Abstract
Shiga toxin-producing Escherichia coli (STEC) strains are responsible for causing hemolytic-uremic syndrome (HUS), and systemic administration of Shiga toxin (Stx)-specific human monoclonal antibodies (HuMAbs) is considered a promising approach for prevention or treatment of the disease in children. The goal of the present study was to investigate the ability of Stx2-specific HuMAbs to protect against infections with STEC strains that produce Stx2 variants. Dose-response studies on five HuMAbs, using the mouse toxicity model, revealed that only the three directed against the A subunit were protective against Stx2 variants, and 5C12 was the most effective among the three tested. Two HuMAbs directed against the B subunit, while highly effective against Stx2, were ineffective against Stx2 variants. In a streptomycin-treated mouse model, parenteral administration of 5C12 significantly protected mice up to 48 h after oral bacterial challenge. We conclude that 5C12, reactive against the Stx2 A subunit, is an excellent candidate for immunotherapy against HUS and that antibodies directed against the A subunit of Stx2 have broad-spectrum activity that includes Stx2 variants, compared with those directed against the B subunit.
Infection with Shiga toxin-producing Escherichia coli (STEC) strains is associated with hemolytic-uremic syndrome (HUS) (2, 24, 27), the leading cause of acute renal failure in young children (11). Shiga toxins (Stx) are cytotoxins and are major virulence factors of STEC. There are two immunologically distinct Stx types, known as Stx1 and Stx2, of which Stx1 is largely homogeneous, whereas the Stx2 group is highly heterogeneous and consists of at least 10 Stx2 gene variants (10, 14, 23, 31, 32, 37, 41, 42, 50). Stx2 is the most prevalent genotype identified in STEC isolated from patients with HUS (9, 40), and Stx2c is the most common Stx2 variant associated with HUS (9). Stx2 variants other than Stx2c are found frequently in asymptomatic STEC carriers but can often cause uncomplicated diarrhea (9) and rarely cause HUS (14, 33, 38, 45). Stx2f, identified in E. coli from pigeons, has been identified only once in humans, in a patient with diarrhea in Canada (10). In addition to pathogenicity to humans, Stx2 is more toxic to mice and piglets than Stx1. Stx2 is about 400 times more lethal to mice than Stx1 when administered systemically (44). STEC strains producing Stx2 alone cause more-severe neurologic symptoms in gnotobiotic piglets than STEC strains producing both Stx1 and Stx2, or Stx1 alone (8).
The nomenclature of Stx2 is confusing; Stx2vha and Stx2vhb (18), which are closely related to Stx2c (42), were originally identified as vtx2ha and vtx2hb (14). They were later shown to be activated by intestinal mucus (21) and named Stx2d (22). However, the Stx2d we refer to in the present study is the Stx2d cluster defined by Pierard et al. (37), which comprises Stx2d-OX3a (32), Stx2d-Ount (37), and Stx2d-O111 (33). An Stx molecule consists of a monomeric A subunit and a pentameric B subunit. Among STEC strains with different Stx2 variants, genetic differences in either the A or the B subunit or in both often confer antigenic and functional differences. The amino acid sequence identities of the A subunits of variants Stx2c (42), Stx2vha (14), Stx2vhb (14), Stx2d-OX3a (32), Stx2d-Ount (37), Stx2d-O111 (33), Stx2e (39), and Stx2f (47) with the A subunit of Stx2 are 100, 99, 99, 95, 93, 95, 94, and 71%, respectively. For the B subunit the amino acid sequence homologies are 96, 96, 96, 87, 88, 88, 87, and 82%, respectively.
The two current therapeutic approaches for HUS involve neutralization of Stx either in the gut or in the bloodstream. The two approaches attempted for Stx inactivation in the gut are (i) utilization of glycoconjugate polymers carrying Pk-trisaccharide sequences that serve as a receptor of Stx (1, 4, 5, 17) and (ii) use of recombinant bacteria displaying a Stx-specific glycolipid (globotriose or globotetraose) receptor (29, 30). We believe that systemic administration of Stx-specific neutralizing antibodies is currently the most promising approach for prevention or treatment of Stx-mediated systemic complications, including HUS (7) and edema disease in pigs (15). Murine Stx1- and Stx2-specific monoclonal antibodies (MAbs) have been shown to neutralize both toxins in vitro and in vivo (13, 28, 43). However, murine MAbs are not considered appropriate for human use. Reshaping of a murine antibody against Stx2 into a humanized form has recently been shown to completely protect mice against a lethal challenge with STEC when the antibody is administered within 24 h after infection (51). The disadvantage of a humanized antibody is that it still has mouse components and reduced affinity (12).
Mukherjee et al. have recently generated a panel of 50 human MAbs (HuMAbs) against Stx1 and Stx2 in transgenic mice (25, 26), from which we have selected a panel of 5 Stx2-specific HuMAbs that were shown to be highly protective for piglets, even when administered 12 h after an oral challenge with Stx2-producing STEC. In the present study, we used the mouse toxicity model (13, 25, 26, 28, 43) and the streptomycin-treated mouse model of STEC infection (22, 48, 49) to investigate the abilities of these five HuMAbs to protect against Stx2 variants.
