Abstract
A series of overlapping recombinant antigens, 61 to 74 residues in length, representing polymorphic outer membrane protein 90 (POMP90) of Chlamydophila abortus and two recombinant peptides spanning gene fragment p91Bf99 of POMP91B were assessed by immunoblotting to determine the antigen-binding sites of 20 monoclonal antibodies to POMP90, -91A, and -91B. The epitopes were further restricted by scanning 52 overlapping synthetic 12-mer peptides representing the N-terminal part of POMP90, and the 12-mer epitopes were then analyzed by using hexapeptides to the resolution of a single amino acid. Ten epitopes were defined: 1, TSEEFQVKETSSGT; 2, SGAIYTCEGNVCISYAGKDSPL; 3, SLVFHKNCSTAE; 4, AIYADKLTIVSGGPTLFS; 5, SPKGGAISIKDS; 6, ITFDGNKIIKTS; 7, LRAKDGFGIFFY; 7a, DGFGIF; 7b, GIFFYD; 8, IFFYDPITGGGS; 8a, FFYDPIT; 9, GKIVFSGE; and 10, DLGTTL. The 20-mer peptide LRAKDGFGIFFYDPITGGGS was a major epitope that was recognized by seven antibodies. Epitopes 7 to 10 were conserved in reference strains of the former species C. psittaci, whereas the strong antigenic peptides FYDPIT and IVFSGE were conserved among members of the genus Chlamydophila. Epitopes 3 to 8 were located within the best-scoring beta-helical wrap (residues 148 to 293) predicted for POMP91B by the program BETAWRAP. Other studies have suggested an association of the POMPs with type V secretory autotransporter proteins. The results presented in this study provide some evidence for a passenger domain that is folded as a beta-helix pyramid with compact antigenic organization.
Chlamydiae are obligate intracellular gram-negative bacterial pathogens that are responsible for a wide variety of important human and animal infections. They have a unique developmental cycle that takes place within a vacuole, named the inclusion, and alternates between the rigid, infectious elementary body (EB) and the metabolically active, noninfectious reticulate body (RB) (24). After a recent reclassification of the order Chlamydiales, the family Chlamydiaceae contains two genera, Chlamydia and Chlamydophila (5). The genus Chlamydia comprises three species, Chlamydia trachomatis, C. muridarum, and C. suis. The new genus Chlamydophila contains the species Chlamydophila psittaci, C. abortus, C. caviae, C. felis, C. pecorum, and C. pneumoniae. The most economically important animal pathogen of small ruminants is C. abortus (formerly C. psittaci serotype 1), which causes abortion late in gestation because of placental infection (ovine enzootic abortion). Small ruminants are also commonly infected with C. pecorum, which is responsible for a variety of conditions, including conjunctivitis, polyarthritis, pneumonia, and clinically inapparent intestinal infections (25). The diagnosis of animals infected with C. abortus must therefore differentiate between the two Chlamydophila species. In this context, our interest has been drawn to a particular 90-kDa antigen family that was originally identified in animal sera as highly immunoreactive and specific for C. abortus (3, 4, 21). A multigene family of four genes coding for three proteins, named at first putative outer membrane proteins (POMPs), was identified and isolated (12, 13). With the completion of the sequencing of the genomes of C. trachomatis and C. pneumoniae, it became apparent that these genes were the first identified members of a larger protein superfamily, referred to as the polymorphic membrane protein (Pmp) family. The features common to all members of the family are the conserved motifs FXXN and GGAI, which are repeated in the N-terminal half of the proteins. Nine genes, termed pmpA to pmpI, are present in C. trachomatis, and 21 genes (pmp-1 to pmp-21) are found in the larger genome of C. pneumoniae (11, 23). This relatively high number of genes in the small genomes of both Chlamydia and Chlamydophila has suggested a critical role in bacterial growth and development (8). Henderson and Lam (10) highlighted the similarities of the POMP and Pmp proteins with autotransporter proteins of the type V secretion system. In autotransporters, the presumed integral membrane beta-barrel domain located at the C terminus mediates the translocation of the N-terminal variable passenger domain, which may be cleaved once it is exported. Further supportive evidence of the similarity of the Pmp proteins to autotransporters follows from the proteomic analysis of C. trachomatis and C. pneumoniae Pmp proteins showing that some of the proteins migrate at molecular weights significantly lower than those predicted by their gene sequences, which is consistent with the proteins being cleaved (8, 17, 20, 27).
