a complete loss of retinal function within 18 h. The intraocular inflammatory cell influx and retinal destruction in plcR-deficient endophthalmitis approached the severity observed in wild-ype infections, but not until 36 h postinfection. Gross and histological examinations of eyes infected with plcR mutants demonstrated that the anterior and posterior segment changes were muted compared to the changes observed in eyes infected with the wild types. The loss of plcR-regulated factors significantly attenuated the severity of Bacillus endophthalmitis. The results therefore suggest that plcR may represent a target for which adjunct therapies could be designed for the prevention of blindness during Bacillus endophthalmitis.
Endophthalmitis is a severe infection caused by the introduction of bacteria into the eye following trauma or surgery or by metastatic spread into the eye during septicemia. Bacillus causes a uniquely explosive form of the disease that, despite aggressive therapeutic and surgical intervention, frequently results in loss of functional vision, if not blindness, within 1 to 2 days (12, 14, 15, 24, 27).
The unique virulence of Bacillus cereus endophthalmitis has traditionally been attributed to toxin production by the organism during growth in the eye (14, 15, 24, 27). B. cereus produces several potential virulence factors that may contribute to the disease (18, 30). To date, three membrane-damaging toxins, hemolysin BL (HBL), phosphatidylinositol-specific phospholipase C (PI-PLC), and phosphatidylcholine-specific phospholipase C (PC-PLC), have been discounted as individual contributors to the pathogenesis of experimental endophthalmitis (9, 10). Intraocular infection with the wild type or with isogenic Bacillus mutants specifically deficient in HBL, PI-PLC, or PC-PLC resulted in similar degrees of destruction of retinal architecture, complete loss of retinal function, and significant intraocular inflammation within 12 to 18 h (9, 10).
These findings suggested that individual toxins may not be responsible for the significant pathology observed in Bacillus endophthalmitis. In the case of Staphylococcus aureus, an absence of multiple toxins resulting from global regulation deficiencies significantly affected virulence in a number of experimental infection models. The expression of several potential S. aureus virulence factors is controlled by the accessory gene regulator (agr) quorum-sensing system (3). Mutants defective in agr exhibited reduced expression of a number of toxins and subsequently reduced virulence in several nonocular infection models (1, 13, 17, 19). Similarly, in experimental endophthalmitis (6, 7) and keratitis (23) models initiated by S. aureus strains deficient in agr, virulence was also highly attenuated.
Extracellular toxin production in Bacillus is controlled by plcR, a global regulator that activates the transcription of a number of putative virulence factors, including membrane-damaging toxins, enzymes, and cell surface proteins (2). plcR has been identified in both B. cereus and Bacillus thuringiensis, two pathogens that have been shown to cause similar degrees of explosive inflammation and tissue destruction in experimental endophthalmitis models (9-11). In this study we examined the relationship of plcR virulence factor regulation to the pathogenesis of Bacillus endophthalmitis.
MATERIALS AND METHODS
Bacterial strains and mutant construction.
The B. cereus and B. thuringiensis strains used in this study have been described previously (25). B. cereus ATCC 14579 (American Type Culture Collection, Manassas, Va.) and B. thuringiensis BT407 were employed as wild-type strains. plcR-deficient mutants of B. cereus (BCplcR::kanR) and B. thuringiensis (BTplcR::kanR) were constructed by insertional inactivation of plcR with the kanamycin resistance cassette aphA3, as previously described (25).
Culture media and reagents.
For cultivation of B. cereus and B. thuringiensis, brain heart infusion (BHI) (Difco, Detroit, Mich.) was used with or without appropriate antibiotic selection. Inocula for intravitreal injections were prepared in BHI without antibiotics. For phenotypic screening of wild-type and mutant strains, motility agar, skim milk agar, egg yolk agar, and 2.0% sheep erythrocyte agar were used.
Phenotypic analysis of Bacillus strains.
The methods used for analysis of hemolytic, proteolytic, enzymatic, and motile activities of Bacillus filtered culture supernatants and individual colonies have been described previously (10). Briefly, hemolytic activity was determined by quantifying hemoglobin release from sheep erythrocytes. Proteolytic activity was determined on hide azure powder and on BHI agar supplemented with 2.5% skim milk. PI-PLC activity was determined colorimetrically by quantifying acetylcholinesterase release. PC-PLC activity was determined by turbidity on egg yolk agar. Sphingomyelinase (SPH) activity was determined colorimetrically by quantifying trinitrophenylaminolauroyl-sphingomyelin hydrolysis and by the CAMP test. Relative motility was determined by measuring colony diameter on motility agar. Phenotypic differences between the wild-type strains and the plcR-deficient mutants are summarized in Table 1.
TABLE 1.
Phenotypic analysis of B. cereus and B. thuringiensis wild-type strains and plcR-deficient isogenic mutantsa
| Strain | Hemolytic titerb | Concn of:
|
Motility (mm)g | |||
|---|---|---|---|---|---|---|
| PI-PLC (μg/ml)c | PC-PLC (μg/ml)d | Sphingomyelinase (μg/ml)e | Protease (U/ml)f | |||
| B. cereus ATCC 14579 | 256 | 11.9 ± 1.8 | 1.13 ± 0.25 | 0.35 ± 0.06 | 0.29 ± 0.04 | 14.23 ± 0.25 |
| BCplcR::kanR | 8 | 2.4 ± 0.2 | NDh | ND | 0.01 ± 0.002 | 7.5 ± 0.2 |
| B. thuringiensis BT407 | 64 | 11.6 ± 0.3 | 1.69 ± 0.21 | ND | 0.23 ± 0.06 | 11.53 ± 0.15 |
| BTplcR::kanR | 4 | 2.7 ± 0.4 | ND | ND | 0.02 ± 0.02 | 7.5 ± 0.1 |
For all phenotypic analyses, three or more separate assays were performed.