MATERIALS AND METHODS
Bacteria.
Enterohemorrhagic E. coli (EHEC) O91:H21 strain B2F1, which produces Stx2 variants Stx2vha and Stx2vhb (14), was obtained from the American Type Culture Collection (ATCC 51435). A streptomycin-resistant clone of wild-type B2F1 was produced by serially passaging B2F1 on a Luria-Bertani (LB) broth agar plate containing 30 to 100 μg of streptomycin/ml. EHEC O157 strains E32511 (producing both Stx2c and Stx2) (42) and 93-8059 (producing Stx2 only) were obtained from Andrew MacKenzie (Child and Youth Clinical Trial Network, Ottawa, Canada).
Crude preparation of Stx.
A culture supernatant of B2F1 was used as a source of Stx2vha plus Stx2vhb (Stx2vha + Stx2vhb). A colony of wild-type B2F1 grown in 3 ml of LB broth for 7 h in a shaker at 37°C was transferred to a sterile flask containing LB broth at a dilution of 1/500 and incubated overnight in a shaker at 37°C. The culture was centrifuged at 1,750 × g for 30 min, and the supernatant was filter sterilized by passage through a 0.22-μm-pore-size filter. Similarly, a culture supernatant of E32511 was used as a source of Stx2c and Stx2, and a culture supernatant of 93-8059 was used as a source of Stx2.
Stx2-specific HuMAbs.
Production of 37 hybridomas secreting Stx2-specific HuMAbs has been described elsewhere (25). Three HuMAbs against the A subunit (3E9, 2F10, and 5C12) and two against both the A and B subunits (5H8 and 6G3) have been shown to be the most efficient at neutralizing Stx2 in vitro and in vivo (25). These were selected for the present study. All five HuMAbs were of the human immunoglobulin G1(κ) [IgG1(κ)] isotype. HuMAb-containing ascites fluid was prepared by injecting hybridoma cells into the peritoneal cavities of pristane (Sigma-Aldrich Co.)-primed ICR SCID mice (Taconic, Germantown, N.Y.).
Quantitation of Stx2-specific HuMAbs by ELISA.
The human IgG1(κ) concentration of each HuMAb in mouse ascites was measured by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated overnight at 4°C with 100 μl of the mouse MAb JDC-1 (IgG1 isotype) against human IgG1 (BD PharMingen) at 5 μg/ml. Plates were washed with phosphate-buffered saline-0.05% Tween 20 (PBS-T) and blocked with 100 μl of 2% nonfat dry milk powder in PBS-T/well at 37°C. After a wash, ascites samples diluted 1:100 in PBS-T were serially diluted twofold in duplicate rows of the plate (100 μl/well). A human IgG1(κ) (Sigma, St. Louis, Mo.) standard was similarly titrated on each plate from a starting concentration of 1 μg/ml. The plates were incubated at 37°C for 1 h and washed again. Horseradish peroxidase-conjugated goat anti-human IgG (Southern Biotech, Birmingham, Ala.), which was affinity purified and cross-adsorbed with human IgA, IgM, and IgD, was added at 100 μl/well at a dilution of 1/1,000. After incubation at 37°C for 1 h and a wash, plates were developed with a substrate solution (0.2% o-phenylenediamine-0.05% hydrogen peroxide in citric acid-phosphate buffer [pH 5.0]). The chromogenic reaction was stopped by using 50 μl of 2 M sulfuric acid, and absorbance was read at 490 nm. By using the linear portion of the IgG1(κ) standard curve, the total IgG1(κ) content of each HuMAb in ascites was determined and expressed as milligrams or micrograms of IgG1(κ) per milliliter of ascites fluid.
HeLa cell cytotoxicity neutralization assay.
An in vitro HeLa cell cytotoxicity assay was used to evaluate the ability of each HuMAb to neutralize the toxic effects of Stx2vha + Stx2vhb exerted against HeLa cells. Briefly, HeLa cells were plated at 1.4 × 104/well on 96-well plates in McCoy's 5A medium (Mediatech, Inc., Herndon, Va.) containing 10% fetal bovine serum (Harlan Bioproducts for Science, Inc., Madison, Wis.) and incubated overnight at 37°C under 5% CO2. A culture supernatant of B2F1 containing Stx2vha + Stx2vhb was titrated on HeLa cells to determine a dilution that killed ∼70% of HeLa cells. Dead cells were removed by a wash with PBS, and crystal violet was used to stain viable cells (16). A mixture of the culture supernatant at a dilution that killed ∼70% of HeLa cells and the HuMAb (5 μg/ml) or IgG1(κ) (5 μg/ml) as an isotype control (Sigma) was preincubated for 1 h at 37°C under 5% CO2, then added to the cells, and incubated overnight at 37°C under 5% CO2. A rabbit anti-Stx2 serum at a dilution of 1/400 was used as a positive control. The assay was similarly performed with a culture supernatant of EHEC O157 strain 93-8059 (a Stx2 producer), which served as another control. Plates were developed by crystal violet staining, and absorbance (optical density) was read at 690 nm. The percent neutralization of Stx2vha-, Stx2vhb-, and Stx2-mediated HeLa cell cytotoxicity by the HuMAb was then determined. Similarly, the HeLa cell cytotoxicity neutralization assay was performed utilizing culture supernatants of E32511 and 93-8059.