Current knowledge concerning the number and sizes of the POMPs in the animal pathogens of the genus Chlamydophila is incomplete, since most of their genomes have yet to be sequenced. To date, 20 orthologous genes have been identified in C. caviae (T. Read, P. Bavoil, et al., unpublished data), while preliminary analysis of the completed C. abortus genome has identified at least 18 genes (J. Parkhill, D. Longbottom, et al., unpublished data). At least six POMPs were identified in C. psittaci strain 6BC by labeling with an outer membrane-specific probe, 3-(trifluoromethyl)-3-m-[125I]iodophenyl)diazirine (26).
Three of the originally identified POMPs, POMP90, -91A, and -91B (13), share >80% homology and migrate very closely in conventional sodium dodecyl sulfate (SDS) gels but can be separated by two-dimensional (2D) electrophoresis (7). Probing of 2D immunoblots with specific monoclonal antibodies (MAbs) has identified a fourth protein, with a molecular mass of 105 kDa, that was characterized by antigenic cross-reactivity as being POMP related (7). This protein was readily fragmented by trypsin, in contrast to the other POMPs, following protease treatment of whole EBs (30). Specific binding of the lectin concanavalin A suggested that the four proteins bear carbohydrate (30). Discrepant results have been reported concerning the localization of the POMPs on the outer membrane surface of C. abortus by immunoelectron microscopy. MAbs were reported to be able to highly decorate RBs but not EBs (2), contrary to a second study that showed similar immunolabeling on both RBs and EBs (14). Since these studies used different MAbs, it was suggested that the antigenic sites recognized by the respective MAbs may differ in their location in the primary amino acid sequence. This prompted us to identify the antigenic sites of the MAbs in question by use of recombinant fragments and synthetic peptides. In this paper, we present a detailed antigenic map of the amino-terminal part of the POMPs.
MATERIALS AND METHODS
Cell culture.
McCoy cells (American Type Culture Collection [ATCC], Manassas, Va.) were grown in Eagle's minimal essential medium supplemented with 5% (vol/vol) fetal calf serum, 2 mM glutamine, 2 mM nonessential amino acids, 0.01% gentamicin (Garamycin), and 0.02% vancomycin. HeLa cells (ATCC) were grown in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal calf serum, 2 mM glutamine, 2 mM nonessential amino acids, and 2 μg of gentamicin ml−1. Both cell lines were monitored regularly for mycoplasma contamination with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; Boehringer Mannheim).
Chlamydial strains.
C. abortus B577T (VR656), C. psittaci 6BCT (VR125) and MNT (VR122), C. caviae GPICT (VR813), C. felis FePnT (VR120), C. pneumoniae TW-183T (VR2282), and C. trachomatis L2/434 (VR920B) and D/UW-3 (VR885) were all purchased from ATCC. C. pecorum strain T13 was a kind gift from M. J. Clarkson (University of Liverpool, Liverpool, United Kingdom). All chlamydial strains were grown in McCoy cell monolayers, except C. pneumoniae, which was propagated in HeLa cells. Cells were infected with chlamydiae by centrifugation at 1,000 × g for 1 h in phosphate buffer supplemented with sucrose and glutamate (0.2 M sucrose, 5 mM glutamate, 0.02 M phosphate, pH 7.2). The cultures were incubated for 48 h in the presence of cycloheximide (1 μg ml−1) and for 24 h without cycloheximide. C. trachomatis and C. caviae GPICT were grown for 48 h. Crude stocks of EBs were prepared as described previously (29).
MAbs.