Determined by a hemolytic assay of filtered supernatants of 8-h stationary-phase Bacillus cultures. A twofold difference in titer was considered significant.
Determined by a chromogenic PI-PLC assay. The values are means ± standard deviations for filtered supernatants of 10-h Bacillus cultures. The PI-PLC activities of the wild-type strains were significantly greater than those of the plcR-deficient mutants (P < 0.0001, as determined by Student's t test).
Determined by an egg yolk agar well diffusion assay for PC-PLC activity. The values are means ± standard deviations for filtered supernatants of 10-h Bacillus cultures.
Determined by a chromogenic SPH assay and CAMP test. No SPH was detected in filtered supernatants of 8-h cultures of strains BCplcR::kanr, BT407, and BTplcR::kanR. Negative CAMP reactions were also observed with these strains.
Determined by a hide azure blue protease assay. The values are means ± standard deviations for filtered supernatants of 8-h Bacillus cultures. The protease activities of the wild-type strains were significantly greater than those of the plcR-deficient mutants (P < 0.0001, as determined by Student's t test).
Determined by swarming on motility agar following an 8-h incubation at 37°C. The values are diameters and are means ± standard deviations. The diameters of wild-type colonies were significantly larger than those of the colonies of the plcR-deficient mutants (P ≤ 0.002, as determined by Student's t test).
ND, not detected.
Experimental Bacillus endophthalmitis.
Experimental B. cereus or B. thuringiensis endophthalmitis was induced in New Zealand White rabbits, as previously described (9-11). Animals were maintained in strict accordance with institutional animal care facility guidelines and the Association for Research in Vision and Ophthalmology Statement on the Use of Laboratory Animals in Ophthalmic Research (4). Rabbits were anesthetized with a mixture of ketamine (Ketaved; 35 mg/kg of body weight; Phoenix Scientific Inc., St. Joseph, Mo.) and xylazine (Rompun; 5 mg/kg of body weight; Bayer Corp., Shawnee Mission, Kans.). Before each surgical procedure, topical anesthetic (Ophthetic; 0.5% proparacaine HCl; Allergan Inc., Hormigueros, Puerto Rico) was applied. Before each intravitreal injection, 100 μl of aqueous humor was withdrawn by paracentesis. Approximately 100 CFU of B. cereus or B. thuringiensis in BHI was delivered by slow infusion via a 30-gauge needle through the pars plana directly into the midvitreous. The contralateral eye either was injected with BHI (surgical control) or remained untouched (absolute controls). At various times after injection, infection courses were analyzed by biomicroscopy, electroretinography (ERG), histology, and bacterial and inflammatory cell enumeration, as described below.
Biomicroscopy and ERG.
Eyes were observed prior to and throughout the course of infection by using a Topcon SL-5D slit lamp biomicroscope (Kogaku Kikai K.K., Tokyo, Japan). ERG was employed to measure retinal function, as previously described (9-11). b-Wave amplitudes were recorded for each eye prior to injection and at various times postinjection by using scotopic bright-flash ERG (EPIC2000; LKC Technologies, Inc., Gaithersburg, Md.). After dilation and 30 min of dark adaptation, b-wave responses to single light flashes (1 flash/s) were measured, and b-wave amplitudes were recorded as the averages of 14 repeated measures. The percentage of retinal function retained was calculated as follows: 100 − {[1 − (experimental b-wave amplitude/control b-wave amplitude)] × 100} (9-11). The latency of retinal responses corresponding to percentages of implicit time (τ) was calculated as follows: [1 − (experimental τ/control τ)] × 100 (10).
Bacterial and inflammatory cell enumeration.
Enumeration of B. cereus and B. thuringiensis cells in ocular tissues has been described previously (9-11). Briefly, globes were enucleated, and the vitreous was removed and homogenized. Bacilli in the vitreous were quantified by track plating serial 10-fold dilutions onto BHI (22). Retention of mutant phenotypes was confirmed by phenotypic assays and subculturing onto antibiotic media. Inflammatory cells in paracentesed aqueous humor samples (10-μl aliquots) were stained with 0.4% trypan blue and quantified with a hemocytometer (10).
Thin-section histopathology.
Globes recovered for histological analysis were fixed in 10% formalin for 24 h. Eyes were sectioned and stained with hematoxylin and eosin and with tissue Gram stain by using standard procedures (28).
Statistical analysis.
The values for parameters used to analyze progressive infections are the mean ± standard error of the mean (SEM) for four or more eyes per time point, unless otherwise specified. The Wilcoxon rank sum test was used for statistical comparison of infection groups. Student's t test was used for statistical comparison of phenotypic data. A P value of ≤0.05 was considered significant.
RESULTS
Experimental B. cereus and B. thuringiensis endophthalmitis.
Reproducible B. cereus endophthalmitis was achieved by intravitreal injection of 1.94 ± 0.08 log10 CFU of strain ATCC 14579. The inflammatory symptoms observed in the infected eyes were similar to those observed during experimental endophthalmitis when B. cereus strain MGBC145, an ocular isolate, was the infecting strain (9, 11). By 6 h, the inflammatory symptoms included 10 to 20 anterior chamber inflammatory cells per slit lamp field, increased vitreous haze, and a slightly decreased fundus reflex in all infected eyes. By 12 h, the inflammatory symptoms had progressed from moderate to severe levels, with severe iritis, vitreous opacities, significant inflammatory cell influx into the cornea and anterior chamber, and no fundus reflex. By 18 h, inflamed periorbital tissues and a panophthalmitis were observed. Infections were therefore not allowed to progress further. No pathological changes were observed in surgical or absolute controls at 6, 12, or 18 h postinfection.