Mouse toxicity model.
The mouse toxicity model (13, 25, 26, 28, 43) was used to determine the most efficacious HuMAb for neutralizing the effects of Stx2vha + Stx2vhb in vivo. Dose-response studies were performed with groups of 10 3- to 4-week-old female Swiss Webster mice (Taconic) to determine the amount of Stx2vha + Stx2vhb in the B2F1 culture supernatant required to induce 100% mortality in untreated animals. A volume of 160 μl of the B2F1 culture supernatant was sufficient (data not shown). The efficacies of HuMAbs were evaluated by administering every Stx2-specific HuMAb intraperitoneally (i.p.) to each of 10 3- to 4-week-old Swiss Webster mice at a dose of 1.25, 2.5, 5, 10, or 20 μg/mouse in 200 μl of PBS, followed 18 h later by i.p. administration of 160 μl of the B2F1 culture supernatant. A control group of 10 mice received human myeloma IgG1(κ) (20 μg/mouse; Sigma), and another control group received 200 μl of PBS alone. Both control groups were also challenged with 160 μl of the B2F1 culture supernatant. Mice were observed twice daily for survival.
Streptomycin-treated mouse model of STEC infection.
A streptomycin-treated mouse model of STEC infection (22, 48, 49) was used to investigate the time-dependent efficacy of the most efficacious Stx2-specific HuMAb following infection with B2F1. Four-week-old DBA/2J mice were given drinking water containing 5 mg of streptomycin/ml for 24 h and were then denied food for 12 to 18 h. The mice received 1010 CFU of a streptomycin-resistant clone of B2F1 (0.1 ml) in 20% sucrose solution by oral administration. The animals were then permitted access to food and water containing 5 mg of streptomycin/ml ad libitum for the duration of the experiment (12 days). The efficacy of the most efficacious Stx2-specific HuMAb, 5C12, was tested following i.p. administration at a dose rate of 2.1 mg/kg of body weight following 0, 12, 24, 48, and 72 h of oral infection with 1010 CFU of B2F1. A group of 10 mice was used for each time point. A control group of 10 mice received human myeloma IgG1(κ) (30 μg/mouse injected i.p.; Sigma) at 0 h following infection with B2F1. Mice were observed three times per day for survival.
Immunoblotting.
In addition to in vitro and in vivo neutralization of Stx2vha + Stx2vhb by HuMAbs, the reactivity of each HuMAb with Stx2vha + Stx2vhb was determined by immunoblotting. Stx2, purified as described elsewhere (6), and a 55-fold-concentrated culture supernatant of B2F1 as a source of Stx2vha + Stx2vhb were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and electrophoretically transferred to a 0.2-μm-pore-size nitrocellulose membrane (Bio-Rad Laboratories, Richmond, Calif.). After transfer, the membrane was blocked with 5% nonfat dry milk powder in PBS-T at room temperature for 1 h, washed, and incubated with each HuMAb (2.5 μg/ml of PBS-T) at room temperature for 1 h. Human IgG1(κ) (Sigma) was used as a control. After a wash, strips were incubated with horseradish peroxidase-conjugated goat anti-human IgG (Southern Biotech) at a dilution of 1/1,000 for 1 h at room temperature and then washed and developed with the 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase-substrate system (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.).
Statistical analysis.
The grouped survival data were analyzed by the Mantel-Cox test and by using the PROC Freq procedure of SAS statistical software. Resulting P values of <0.05 were considered significant.
RESULTS
Reactivity in immunoblotting.
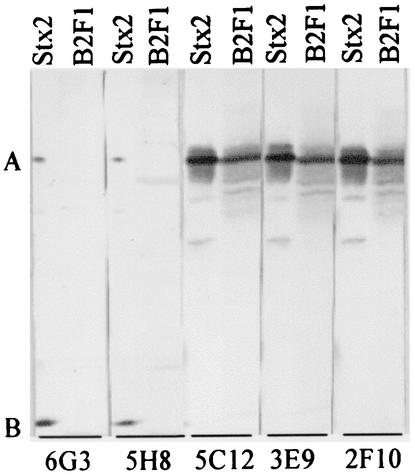
HuMAbs 5C12, 3E9, and 2F10 reacted with the A subunits of Stx2 and Stx2vha + Stx2vhb (Fig. 1). HuMAbs 6G3 and 5H8 reacted strongly with the B subunit and mildly with the A subunit of Stx2 but did not react with any of the subunits of Stx2vha + Stx2vhb.
FIG. 1.
Immunoblot reactivities of HuMAbs with Stx2 and Stx2 variants. Lanes Stx2 and lane B2F1 represent purified Stx2 and a concentrated culture supernatant of B2F1 containing Stx2 variants (Stx2vha and Stx2vhb), respectively. HuMAbs used to react with the Stx are given below the blot. Bands A and B represent the A and B subunits, respectively.