Table 1 lists the 20 anti-POMP MAbs under study and their isotypes and details their origins and previous characterizations. Seventeen of the MAbs have been described previously (7, 21, 29). MAb LB4B4 was kindly provided by A. Rodolakis (Institut National de la Recherche Agronomique, Nouzilly, France). MAbs G11 and H11 were generated in BALB/c mice by intrasplenic deposition of nitrocellulose-bound antigen after 2D electrophoretic separation and immunoblotting. Mice received an intraperitoneal boost of dissolved excised nitrocellulose pieces emulsified in incomplete Freund's adjuvant and a final intraperitoneal boost of 40 μg of purified EBs 4 days before fusion. Spleen cells were fused with NS-O cells in polyethylene glycol, and hybridomas were grown by using standard protocols in a Mini-perm apparatus (Heraeus, Osterode, Germany). The isotypes were determined with an ELISA kit (Pierce).
TABLE 1.
List of anti-POMP MAbs under study
| Immunogen and MAb | Isotypea | Origin or reference |
|---|---|---|
| C. abortus | ||
| JA6C7 | IgG2a | 2, 21, 30 |
| EB3G2 | IgG2a | 2, 21, 30 |
| IC4C7 | IgG2a | 2, 21 |
| 181 | IgG2b | 13, 14, 29, 30 |
| 191 | IgG3 | 13, 29 |
| 192 | IgG2a | 13, 29 |
| G11 | IgG2a | This study |
| H11 | IgG1 | This study |
| FC1A2 | IgG2a | 2, 21 |
| DA4E8 | IgG2a | 2, 21 |
| LB4B4 | IgG2a | This study |
| C. psittaci | ||
| 73/04 | IgG1 | 13, 29 |
| 84/019 | IgG2b | 13, 29 |
| 73/0200 | IgG2b | 13, 29 |
| 73/0040 | IgG1 | 13, 14, 29 |
| 73/0248 | IgG2a | 13, 29 |
| 73/053 | NC | 13, 29 |
| 76/073 | IgG2b | 7, 13, 29, 30 |
| 76/049 | IgG2b | 13, 29 |
| 76/030 | NC | 13, 29 |
IgG, immunoglobulin G; NC, nonconclusive results.
Animal sera.
Sera were pooled from six sheep flocks that were diagnosed with chlamydial abortion. Anti-C. pecorum sera were raised in specific-pathogen-free (SPF) lambs, two of which were experimentally infected with arthritogenic C. pecorum strain P787, two were experimentally infected with conjunctivitis-causing strain 84/796, and one was experimentally infected with enteric strain 84/604 (19). Affinity-purified antibodies against the 105-kDa POMP were prepared as previously described (7).
Immunoperoxidase assay.
McCoy or HeLa cells grown in 96-well tissue culture plates were infected by centrifugation with each one of the chlamydial strains. The cell monolayers, 30 to 50% infected, were fixed with methanol and incubated for 1 h at 37°C with serial dilutions of the MAbs in phosphate-buffered saline (0.14 M NaCl, 26 mM KCl, 1.4 mM KH2PO4, 8 mM Na2HPO4) containing 0.05% Tween 20. Incubation with a peroxidase-conjugated second antibody and visualization with 3,3′-diaminobenzidine (DAB) followed, as described previously (29).
Production of recombinant fragments and immunoblotting.
Amplification of the truncated p91Bf99 gene fragment (p91Bf99s), cloning, expression, and purification of the glutathione S-transferase (GST) fusion protein have been described previously in detail (15). Primers and plasmid construction of the 12 200- to 300-bp overlapping fragments representing the entire gene for POMP90, as well as cloning into pGEX-4T-1 and expression as GST fusion proteins, have been published recently (16). GST was also expressed from the empty vector (i.e., no insert) as a control. Protein concentrations were determined with the bicinchoninic acid protein assay reagent (Perbio Science United Kingdom Ltd.) with bovine serum albumin as the standard. Little expression could be achieved for fragment POMP90-12, and fragment POMP90-8 was expressed in insufficient amounts for screening of all of the MAbs. They were therefore not tested. Analysis by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent electrophoretic transfer and immunoblotting were performed in accordance with previously published procedures (29). Captured antibodies were detected either with DAB or by chemiluminescence (Super Signal; Pierce).
Peptide synthesis.