Reproducible B. thuringiensis endophthalmitis with BT407 as the infecting strain has recently been described (10). In the present study, reproducible infection was achieved by intravitreal injection of 2.06 ± 0.04 log10 CFU of strain BT407. A mild posterior segment inflammatory response with 5 to 10 anterior chamber inflammatory cells per slit lamp microscope field was observed at 6 h. At this time, all eyes had a normal fundus reflex. The inflammatory changes in the anterior and posterior segments progressed from 6 to 12 h, with a diminishing fundus reflex and increasing vitreous haze. From 12 to 18 h, the inflammatory symptoms were severe in all eyes, as previously described (10). Corneal ring infiltrates and severe inflammation of periorbital tissues were present in at least one-half of the eyes infected with BT407. Because of the impending panophthalmitis at 18 h, infections were not allowed to progress further. No pathological changes were observed in surgical or absolute controls at 6, 12, or 18 h postinfection.
Experimental plcR-deficient endophthalmitis.
The endophthalmitis induced by plcR-deficient mutants of B. cereus and B. thuringiensis progressed considerably slower than wild-type bacterial endophthalmitis progressed. Reproducible endophthalmitis was achieved by intravitreal injection of 1.99 ± 0.08 log10 CFU of strain BCplcR::kanR or 1.97 ± 0.04 log10 CFU of strain BTplcR::kanR. At 6 h postinfection, most eyes infected with plcR-deficient strains lacked notable inflammatory symptoms. The remaining eyes exhibited a mild anterior segment cellular infiltrate and a normal fundus reflex. In general, the inflammatory symptoms in eyes infected with the plcR-deficient strains progressed slowly from 6 to 24 h postinfection, with the number of infiltrating cells in the anterior chamber increasing from approximately 5 cells per field to approximately 20 cells per field over this time period. Conjunctival inflammation progressed from mild to moderate over this time period, and periorbital tissues were only slightly inflamed. Fundus reflexes in all eyes were slightly diminished.
Because the inflammatory symptoms of eyes infected with plcR-deficient Bacillus strains had not achieved severe levels by 24 h postinfection, the infections were allowed to progress further. The level in the anterior segment cellular infiltrate remained approximately 20 to 25 cells per field for 42 h. Fundus reflexes were absent by 36 h. Conjunctival chemosis and injection remained at moderate levels throughout 42 h of infection and did not achieve the severity of wild-type infections. Developing corneal ring abscesses were evident in one-third of eyes infected with the plcR-deficient mutants at 42 h postinfection.
Growth of wild-type and plcR-deficient Bacillus strains.
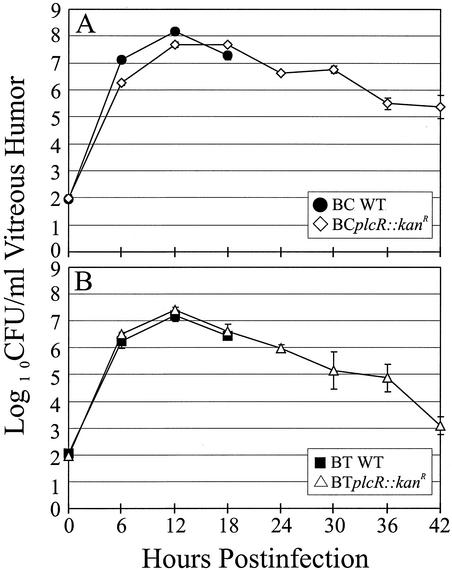
The growth of each wild-type Bacillus strain and the growth of its plcR-deficient mutant in BHI were similar (data not shown). The intravitreal concentrations of BCplcR::kanR were significantly lower than those of wild-type B. cereus at 6 and 12 h postinfection (P ≤ 0.004) but were similar to those of wild-type B. cereus at 18 h postinfection (P = 0.09) (Fig. 1A). Growth of BCplcR::kanR peaked at 12 to 18 h postinfection, after which a gradual decline in the intravitreal concentration occurred. The concentration of BCplcR::kanR cells had decreased to 0.03% of the peak intravitreal concentration by 42 h. The intraocular growth of strain BT407 and the intraocular growth of its plcR-deficient mutant were similar from zero time to 18 h postinfection (P ≥ 0.12) (Fig. 1B). Growth of BTplcR::kanR peaked at 12 h postinfection. After 12 h, the intravitreal bacterial concentrations decreased to 0.0046% of the peak intravitreal concentration by 42 h.
FIG. 1.
Intraocular growth of B. cereus and B. thuringiensis wild-type strains and their plcR-deficient mutants. Approximately 100 CFU of each Bacillus strain was injected intravitreally. Wild-type Bacillus strains were quantified every 6 h for 18 h, while plcR-deficient strains were quantified every 6 h for 42 h of intraocular growth. The strains analyzed were B. cereus ATCC 14579 (BC WT) and its plcR-deficient mutant (BCplcR::kanR) (A) and B. thuringiensis BT407 (BT WT) and its plcR-deficient mutant (BTplcR::kanR) (B). All values are means ± SEM for four or more eyes per group.
Retinal function analysis.
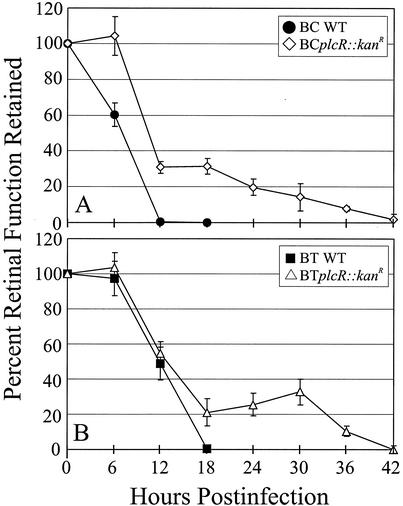
The ERG results for eyes infected with wild-type Bacillus strains or their plcR-deficient mutants are summarized in Fig. 2 and 3. The retinal responsiveness of eyes infected with wild-type B. cereus or B. thuringiensis was similar to that observed in previous studies (9-11). The retinal responsiveness of all surgical and absolute control eyes was similar the preoperative responsiveness throughout the duration of the experiment (data not shown). Retinal function was eliminated completely in eyes infected with wild-type B. cereus by 12 h postinfection and by 18 h postinfection in eyes infected with wild-type B. thuringiensis.