Neutralization of Stx2vha- and Stx2vhb-mediated HeLa cell cytotoxicity.
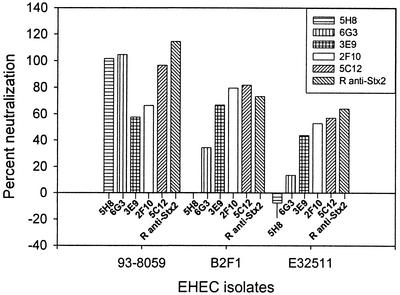
Each of the Stx2-specific HuMAbs was effective at neutralizing the activity of Stx2 present in the culture supernatant of 93-8059; however, differences in relative potency were observed (5H8 and 6G3 showed the highest potency, followed, in descending order, by 5C12, 2F10, and 3E9) (Fig. 2). Similarly, consistent relative differences were observed among the Stx2 A-subunit-specific HuMAbs with respect to neutralization of Stx2vha + Stx2vhb present in the culture supernatant of B2F1 and neutralization of Stx2c present in the culture supernatant of E32511 (5C12 and 2F10 showed approximately equal potencies, while that of 3E9 was lower). In contrast, the B-subunit-specific HuMAb 5H8 did not neutralize Stx2vha + Stx2vhb or Stx2c, and 6G3 neutralized them at very low levels. Although 5C12 neutralized Stx2 completely, residual cytotoxicity was observed with Stx2vha + Stx2vhb and Stx2c; this might have been due to the presence of toxic factors other than Stx2 variants in the culture supernatants of B2F1 and E32511, since the rabbit anti-Stx2 serum also neutralized Stx2 completely but did not completely neutralize the Stx2 variants.
FIG. 2.
Neutralization of HeLa cell cytotoxicity mediated by Stx2 (produced by 93-8059), Stx2vha + Stx2vhb (produced by B2F1), and Stx2 plus Stx2c (produced by E32511) by Stx2-specific HuMAbs. The B-subunit-specific HuMAbs 5H8 and 6G3 neutralized Stx2 completely. However, 5H8 did not neutralize Stx2vha, Stx2vhb, or Stx2c, and 6G3 neutralized them mildly. The A-subunit-specific HuMAb 5C12 and rabbit anti-Stx2 serum (R anti-Stx2) strongly neutralized all Stx types. The other A-subunit-specific HuMAbs, 2F10 and 3E9, were also very effective.
Neutralization of Stx2vha + Stx2vhb in vivo.
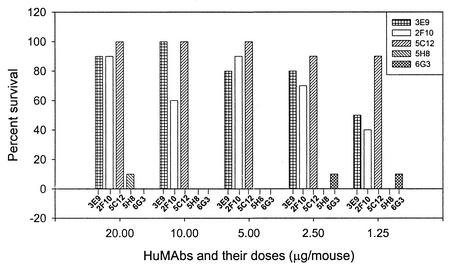
Considering the identical in vitro neutralization patterns of the Stx2-specific HuMAbs against Stx2c and Stx2vha + Stx2vhb (Fig. 2), further studies to examine the relative potency of each HuMAb in vivo were performed only against Stx2vha + Stx2vhb, by utilizing the mouse toxicity model. At each dose, Stx2 A-subunit-specific HuMAbs 5C12, 2F10, and 3E9 significantly protected mice, as evidenced by comparison with the PBS control (average survival, 2.30 ± 0.35 days) and the HuMAb IgG1(κ) control (average survival, 2.35 ± 0.34 days) (P < 0.0001) (Fig. 3). In contrast, Stx2 B-subunit-specific HuMAbs 5H8 and 6G3 did not protect mice significantly at any dose level. HuMAbs 2F10 and 3E9 exhibited very similar dose-dependent effects on relative average survival; they did not differ significantly from each other at any dose level except 10 μg/mouse (P < 0.0001). In contrast, 5C12 did not show dose dependency; it protected 90% of the mice even at the lowest dose administered (1.25 μg/mouse). 5C12 provided better protection than 2F10 and 3E9, differing significantly from them at all dose levels except for 3E9 at doses of 10 and 2.5 μg/mouse. At the lowest dose (1.25 μg/mouse) tested, 5C12 was far superior to 2F10 and 3E9 (P < 0.0001).
FIG. 3.
Percent survival of mice given 20, 10, 5, 2.5, or 1.25 μg of HuMAb 3E9, 2F10, 5C12, 5H8, or 6G3 i.p., followed 18 h later with a 100% lethal dose of the Stx2vha + Stx2vhb-containing culture supernatant of EHEC isolate B2F1, also given i.p. Stx2 A-subunit-specific HuMAbs 5C12, 2F10, and 3E9 significantly protected mice relative to the PBS control (average survival, 2.30 ± 0.35 days) and the HuMAb IgG1(κ) control (average survival, 2.35 ± 0.34 days) (P < 0.0001). HuMAbs 5H8 and 6G3 did not protect mice. HuMAbs 2F10 and 3E9 exhibited very similar dose-dependent effects on relative average survival; they did not differ significantly from each other at any dose level except 10 μg/mouse (P < 0.0001). In contrast, 5C12 did not show dose dependency; it protected 90% of the mice even at the lowest dose administered (1.25 μg/mouse). At the lowest dose (1.25 μg/mouse) tested, 5C12 was far superior to 2F10 and 3E9 (P < 0.0001).