Noncleavable peptides were synthesized on polyethylene rods with 9-flouorenylmethyloxy carbonyl chemistry as described by Geysen et al. (6) and in accordance with the instructions of the supplier (PEPSCAN, Chiron Technologies Pty. Ltd., Clayton, Victoria, Australia). The following syntheses were performed: (i) a set of 52 dodecamer peptides overlapping each other by 4 amino acids (aa) and covering the N-terminal part (aa 1 to 420) of the deduced amino acid sequence of POMP90 (13); (ii) 10 18-mer peptides overlapping by 14 residues and spanning aa 40 to 93 of POMP91B; (iii) 7 dodecamer peptides overlapping by 4 residues and spanning aa 39 to 98 of POMP91B; (iv) 1 18-mer, 2 16-mers, and 4 12-mers offset by 2 residues and spanning aa 197 to 216 of POMP90; (v) a set of 3 dodecapeptides offset by 2 residues and covering aa 41 to 56 each of POMP90, -91A, and -91B; (vi) a set of 7 hexapeptides offset by 1 residue and spanning each one of the 5 dodecamers YAKDLTIVSGGP, SPKGGAISIKDS, ITFDGNKIIKTS, YTGKIVFSGEKL, and STVVMDLGTTLQ; and (vii) 15 hexapeptides overlapping by 5 residues and spanning the 20-mer LRAKDGFGIFFYDPITGGGS. Antibodies bound to the pins were detected as described previously (19). The mean of the lowest 25% of the A405 readings was defined as the background. The results for the MAbs were expressed as the net A405 after subtraction of the background. For each serum, the background was determined first and the results were expressed as the ratio of the A405 readings to the background (A405/Abackground).
Sequence analysis.
The genomic sequences of C. pneumoniae Pmp proteins were obtained from the National Center for Biotechnology Information at http://ncbi.nlm.nih.gov, and the whole genome of C. caviae was accessed from The Institute for Genomic Research at http://tigr.org. The ProDom database, for alignment of the epitopes, was accessed at http://prodes.toulouse.inra.fr, and the program BETAWRAP, for the prediction of parallel beta-helices, is available at http://betawrap.lcs.mit.edu.
RESULTS
Identification of epitopes on recombinant protein fragments and synthetic peptides.
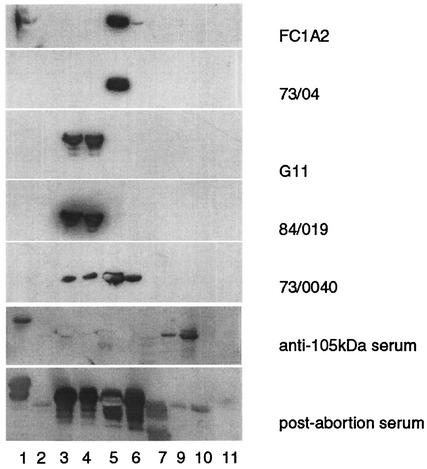
For epitope mapping, each MAb was screened by immunoblotting against a series of overlapping recombinant protein fragments representing POMP90, which were expressed as GST fusion proteins as described recently (16). Specifically, seven fusion proteins spanning the N-terminal amino acid sequence of POMP90 (rOMP90 1-7) and three fragments of the C-terminal part (rOMP90 9-11) were assessed for antibody binding. The lengths of the expressed peptides varied from 61 to 74 aa. Their immunoreactivity was tested (i) with a pool of postabortion ewe sera and (ii) with ewe sera that were affinity purified against the 105-kDa POMP-related protein (Fig. 1). The strongest reaction of the postabortion sera was with fragments 3, 4, 5, and 6, in agreement with previous results (16). The affinity-purified antibodies