FIG. 2.
Retinal function analysis of wild-type and plcR-deficient Bacillus endophthalmitis. ERG was performed every 6 h throughout the course of infection. Rapid decreases in b-wave amplitude were observed in eyes infected with wild-type Bacillus strains for 18 h, while gradual decreases were observed over a 42-h period in eyes infected with plcR mutant strains. All values are means ± SEM for four or more eyes per group. BC WT, B. cereus ATCC 14579; BCplcR::kanR, plcR-deficient mutant of B. cereus ATCC 14579; BT WT, B. thuringiensis BT407; BTplcR::kanR, plcR-deficient mutant of B. thuringiensis BT407.
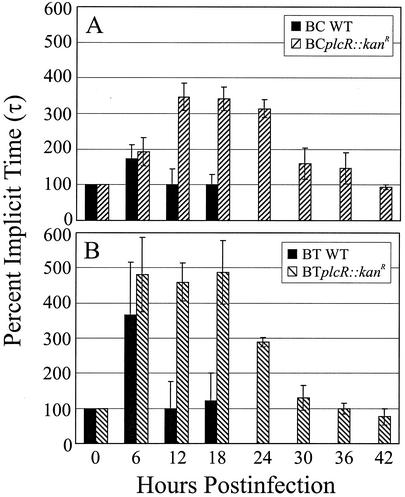
FIG. 3.
Analysis of latent b-wave responses in wild-type and plcR-deficient Bacillus endophthalmitis. Implicit times (τ) from a-wave valleys to b-wave peaks of electroretinograms are shown. Increases in implicit times were observed at 6 h only for eyes infected with wild-type strains and were extended from 6 to 24 h for eyes infected with plcR-deficient strains. Increases in retinal response latencies may reflect changes in functional integrity associated with the rate of retinal response. All values are means ± SEM for four or more eyes per group. BC WT, B. cereus ATCC 14579; BCplcR::kanR, plcR-deficient mutant of B. cereus ATCC 14579; BT WT, B. thuringiensis BT407; BTplcR::kanR, plcR-deficient mutant of B. thuringiensis BT407.
The retinal responsiveness of eyes infected with BCplcR::kanR was significantly greater than that of eyes infected with wild-type B. cereus at 6, 12, and 18 h (P ≤ 0.004) (Fig. 2A). Eyes infected with BCplcR::kanR exhibited retinal function similar to the preoperative function at 6 h (P = 0.81). From 6 to 12 h postinfection, these eyes exhibited a sharp drop in retinal responsiveness to a level of approximately 30%. After 18 h, the retinal function declined slowly, and by 42 h the retinal function was less than 2%.
The retinal responsiveness of eyes infected with BTplcR::kanR was similar to the preoperative responsiveness and to the responsiveness of controls and of eyes infected with wild-type B. thuringiensis at 6 h postinfection (P ≥ 0.64). The retinal responses of BTplcR::kanR-infected eyes were also similar to those of eyes infected with wild-type B. thuringiensis at 12 h (P ≥ 0.61) (Fig. 2B). The retinal responsiveness of eyes infected with BTplcR::kanR was significantly greater than that of eyes infected with wild-type B. thuringiensis at 18 h postinfection (P = 0.04). From 6 to 18 h postinfection, these eyes exhibited a precipitous drop in retinal responsiveness to approximately 22%. Retinal function did not change from 18 to 30 h (P ≥ 0.38), but it declined slowly thereafter and there was a complete loss of retinal function by 42 h.
Changes in the latency of retinal responses are summarized in Fig. 3. Increases in latency were detected in eyes infected with both Bacillus wild-type strains at 6 h postinfection only. At 6 h, the implicit times for eyes infected with the wild-type strains and their respective plcR-deficient mutants were similar (P = 0.18 for B. cereus wild type versus BCplcR::kanR; P = 0.57 for B. thuringiensis wild type versus BTplcR::kanR). The implicit times for eyes infected with each wild-type Bacillus strain were similar to the preoperative values and the control values at 12 and 18 h postinfection (P ≥ 0.09).
The implicit times for eyes infected with BCplcR::kanR increased significantly from time zero to 12 h postinfection (P ≤ 0.03), and the values reached a plateau of approximately 350% at 12 to 24 h (P ≥ 0.60) (Fig. 3A). From 30 to 42 h, the implicit times returned to levels similar to preoperative levels (P ≥ 0.22). The implicit times for eyes infected with BTplcR::kanR increased significantly from time zero to 6 h postinfection (P = 0.0004) to nearly 500%, a value that remained constant from 6 to 18 h (P ≥ 0.85) (Fig. 3B). Following a sharp decline, the implicit times returned to levels similar to preoperative levels from 30 to 42 h (P ≥ 0.35). Increases in retinal function latencies may reflect early changes in functional integrity that affect the rate of retinal responses.
Anterior segment inflammation.