Time-dependent efficacy of 5C12 in B2F1-infected mice.
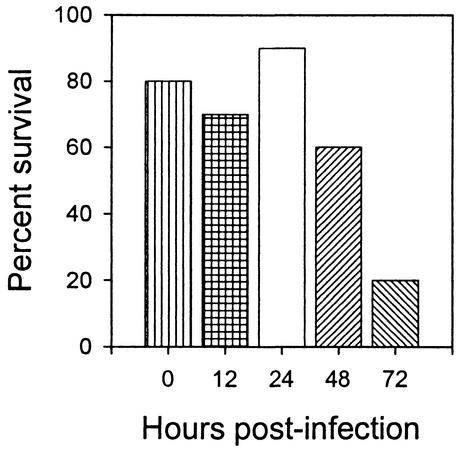
To test for the time-dependent efficacy of 5C12, mice were orally infected with 1010 CFU of B2F1, and HuMAb 5C12 was administered at 0 to 72 h after infection (Fig. 4). All control mice infected and treated i.p. at the same time with control human IgG1(κ) died, with an average survival time of 6 days. In contrast, 5C12 administered 0, 12, 24, or 48 h following infection protected 80% (P = 0.0001), 70% (P = 0.0002), 90% (P < 0.0001), or 60% (P = 0.001) of the mice, respectively (Fig. 4). However, 5C12 administered 72 h following infection protected only 20% of the mice, which was not a significant effect.
FIG. 4.
Percent survival of mice orally infected with Stx2vha + Stx2vhb-producing B2F1 and given 5C12 i.p. at a dose of 2.1 mg/kg of body weight at various times postinfection. All control mice infected and treated i.p. at the same time with control human IgG1(κ) (2.1 mg/kg of body weight) died, with an average survival time of 6 days. In contrast, 5C12 administered 0, 12, 24, or 48 h following infection protected 80% (P = 0.0001), 70% (P = 0.0002), 90% (P < 0.0001), or 60% (P = 0.001) of the mice, respectively. However, 5C12 administered 72 h following infection protected only 20% of the mice, which was not significant.
DISCUSSION
The main goals of the present study were (i) to identify the most effective Stx2-specific HuMAb by using the mouse toxicity model and (ii) to determine the protective ability of the selected HuMAb against Stx2c, the most prevalent Stx2 variant associated with HUS (9), by using the streptomycin-treated mouse model of oral STEC infection (22, 48, 49). Like others (18), we were unsuccessful in adapting the streptomycin-treated mouse model for strain E32511, which produces both Stx2 and Stx2c (data not shown). Consequently, we have used strain B2F1, described by other investigators (22, 48, 49, 51), which expresses both Stx2vha and Stx2vhb (14). B2F1 was ideal because the B subunits of Stx2c, Stx2vha, and Stx2vhb are identical and differ from the B subunit of Stx2 by 2 amino acids (14, 42). In addition, unlike the A subunit of Stx2c, which is identical to that of Stx2, the A subunits of Stx2vha and Stx2vhb differ by 3 amino acids from that of Stx2. Therefore, testing Stx2vha and Stx2vhb against our Stx2 A- and B-subunit-specific HuMAbs determined not only the influence of amino acid differences in the B subunit, but also that for the A subunit, on the relative protective abilities of the HuMAbs. Moreover, STEC strains producing Stx2vha + Stx2vhb have also been associated with HUS (14).
We first evaluated the neutralizing abilities of the five selected HuMAbs against Stx2 variants (Stx2c and Stx2vha + Stx2vhb) in vitro by HeLa cell cytotoxicity neutralization assay, followed by dose-response studies in the mouse toxicity model (13, 25, 26, 28, 43). Of the five HuMAbs tested (three A-subunit and two B-subunit specific), 5C12 was the most effective, and therefore it was selected for further evaluation in the streptomycin-treated mouse model of infection (22, 48, 49). 5C12 was administered at various time points after bacterial challenge, since treatment of patients with STEC infection is expected to occur after exposure to infection, at the onset of bloody diarrhea. Studies with piglets have already shown that these HuMAbs are protective even when given after an oral bacterial challenge with Stx2-producing STEC (25). This is the first report, however, which shows that administration of a specific HuMAb against Stx2 (5C12 at 2.1 mg/kg) can significantly protect mice when given as long as 48 h after bacterial challenge. In contrast, a study using the same mouse model and strain B2F1 has shown that the Stx2-specific humanized MAb TMA-15, given at a dose of 1.0 mg/kg, protects mice when given as long as 24 h after bacterial challenge (51). It is possible that the differences in length of protection afforded by 5C12 and TMA-15 are due to differences in their respective affinities. Although concentration of Stx2 variants in the blood were not determined in the present study, Yamagami et al. (51) have reported that serum Stx2 variant levels are highest in mice at 48 h after STEC infection (51). This suggests that 5C12 can significantly protect mice even when the maximum levels of Stx2 variants are present in the bloodstream. The time window of 48 h for immunotherapeutic intervention has direct implications for children at risk of developing HUS (e.g., those presenting with bloody diarrhea or excreting STEC) and for individuals in contact with them. The development of rapid and sensitive diagnostic methods has made it possible to detect STEC infections almost a week before symptoms of HUS become apparent (34, 35).