to the 105-kDa POMP-related protein bound in particular to rOMP90-1, -9, and -10, providing additional support for an antigenic relationship between this protein and the POMP family, as suggested previously (7, 30). Nineteen of the 20 MAbs were found to bind to the N-terminal rOMP90 fragments, the exception being MAb JA6C7. Some MAbs, for example, FC1A2 and 73/04, reacted with one fragment only, whereas others, like MAbs G11 and 84/019, bound to two fragments with the same intensity (Fig. 1). MAb 73/0040 reacted with fragments 3 to 6, although its strongest reaction was with fragment 5 (Fig. 1). Fragment rOMP90-5 (244Ala-317Ile), recognized by 10 MAbs (73/04, 192, FC1A2, 73/0200, DA4E8, H11, 73/0040, 73/0248, 73/053, and LB4B4), was the most antigenic, in contrast to rOMP90-2, which did not react with any of the MAbs tested. The reactivity of each MAb with the fusion peptides is presented in Table 2, column 3. Antibodies EB3G2 and IC4C7 reacted with rOMP90-1. Previous work had suggested that MAb JA6C7 binds only to POMP91B (30). Two smaller fragments of clone p91Bf99, a 450-bp fragment of pomp91B, were tested: the 310-bp fragment p91Bf99s (15) and a similarly expressed 140-bp fragment named p91Bf99a, which is equivalent to POMP90-1. MAb JA6C7 bound to the smaller fragment, p91Bf99a, but not to p91Bf99s (results not shown). When both corresponding fragments, rOMP90-1 (aa 17 to 87) and p91Bf99a (aa 44 to 89), were tested, MAb JA6C7 reacted with fragment p91Bf99a (Fig. 2 lane 3) but not with rOMP90-1 (lane 2) or with rOMP90-2 (lane 1) or the GST control (lane 4). In order to further restrict the epitope of MAb JA6C7, we synthesized seven dodecapeptides overlapping by four residues and representing the amino acid sequence of p91Bf99a (aa 39 to 98 of POMP91B). MAb JA6C7 did not react with any of these dodecapeptides. Therefore, we synthesized 10 18-mer peptides overlapping by 14 residues and spanning the sequence of aa 40 to 93. Two of these 18-mer peptides, 52-SGAIYTCEGNVCISYAGK-69 and 56-YTCEGNVCISYAGKDSPL73, reacted strongly with MAb JA6C7.
FIG. 1.
Binding patterns of MAbs against recombinant protein fragments representing POMP90, expressed as GST fusion proteins (see Table 2). Purified fragments rOMP90-1 to -7 and rOMP90-9 to -11 were subjected to SDS-12% PAGE and immunoblotted with MAbs FC1A2, 73/04, G11, 84/019, and 73/0040, with affinity-purified antibodies to POMP105kDa, and with a pool of postabortion ewe sera.
TABLE 2.
Reactivity of MAbs with recombinant fragments and synthetic peptides
| Fragment | Domaina (length [amino acids]) | MAb | Dodecapeptide | Hexapeptide(s)b | Epitope (sequence) |
|---|---|---|---|---|---|
| rOMP90-1 | 17S-87L (72) | EB3G2/IC4C7 | 41-TSEEFQVKETSS-52 | ND | 1 (TSEEFQVKETSSGT) |
| 43-EEFQVKETSSGT-54 | |||||
| p91Bf99a | 44E-89G (46) | JA6C7 | 52-SGAIYTCEGNVCISYAGK-69 | None | 2 (SGAIYTCEGNVCISYAGKDSPL) |
| 56-YTCEGNVCISYAGKDSPL-73 (2 × 18-mer, 91B) | |||||
| rOMP90-2 | 72G-137Y (65) | None | |||