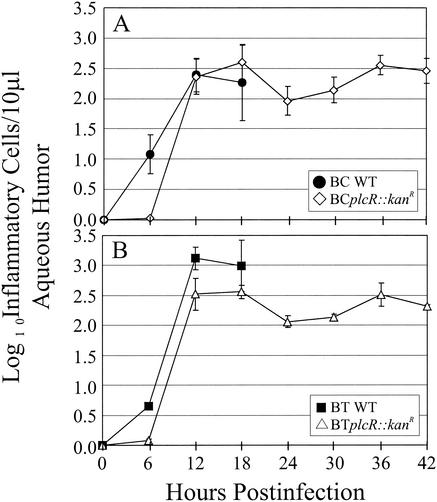
The inflammatory changes in eyes infected with Bacillus in terms of the cellular influx into the anterior segment are summarized in Fig. 4. Although few inflammatory cells were recovered from eyes infected with each Bacillus strain at 6 h postinfection, the numbers of inflammatory cells in eyes infected with wild-type B. cereus were greater than the numbers of inflammatory cells in eyes infected with BCplcR::kanR (P = 0.01) at this time (Fig. 4A). Similarly, the numbers of inflammatory cell were greater in eyes infected with wild-type B. thuringiensis than in eyes infected with BTplcR::kanR at the same time point (Fig. 4B). At 12 and 18 h postinfection, the numbers of inflammatory cells recovered from eyes infected with wild-type Bacillus strains and their plcR-deficient mutants were similar (P ≥ 0.53 for B. cereus wild type versus BCplcR::kanR; P ≥ 0.12 for B. thuringiensis wild type versus BTplcR::kanR). The numbers of inflammatory cell recovered from BCplcR::kanR-infected eyes from 12 to 42 h were similar (P ≥ 0.06). In BTplcR::kanR-infected eyes, the numbers of inflammatory cell were lower at 24 and 30 h than at 18 h postinfection (P ≤ 0.01).
FIG. 4.
Inflammatory cell influx into the anterior segment in wild-type and plcR-deficient Bacillus endophthalmitis. Anterior chamber paracentesis was performed to harvest aqueous humor, and inflammatory cells were quantified every 6 h throughout the course of infection. Rapid increases in the numbers of inflammatory cells were observed by 12 h, regardless of the infecting strain. The numbers of cells in plcR-infected eyes were similar to peak levels throughout 42 h of infection. All values are means ± SEM for four or more eyes per group. BC WT, B. cereus ATCC 14579; BCplcR::kanR, plcR-deficient mutant of B. cereus ATCC 14579; BT WT, B. thuringiensis BT407; BTplcR::kanR, plcR-deficient mutant of B. thuringiensis BT407.
Histological analysis.
The results of whole-organ and retinal histologic analysis of progressive B. cereus and B. thuringiensis endophthalmitis are summarized in Fig. 5 and 6. Immediately following intravitreal injection, eyes in all infection groups had intact retinal layers, no anterior or posterior segment inflammation, and few bacilli in the vitreous (data not shown).
FIG. 5.
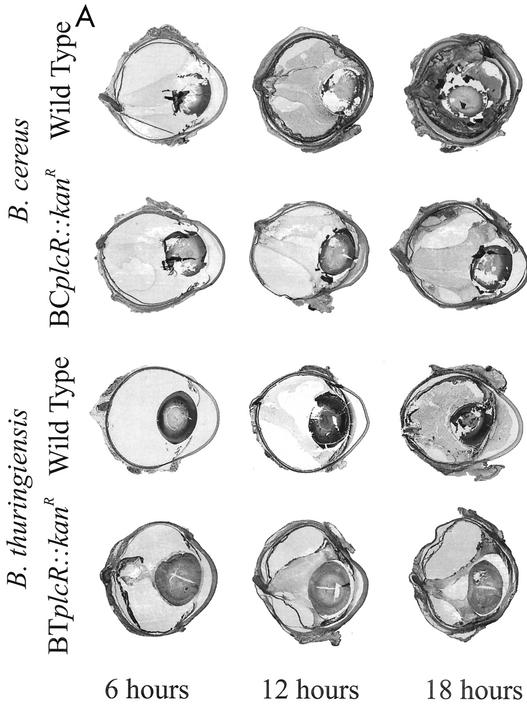
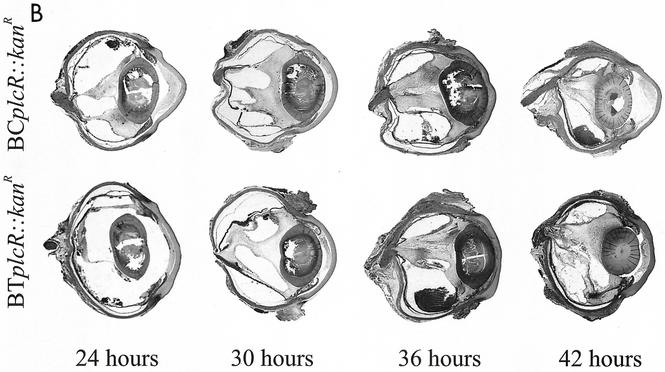
(A) Whole-organ histological analysis of wild-type and plcR-deficient Bacillus endophthalmitis. In eyes infected with wild-type Bacillus, photoreceptor folding was observed in retinal sections by 12 h. By 18 h, severe inflammation was observed, and retinal layers were difficult to differentiate. Eyes infected with plcR-deficient strains showed mild inflammatory changes compared with eyes infected with wild-type strains at 12 and 18 h postinfection. All representative histological sections were stained with hematoxylin and eosin. Magnification, ×8.9. (B) Whole-organ histological analysis of extended plcR-deficient Bacillus endophthalmitis. Inflammatory changes were relatively mild throughout 30 h of infection. From 36 to 42 h, photoreceptor folding, random retinal detachments, and severe inflammation were observed. All representative histological sections were stained with hematoxylin and eosin. Magnification, ×7.5.
FIG. 6.