The three amino acid differences between the A subunits of Stx2vha and Stx2vhb, on the one hand, and the A subunit of Stx2, on the other (14), did not significantly affect the binding of any of the Stx2 A-subunit-specific HuMAbs; all of them neutralized Stx2vha + Stx2vhb both in vitro and in vivo. However, the Stx2 B-subunit-specific HuMAbs (5H8 and 6G3) failed to neutralize Stx2vha + Stx2vhb, suggesting that one or both of the amino acid changes in the B subunits of Stx2vha and Stx2vhb considerably affected the neutralizing capabilities of 5H8 and 6G3. Since the B subunits of Stx2vha and Stx2vhb are identical to the Stx2c B subunit, 5H8 and 6G3 also failed to neutralize Stx2c in vitro. However, Stx2 A-subunit-specific HuMAbs neutralized Stx2c in vitro, because Stx2 and Stx2c have identical A subunits. The failure of 6G3 and 5H8 to neutralize Stx2c in vitro and their stronger immunoblot-reactivity with the B subunit than with the A subunit of Stx2 unequivocally show that the neutralization activities of these two HuMAbs are due to their binding with the B subunit and not the A subunit.
Given that STEC can produce any combination of Stx1, Stx2, and/or Stx2c (9), an ideal therapeutic formulation should, in our view, include HuMAbs specific for Stx1, Stx2, and Stx2c. Mukherjee et al. have recently reported production of protective Stx1-specific HuMAbs (26) for inclusion in such a formulation. Since it appears from this study that A-subunit-specific Stx2 antibodies display inhibitory activity against Stx2c as well, the selection of 5C12 combined with an effective Stx1-specific HuMAb, described in an earlier study (25), could provide broad-spectrum protection against Stx1, Stx2, and Stx2c. However, the efficacy of 5C12 needs to be further investigated in the orally infected piglet model, since piglets are the only species in addition to humans that are naturally susceptible to the systemic effects of Stx produced by E. coli strains that proliferate in the gastrointestinal tract (19, 20), with characteristic attachment-and-effacement lesions (36, 46), which are absent in the mouse (18). The mouse model is also less susceptible to Stx, as judged by the amount of toxin required to cause death compared to that for the piglet (3, 26) and presumably for children. The mouse infection model, however, is useful for screening and evaluation, because it is genetically uniform, available in large numbers, easy to manipulate, requires smaller amounts of reagents, and is less expensive and less labor-intensive. The piglet model, on the other hand, is more appropriate for preclinical evaluation of formulations and for validation, including determination of the likely effective therapeutic dose for humans.
We conclude that 5C12, which is reactive against the Stx2 A subunit, is an excellent candidate for immunotherapy against HUS and that antibodies directed against the A subunit of Stx2, as opposed to those directed against the B subunit, have broad-spectrum activity that includes Stx2 variants.
Acknowledgments
This study was supported by NIH Public Health Service grants RO1-AI41326 and P30-DK-34928.
We thank Jennifer Martineau for technical assistance.