| rOPM90-3 | 129Y-203D (74) | 73/0040 | None | ||
| 84/019 | None | ||||
| G11 | 152-SLVFHKNCSTAE-163 | ND | 3 (SLVFHKNCSTAE) | ||
| 191 | None | ||||
| 181 | 201-YADKLTIVSGGPT-212 | None | 4-(AIYADKLTIVSGGPTLFS) | ||
| rOPM90-4 | 193S-254N (61) | 73/0040 | None | ||
| 84/019 | None | ||||
| G11 | None | ||||
| 181 | 205-LTIVSGGPTLFS-216 | None | 4a (LTIVSGGPTLFS) | ||
| 191 | 225-SPKGGAISIKDS-236 | None | 5 (SPKGGAISIKDS) | ||
| rOPM90-5 | 244A-317I (73) | 73/04 | None | ||
| 192 | 249-ITFDGNKIIKTS-260 | None | 6 (ITFDGNKIIKTS) | ||
| FC1A2 | 281-LRAKDGFGIFFY-292 | None | 7 (LRAKDGFGIFFY) | ||
| 73/0200 | 281-LRAKDGFGIFFY-292 | DGFGIF, GFGIFF, | 7a (DGFGIF) | ||
| DA4E8 | 281-LRAKDGFGIFFY-292 | DGFGIF, GIFFYD, IFFYDP | 7a (DGFGIF) + 7b (GIFFYD) | ||
| 289-IFFYDPITGGGS-300 | |||||
| H11 | 289-IFFYDPITGGGS-300 | GIFFYD | 8 (IFFYDPITGGGS) | ||
| 73/0040 | 289-IFFYDPITGGGS-300 | None | 8-(IFFYDPITGGGS) | ||
| 73/0248 | 289-IFFYDPITGGGS-300 | GIFFYD, IFFYDP, FFYDPI, FYDPIT | 8a (FFYDPIT) | ||
| 73/053 | 289-IFFYDPITGGGS-300 | IFFYDF, FFYDPI, FYDPIT | 8a (FFYDPIT) | ||
| LB4B4 | 313-YTGKIVFSGEKL-324 | GKIVFS, KIVFSG, IVFSGE | 9 (GKIVFSGE) | ||
| rOPM90-6 | 301D-373M (72) | 73/0040 | None | ||
| LB4B4 | 313-YTGKIVFSGEKL-324 | GKIVFS, KIVFSG, IVFSGE | |||
| rOPM90-7 | 360A-431A (71) | 76/030 | 369-STVVMDLGTTLQ-380 | DLGTTL | 10 (DLGTTL) |
| 76/073 | 369-STVVMDLGTTLQ-380 | DLGTTL | |||
| 76/049 | 369-STVVMDLGTTLQ-380 | DLGTTL |
From start codon.
ND, not done. Bold indicates a strong reaction.
FIG. 2.

Specificity of MAb JA6C7 for POMP91B. Recombinant fragments expressed as GST fusion proteins covering residues 17 to 87 (rOMP90-1, lane 2) and 72 to 137 (rOMP90-2, lane 1) of POMP90 and residues 44 to 89 of POMP91B (p91Bf99a, lane 3) and the empty-control vector (GST, lane 4) were subjected to SDS-12% PAGE and immunoblotted with MAb JA6C7.
To identify the exact binding sites of the other MAbs, 52 dodecapeptides overlapping by 4 residues and covering the N-terminal amino acid sequence of POMP90 (aa 1 to 420) were synthesized and screened against the 20 MAbs. Each reacting dodecapeptide was further analyzed at the single-amino-acid level with hexapeptides overlapping by five residues. Seventeen of the MAbs reacting with rOMP90 fragments also recognized 12-mer synthetic peptides (Table 2). Antibodies EB3G2 and IC4C7 reacted with the dodecapeptide 41-TSEEFQVKETSS-52 of POMP90. To ascertain the reactivity of these MAbs to POMP90 and -91B but not to POMP91A, as indicated by 2D immunoblotting (30), we tested them against three dodecapeptides overlapping by 10 residues and corresponding to the sequences of the three POMPs. Both MAbs reacted strongly with two out of the three peptides belonging to POMP90 and -91B but not with the corresponding peptides of POMP91A (data not shown). The epitopes of MAbs EB3G2 and IC4C7 were thus determined to be 41-TSEEFQVKETSSGT-54 and 41-TSDEFEVKETTSGA-54 for POMP90 and -91B, respectively.