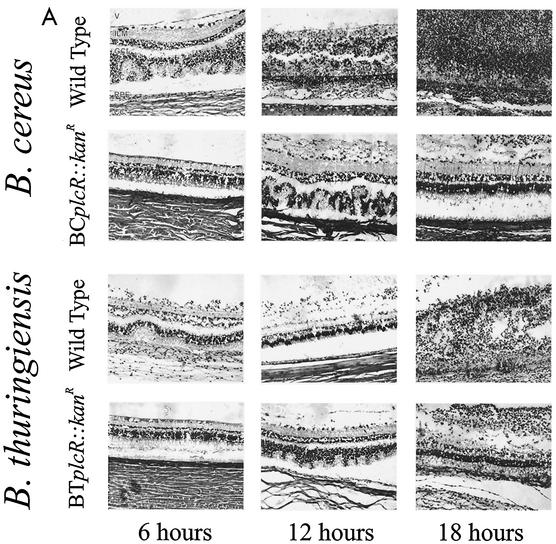
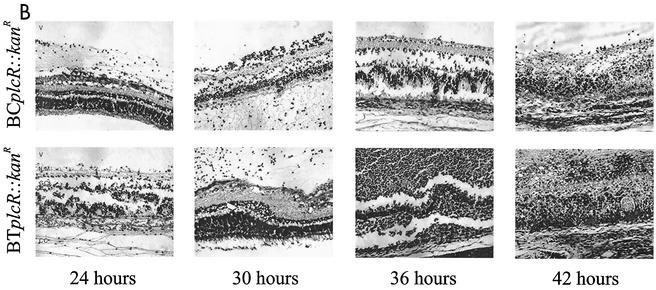
(A) Retinal histological analysis of wild-type and plcR-deficient Bacillus endophthalmitis. Retinas from eyes infected with wild-type Bacillus were highly inflamed and partially detached, and the photoreceptor layers were indistinguishable by 18 h. Retinas from eyes infected with plcR-deficient Bacillus were mildly inflamed and essentially intact at 18 h postinfection. Abbreviations: V, vitreous; ILM, inner limiting membrane; GCL, ganglion cell layer; PC, photoreceptor cell layer; RPE, retinal pigment epithelium; CC, choriocapillaris. Magnification, ×168. (B) Retinal histological analysis of extended plcR-deficient Bacillus endophthalmitis. Retinas from eyes infected with plcR-deficient Bacillus were essentially intact, and there were small areas of photoreceptor layer folding by 30 h postinfection. Significant inflammation and photoreceptor layer folding were apparent by 36 h, and retinal layers were severely disrupted by 42 h postinfection. Magnification, ×128.
At 6 h, eyes infected with wild-type B. cereus exhibited mild to moderate inflammatory cell migration originating from the optic nerve head and ciliary body into the posterior segment. Bacilli were observed throughout the vitreous, and the retinal architecture was moderately disrupted, with some photoreceptor layer folding and bacilli within the retinal layers. By 12 h postinfection, the effects in eyes infected with wild-type B. cereus had reached severe levels, with significant retinal architecture disruption, massive influx of inflammatory cells into the posterior and anterior segments, numerous bacilli throughout all parts of the eye, and inflamed periocular tissues. By 18 h postinfection, the retinal layers were indistinguishable, and the posterior and anterior segments were filled with fibrin, inflammatory cells, and bacilli.
A histological analysis of progressive infection with the wild-type B. thuringiensis strain has been described previously (10). Briefly, at 6 h postinfection, a mild inflammatory response was observed in the posterior segment, and the retinal architecture was intact. At 12 h postinfection, a moderate inflammatory response was observed in both the anterior and posterior segments, with significant retinal photoreceptor layer folding, partial retinal detachment, and bacilli observed throughout the anterior and posterior segments. At 18 h postinfection, the retinal layers were completely destroyed, severe inflammation in both posterior and anterior segments was present, and periocular tissues were inflamed. In general, eyes infected with wild-type B. thuringiensis appeared to be less inflamed than eyes infected with wild-type B. cereus at 12 and 18 h postinfection.
Eyes infected with both Bacillus plcR-deficient mutants were less inflamed than eyes infected with the corresponding wild-type parental strains throughout 18 h of infection. Inflammatory cell influx into the posterior segment from the optic nerve head was observed at 6 h postinfection. At 12 h postinfection, significant inflammation was observed in all eyes, originating from the optic nerve head and ciliary body. Small areas of retinal photoreceptor layer folding were also seen at this time. Bacilli were observed primarily in the midvitreous, with very few bacilli located in the anterior segment and no bacilli observed in the retinal layers. Overall, the inflammatory changes did not increase appreciably between 12 and 30 h postinfection, and the retinal layers remained intact. Inflammatory cells began to invade the corneal stroma from the limbus, and corneal edema increased slightly. Few bacilli were observed in the anterior segment, and no bacilli were observed within the retinal layers from 12 to 30 h postinfection. At 36 and 42 h, significant inflammation in the anterior and posterior segments and photoreceptor layer folding were evident in all eyes infected with the plcR-deficient mutants. Few bacilli were dispersed throughout the vitreous, within retinal layers, and in the anterior segment.
DISCUSSION
Bacillus is unrivaled in its capacity to infect the interior of the eye and invade ocular tissues in a rapid and explosive manner, causing severe inflammation, irreparable retinal damage, and ultimately destruction of the eye in a very short time. Clinical reports have attributed this devastating infection course to toxin production, but there has been little supporting experimental evidence (14, 15, 23, 27). By comparing endophthalmitis caused by wild-type Bacillus and endophthalmitis caused by isogenic mutants deficient only in single toxins of interest, we discounted the contribution of three Bacillus toxins, HBL, PI-PLC, and PC-PLC, to the course and severity of disease (9, 10). Evidence concerning the contribution of bacterial toxins to endophthalmitis demonstrates that in some cases, single toxins are the primary virulence factors (e.g., the Enterococcus faecalis cytolysin [21]). In other cases, multiple toxins work synergistically to achieve virulence (e.g., S. aureus toxins governed by the agr-sar quorum-sensing system [6, 16]).