Editor: J. D. Clements
REFERENCES
- 1.Armstrong, G. D., P. C. Rowe, P. Goodyer, E. Orrbine, T. P. Klassen, G. Wells, A. MacKenzie, H. Lior, C. Blanchard, F. Auclair, et al. 1995. A phase I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J. Infect. Dis. 171:1042-1045. [DOI] [PubMed] [Google Scholar]
- 2.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd, B., G. Tyrrell, M. Maloney, C. Gyles, J. Brunton, and C. Lingwood. 1993. Alteration of the glycolipid binding specificity of the pig edema toxin from globotetraosyl to globotriaosyl ceramide alters in vivo tissue targetting and results in a verotoxin 1-like disease in pigs. J. Exp. Med. 177:1745-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dohi, H., Y. Nishida, Y. Furuta, H. Uzawa, S. Yokoyama, S. Ito, H. Mori, and K. Kobayashi. 2002. Molecular design and biological potential of galacto-type trehalose as a nonnatural ligand of Shiga toxins. Org. Lett. 4:355-357. [DOI] [PubMed] [Google Scholar]
- 5.Dohi, H., Y. Nishida, M. Mizuno, M. Shinkai, T. Kobayashi, T. Takeda, H. Uzawa, and K. Kobayashi. 1999. Synthesis of an artificial glycoconjugate polymer carrying Pk-antigenic trisaccharide and its potent neutralization activity against Shiga-like toxin. Bioorg. Med. Chem. 7:2053-2062. [DOI] [PubMed] [Google Scholar]
- 6.Donohue-Rolfe, A., D. W. Acheson, A. V. Kane, and G. T. Keusch. 1989. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect. Immun. 57:3888-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donohue-Rolfe, A., I. Kondova, J. Mukherjee, K. Chios, D. Hutto, and S. Tzipori. 1999. Antibody-based protection of gnotobiotic piglets infected with Escherichia coli O157:H7 against systemic complications associated with Shiga toxin 2. Infect. Immun. 67:3645-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donohue-Rolfe, A., I. Kondova, S. Oswald, D. Hutto, and S. Tzipori. 2000. Escherichia coli 0157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. J. Infect. Dis. 181:1825-1829. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich, A. W., M. Bielaszewska, W. L. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 10.Gannon, V. P., C. Teerling, S. A. Masri, and C. L. Gyles. 1990. Molecular cloning and nucleotide sequence of another variant of the Escherichia coli Shiga-like toxin II family. J. Gen. Microbiol. 136:1125-1135. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 12.Halloran, P. F., and S. Prommool. 1998. Humanized monoclonals and other biological initiatives. Clin. Biochem. 31:353-357. [DOI] [PubMed] [Google Scholar]
- 13.Islam, M. S., and W. H. Stimson. 1990. Production and characterization of monoclonal antibodies with therapeutic potential against Shiga toxin. J. Clin. Lab. Immunol. 33:11-16. [PubMed] [Google Scholar]
- 14.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 15.Johansen, M., L. O. Andresen, L. K. Thomsen, M. E. Busch, H. Wachmann, S. E. Jorsal, and C. L. Gyles. 2000. Prevention of edema disease in pigs by passive immunization. Can. J. Vet. Res. 64:9-14. [PMC free article] [PubMed] [Google Scholar]
- 16.Keusch, G. T., A. Donohue-Rolfe, M. Jacewicz, and A. V. Kane. 1988. Shiga toxin: production and purification. Methods Enzymol. 165:152-162. [DOI] [PubMed] [Google Scholar]
- 17.Kitov, P. I., J. M. Sadowska, G. Mulvey, G. D. Armstrong, H. Ling, N. S. Pannu, R. J. Read, and D. R. Bundle. 2000. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 403:669-672. [DOI] [PubMed] [Google Scholar]
- 18.Lindgren, S. W., A. R. Melton, and A. D. O'Brien. 1993. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect. Immun. 61:3832-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLeod, D. L., C. L. Gyles, and B. P. Wilcock. 1991. Reproduction of edema disease of swine with purified Shiga-like toxin-II variant. Vet. Pathol. 28:66-73. [DOI] [PubMed] [Google Scholar]
- 20.Marques, L. R. M., J. S. M. Peiris, S. J. Cryz, and A. D. O'Brien. 1987. Escherichia coli strains isolated from pigs with edema disease produce a variant of Shiga-like toxin II. FEMS Microbiol. Lett. 44:281-283. [Google Scholar]
- 21.Melton-Celsa, A. R., S. C. Darnell, and A. D. O'Brien. 1996. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect. Immun. 64:1569-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melton-Celsa, A. R., J. E. Rogers, C. K. Schmitt, S. C. Darnell, and A. D. O'Brien. 1998. Virulence of Shiga toxin-producing Escherichia coli (STEC) in orally-infected mice correlates with the type of toxin produced by the infecting strain. Jpn. J. Med. Sci. Biol. 51:S108-S114. [DOI] [PubMed] [Google Scholar]
- 23.Meyer, T., H. Karch, J. Hacker, H. Bocklage, and J. Heesemann. 1992. Cloning and sequencing of a Shiga-like toxin II-related gene from Escherichia coli O157:H7 strain 7279. Zentbl. Bakteriol. 276:176-188. [DOI] [PubMed] [Google Scholar]
- 24.Milford, D. V., C. M. Taylor, B. Guttridge, S. M. Hall, B. Rowe, and H. Kleanthous. 1990. Haemolytic uraemic syndromes in the British Isles 1985-8: association with verocytotoxin producing Escherichia coli. Part 1. Clinical and epidemiological aspects. Arch. Dis. Child. 65:716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee, J., K. Chios, D. M. Fishwild, D. Hudson, S. L. O'Donnell, S. Rich, A. Donohue-Rolfe, and S. Tzipori. 2002. Human Stx2-specific monoclonal antibodies prevent systemic complications of Escherichia coli O157:H7 infection. Infect. Immun. 70:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee, J., K. Chios, D. M. Fishwild, D. Hudson, S. L. O'Donnell, S. Rich, A. Donohue-Rolfe, and S. Tzipori. 2002. Production and characterization of protective human antibodies against Shiga toxin 1 (Stx1). Infect. Immun. 70:5896-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostroff, S. M., P. I. Tarr, M. A. Neill, J. H. Lewis, N. Hargrett-Bean, and J. M. Kobayashi. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 160:994-998. [DOI] [PubMed] [Google Scholar]