In a manner similar to S. aureus virulence factor regulation, production of a number of Bacillus toxins is controlled by the transcriptional regulator plcR. Our phenotypic results demonstrated that a mutation in plcR resulted in a loss of the activities of SPH (in B. cereus only), PI-PLC, and PC-PLC and in significant decreases in hemolytic and proteolytic activities. In the absence of these toxins, intraocular inflammation and decreases in retinal function did occur, but at notably lower rates. Taken together, these results suggest that Bacillus factors under the control of plcR likely work in concert to achieve the level of virulence observed in experimental endophthalmitis. plcR has previously been shown to regulate the opportunistic pathogenicity of B. cereus and B. thuringiensis (25). However, unlike the case of the nearly complete attenuation of endophthalmitis pathology by a mutation in the S. aureus agr-sar global regulatory system (6, 7), a mutation in plcR did not eliminate Bacillus endophthalmitis virulence. These results highlight the conclusion that additional factors not regulated by plcR likely contributed to intraocular virulence in these protracted infections.
In this study, a mutation in plcR not only depressed toxin production but also negatively influenced bacterial motility. In vitro analysis showed that both plcR mutants were less motile. In the eyes, much smaller numbers of bacilli were observed in the anterior segment, and significant numbers were not observed within disrupted retinal layers in eyes infected with the two plcR-deficient mutants; these results are different from those observed with wild-type endophthalmitis. Therefore, decreased motility of the plcR-deficient mutants may also have contributed to the attenuated virulence of these strains. The present results are consistent with findings obtained in preliminary analyses of a nonmotile B. cereus transposon mutant (12) and a nonmotile flagellar (flhA) B. thuringiensis mutant (M. C. Callegan, D. C. Cochran, S. T. Kane, M. S. Gilmore, E. Gelhardi, D. J. Beecher, F. Celandroni, and S. Senesi, Abstr. Assoc. Res. Vis. Ophthalmol. Annu. Meeting, abstr. 1382, 2001) in the experimental endophthalmitis model. These nonmotile Bacillus mutants were significantly less virulent than their motile wild-type parental strains in the eye.
Establishment of a Bacillus endophthalmitis infection course with measurable retinal function at the later stages of infection (i.e., after 18 h) was unexpected. Infections initiated by plcR-deficient mutants resulted in measurable retinal responses throughout 36 h of infection. By 42 h, however, retinal function was eliminated or nearly eliminated in these eyes. Retinal malfunction in the absence of plcR-regulated factors could be due to the presence of factors not controlled by plcR, to slower inflammatory cell invasion, to an attenuated infection, to the lack of bacterial migration into the tissues, or to a combination of these factors.
Retinal response latencies observed during endophthalmitis caused by plcR-deficient mutants were increased over an extended period, a result unlike the results observed at 6 h only for wild-type endophthalmitis in this and previous studies (10). It should be noted that no changes in latency were observed during the later stages of wild-type infections because the retinas were nonfunctional. Retinal response latency, commonly detected as a delay in the b-wave response by ERG, is an important indicator of functional integrity (5, 8, 20, 26). In eyes infected with wild-type B. thuringiensis and both plcR-deficient mutants at 6 h, the b-wave amplitudes were similar to those of controls, but the latencies were increased significantly compared with those of the controls. This result may indicate that there are early retinal changes that affect the rate of retinal response but not the extent of this response. The unusually long period of latency observed in plcR-deficient endophthalmitis after 6 h may reflect slow cellular damage in a retina that continues to function. It is during this time that in an attenuated infection, therapies designed for preserving retinal stability and function may be of use.
Bacterial pathogens have evolved unique regulatory systems to coordinate the production of factors necessary for survival Pin different environments. plcR-regulated toxins and motility are only two virulence traits that Bacillus may use to its advantage in a hostile environment, such as that encountered during endophthalmitis. Attenuation of the severity of endophthalmitis resulting from blockade or alteration of plcR function may provide a necessary window of therapeutic opportunity. It has recently been shown that activation of plcR requires a signaling peptide (PapR), which acts as a quorum-sensing autoinducer (29). Activation of plcR therefore represents an attractive target for which information-based therapies could be designed for concomitant use with antibiotics, anti-inflammatory agents, and retinal cell stabilizers in order to prevent blindness during devastating Bacillus endophthalmitis.
.
Acknowledgments
We thank Mark Dittmar (DMEI Animal Facility), Paula Pierce (DMEI Pathology Laboratory), and Raniyah Ramadan (OUHSC Oklahoma Center for Neuroscience) for technical assistance and Michael Engelbert, Dan Carr, and James Chodosh for helpful discussions.
This work was supported by the National Institutes of Health (grant R01EY12985), by Fight for Sight/Prevent Blindness America, and by an unrestricted Career Development Award from Research to Prevent Blindness, Inc. (to M.C.C.).
Editor: D. L. Burns
REFERENCES
- 1.Abdelnour, A., S. Arvidson, T. Bremell, C. Ryden, and A. Tarkowski. 1993. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect. Immun. 61:3879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agaisse, H., M. Gominet, O. A. Okstad, A. B. Kolsto, and D. Lereclus. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043-1053. [DOI] [PubMed] [Google Scholar]
- 3.Arvidson, S., and K. Tegmark. 2001. Regulation of virulence determinants in Staphylococcus aureus. Int. J. Med. Microbiol. 291:159-170. [DOI] [PubMed] [Google Scholar]
- 4.Association for Research in Vision and Ophthalmology. 2002, posing date. Statement for the use of animals in ophthalmic and visual research. [Online.] Association for Research in Vision and Ophthalmology, Rockville, Md. http://www.arvo.org/AboutARVO/animalst.htm.