- 28.Padhye, V. V., T. Zhao, and M. P. Doyle. 1989. Production and characterisation of monoclonal antibodies to Verotoxins 1 and 2 from Escherichia coli of serotype O157:H7. J. Med. Microbiol. 30:219-226. [DOI] [PubMed] [Google Scholar]
- 29.Paton, A. W., R. Morona, and J. C. Paton. 2001. Neutralization of Shiga toxins Stx1, Stx2c, and Stx2e by recombinant bacteria expressing mimics of globotriose and globotetraose. Infect. Immun. 69:1967-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton, A. W., R. Morona, and J. C. Paton. 2000. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat. Med. 6:265-270. [DOI] [PubMed] [Google Scholar]
- 31.Paton, A. W., J. C. Paton, P. N. Goldwater, M. W. Heuzenroeder, and P. A. Manning. 1993. Sequence of a variant Shiga-like toxin type-I operon of Escherichia coli O111:H. Gene 129:87-92. [DOI] [PubMed] [Google Scholar]
- 32.Paton, A. W., J. C. Paton, M. W. Heuzenroeder, P. N. Goldwater, and P. A. Manning. 1992. Cloning and nucleotide sequence of a variant Shiga-like toxin II gene from Escherichia coli OX3:H21 isolated from a case of sudden infant death syndrome. Microb. Pathog. 13:225-236. [DOI] [PubMed] [Google Scholar]
- 33.Paton, A. W., J. C. Paton, and P. A. Manning. 1993. Polymerase chain reaction amplification, cloning and sequencing of variant Escherichia coli Shiga-like toxin type II operons. Microb. Pathog. 15:77-82. [DOI] [PubMed] [Google Scholar]
- 34.Paton, A. W., R. M. Ratcliff, R. M. Doyle, J. Seymour-Murray, D. Davos, J. A. Lanser, and J. C. Paton. 1996. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J. Clin. Microbiol. 34:1622-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paton, A. W., M. C. Woodrow, R. M. Doyle, J. A. Lanser, and J. C. Paton. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips, A. D., S. Navabpour, S. Hicks, G. Dougan, T. Wallis, and G. Frankel. 2000. Enterohaemorrhagic Escherichia coli O157:H7 target Peyer's patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut 47:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierard, D., G. Muyldermans, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramachandran, V., M. A. Hornitzky, K. A. Bettelheim, M. J. Walker, and S. P. Djordjevic. 2001. The common ovine Shiga toxin 2-containing Escherichia coli serotypes and human isolates of the same serotypes possess a Stx2d toxin type. J. Clin. Microbiol. 39:1932-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reischl, U., M. T. Youssef, J. Kilwinski, N. Lehn, W. L. Zhang, H. Karch, and N. A. Strockbine. 2002. Real-time fluorescence PCR assays for detection and characterization of Shiga toxin, intimin, and enterohemolysin genes from Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 40:2555-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russmann, H., H. Schmidt, J. Heesemann, A. Caprioli, and H. Karch. 1994. Variants of Shiga-like toxin II constitute a major toxin component in Escherichia coli O157 strains from patients with haemolytic uraemic syndrome. J. Med. Microbiol. 40:338-343. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, H., J. Scheef, S. Morabito, A. Caprioli, L. H. Wieler, and H. Karch. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt, C. K., M. L. McKee, and A. D. O'Brien. 1991. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect. Immun. 59:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strockbine, N. A., L. R. Marques, R. K. Holmes, and A. D. O'Brien. 1985. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect. Immun. 50:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, A., T. Cheasty, H. Chart, and B. Rowe. 1994. Isolation of Vero cytotoxin-producing Escherichia coli serotypes O9ab:H− and O101:H− carrying VT2 variant gene sequences from a patient with haemolytic uraemic syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 13:1074-1076. [DOI] [PubMed] [Google Scholar]
- 46.Tzipori, S., R. Gibson, and J. Montanaro. 1989. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect. Immun. 57:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unkmeir, A., and H. Schmidt. 2000. Structural analysis of phage-borne stx genes and their flanking sequences in Shiga toxin-producing Escherichia coli and Shigella dysenteriae type 1 strains. Infect. Immun. 68:4856-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadolkowski, E. A., J. A. Burris, and A. D. O'Brien. 1990. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 58:2438-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wadolkowski, E. A., L. M. Sung, J. A. Burris, J. E. Samuel, and A. D. O'Brien. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 58:3959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinstein, D. L., M. P. Jackson, J. E. Samuel, R. K. Holmes, and A. D. O'Brien. 1988. Cloning and sequencing of a Shiga-like toxin type II variant from an Escherichia coli strain responsible for edema disease of swine. J. Bacteriol. 170:4223-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamagami, S., M. Motoki, T. Kimura, H. Izumi, T. Takeda, Y. Katsuura, and Y. Matsumoto. 2001. Efficacy of postinfection treatment with anti-Shiga toxin (Stx) 2 humanized monoclonal antibody TMA-15 in mice lethally challenged with Stx-producing Escherichia coli. J. Infect. Dis. 184:738-742. [DOI] [PubMed] [Google Scholar]