- 5.Block, F., and M. Schwarz. 1998. The b-wave of the electroretinogram as an index of retinal ischemia. Gen. Pharmacol. 30:281-287. [DOI] [PubMed] [Google Scholar]
- 6.Booth, M. C., A. L. Cheung, K. L. Hatter, B. D. Jett, M. C. Callegan, and M. S. Gilmore. 1997. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect. Immun. 65:1550-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth, M. C., R. V. Atkuri, S. K. Nanda, J. J. Iandolo, and M. S. Gilmore. 1995. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Investig. Ophthalmol. Vis. Sci. 36:1828-1836. [PubMed] [Google Scholar]
- 8.Bresnick, G. H., and M. Palta. 1987. Temporal aspects of the electroretinogram in diabetic retinopathy. Arch. Ophthalmol. 105:660-664. [DOI] [PubMed] [Google Scholar]
- 9.Callegan, M. C., B. D. Jett, L. E. Hancock, and M. S. Gilmore. 1999. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection as assessed using an endophthalmitis model. Infect. Immun. 67:3357-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callegan, M. C., D. C. Cochran, S. T. Kane, M. S. Gilmore, M. Gominet, and D. Lereclus. 2002. Contribution of membrane-damaging toxins to Bacillus endophthalmitis pathogenesis. Infect. Immun. 70:5381-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callegan, M. C., M. C. Booth, B. D. Jett, and M. S. Gilmore. 1999. Pathogenesis of gram-positive bacterial endophthalmitis. Infect. Immun. 67:3348-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callegan, M. C., S. T. Kane, D. C. Cochran, and M. S. Gilmore. 2002. Molecular mechanisms of Bacillus endophthalmitis pathogenesis. DNA Cell Biol. 21:367-373. [DOI] [PubMed] [Google Scholar]
- 13.Cheung, A. L., K. J. Eberhardt, E. Chung, M. R. Yeaman, P. M. Sullam, M. Ramos, and A. S. Bayer. 1994. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 94:1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan, C. L., W. M. Madden, G. F. Hatem, and J. C. Merritt. 1987. Endogenous Bacillus cereus panophthalmitis. Ann. Ophthalmol. 19:65-68. [PubMed] [Google Scholar]
- 15.Davey, R. T., and W. B. Tauber. 1987. Posttraumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev. Infect. Dis. 9:110-123. [DOI] [PubMed] [Google Scholar]
- 16.Giese, M. J., J. A. Berliner, A. Riesner, E. A. Wagarm, and B. J. Mondino. 1999. A comparison of the early inflammatory effects of an agr−/sar− versus a wild type strain of Staphylococcus aureus in a rat model of endophthalmitis. Curr. Eye Res. 18:177-185. [DOI] [PubMed] [Google Scholar]
- 17.Gillaspy, A. F., S. G. Hickmon, R. A. Skinner, J. R. Thomas, C. L. Nelson, and M. S. Smeltzer. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 63:3373-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilmore, M. S., M. C. Callegan, and B. D. Jett. 1999. Multicomponent cytolysins of Enterococcus faecalis and Bacillus cereus, p. 419-434. In J. E. Alouf and J. H. Freer (ed.), Comprehensive sourcebook: bacterial protein toxins. Academic Press, London, United Kingdom.
- 19.Heyer, G., S. Saba, R. Adamo, W. Rush, G. Soong, A. Cheung, and A. Prince. 2002. Staphylococcus aureus agr and sarA functions are required for invasive infection but not inflammatory responses in the lung. Infect. Immun. 70:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iijima, H., S. Yamaguchi, and O. Hosaka. 1993. Photopic electroretinogram implicit time in retinitis pigmentosa. Jpn. J. Ophthalmol. 37:130-135. [PubMed] [Google Scholar]
- 21.Jett, B. D., H. G. Jensen, R. E. Nordquist, and M. S. Gilmore. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 60:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 23.O'Callaghan, R. J., M. C. Callegan, J. M. Moreau, L. C. Green, T. J. Foster, O. M. Hartford, L. S. Engel, and J. M. Hill. 1997. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect. Immun. 65:1571-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Day, D. M., R. S. Smith, C. R. Gregg, P. C. B. Turnbull, W. S. Head, J. A. Ives, and P. C. Ho. 1981. The problem of Bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology 88:833-838. [DOI] [PubMed] [Google Scholar]
- 25.Salamitou, S., F. Ramisse, M. Brehelin, D. Bourguet, N. Gilois, M. Gominet, E. Hernandez, and D. Lereclus. 2000. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146:2825-2832. [DOI] [PubMed] [Google Scholar]
- 26.Satoh, S., H. Iijima, M. Imai, K. Abe, and T. Shibuya. 1994. Photopic electroretinogram implicit time in diabetic retinopathy. Jpn. J. Ophthalmol. 38:178-184. [PubMed] [Google Scholar]
- 27.Shamsuddin, D., C. U. Tuazon, C. Levy, and J. Curtin. 1982. Bacillus cereus panophthalmitis: source of the organism. Rev. Infect. Dis. 4:97-103. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan, D. C., and B. B. Hrapchak. 1987. Theory and practice of histotechnology. Batelle Press, Columbus, Ohio.
- 29.Slamti, L., and D. Lereclus. 2002. A cell-cell signaling peptide activates the plcR virulence regulon in bacteria of the Bacillus cereus group. EMBO J. 21:4550-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnbull, P. C. B., and J. M. Kramer. 1983. Non-gastrointestinal Bacillus cereus infections: an analysis of exotoxin production by strains isolated over a two-year period. J. Clin. Pathol. 36:1091-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]