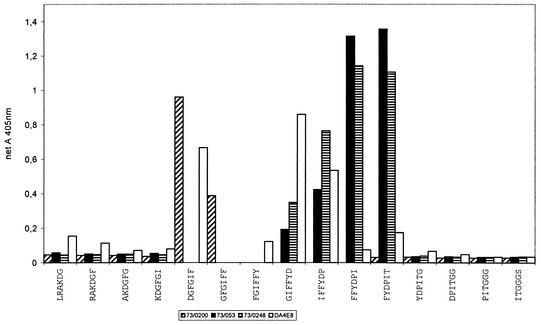
Antibodies 84/019 and 73/04 failed to react with any 12-mer synthetic peptide, although both were positive on recombinant fragments, as mentioned above. It is more likely that this result was due to the downsizing of the antigen (73 to 74 aa to 12 aa) rather than to an insufficient overlap between successive peptides. A new synthesis with larger peptides, however, was not attempted in the case of these MAbs. Twelve-mer peptide epitopes were identified for three out of four MAbs binding to rOMP90 fragments 3 and 4. Peptide 152-SLVFHKNCSTAE-163 (within fragment 3 of POMP90) reacted with MAb G11, and MAb 181 bound to peptide 201-YADKLTIVSGGP-212 (within fragments 3 and 4 of POMP90). Since it has been shown that the epitope of MAb 181 was accessible by EM on the surface of the EB (14), it was studied in more detail. One 18-mer, two 16-mer, and four 12-mer peptides, offset by two residues and spanning residues 197 to 216 between fragments 3 and 4, were synthesized and tested. The 18-mer 199-AIYADKLTIVSGGPTLFS-216 and the 12-mer 205-LTIVSGGPTLFS-216 were the strongest reactors. MAb 191 bound peptide 225-SPKGGAISIKDS-236 (within fragment 4 of POMP90), and MAb 192 reacted with the dodecapeptide 249-ITFDGNKIIKTS-260 in the overlapping region between fragments 4 and 5. None of the epitopes of MAbs 181, 191, and 192 could be further restricted on hexapeptides (Table 2, column 5). The epitopes of 9 of the 10 MAbs binding to the major antigenic fragment rOMP90-5 were delineated. Seven MAbs reacted with the 20-mer 281-LRAKDGFGIFFYDPITGGGS-300, namely, anti-ovine MAbs FC1A2, DA4E8, and H11 and anti-psittacine MAbs 73/0200, 73/0040, 73/0248, and 73/053. When these MAbs were tested further on hexapeptides spanning the 20-mer, each MAb showed a specific reactivity pattern, with the exception of MAbs FC1A2 and 73/0040, which did not react with any hexapeptide (Table 2, column 5). MAb DA4E8 spanned the sequence DGFGIFFYDP and reacted with the hexapeptides DGFGIF, GIFFYD, and IFFYDP but not with the intervening peptides (Fig. 3). MAb H11 had a low affinity for peptide GIFFYD, while MAb 73/0200 reacted with two hexapeptides (Fig. 3), DGFGIF and GFGIFF. Four peptides, GIFFYD, IFFYDP, FFYDPI, and FYDPIT, were recognized by MAb 73/0248 (Fig. 3). The latter three peptides also reacted with MAb 73/053 (Fig. 3). MAb LB4B4 bound to dodecapeptide 313-YTGKIVFSGEKL-324 in the overlapping domain between fragments 5 and 6 and reacted strongly with the hexapeptides GKIVFS, KIVFSG, and IVFSGE. Finally, the hexapeptide DLGTTL, within the epitope 369-STVVMDLGTTLQ-380 in rOMP90-7, was the only ligand of MAbs 76/030, 76/073, and 76/049. In summary, 10 epitopes were determined in the N-terminal part of POMP90, as shown in Table 2, column 6.
FIG. 3.
Binding of MAbs DA4E8, 73/0200 73/0248, and 73/053 to synthetic hexapeptides offset by one amino acid and spanning residues 281 to 300 of POMP90. Reactivity of the MAbs with the peptides is expressed as the net A405.
Serological reactivity.
To find suitable peptides for serological diagnosis, differentiating between infections with C. abortus and infections with C. pecorum, we scanned the 52 dodecapeptides with sera from six different flocks known to be infected with C. abortus. Six out of the 52 peptides, SNSLSFANDAQT, SLVFHKNCSTAE, YADKLTIVSGGP, IFFYDPITGGGS, YTGKIVFSGEKL, and NLDINIASLGGG, were selected on the basis of their absorbance values, which were 50% above the background (Fig. 4). When tested with serum samples from five SPF lambs infected with arthritogenic and conjunctivitis-causing strains of C. pecorum, peptides SLVFHKNCSTAE and NLDINIASLGGG reacted significantly less strongly (P < 0.05, two-tailed t test). The differentiation by peptide YADKLTIVSGGP was of marginal significance (P < 0.1). These data suggest that these peptides may be of potential diagnostic value. The overall seroreactivity of peptide IFFYDPITGGGS was relatively higher than that of the other peptides. It reacted more strongly with postabortion sera than did peptides SNSLSFANDAQT, YTGKIVFSGEKL, and NLDINIASLGGG and more strongly than four other peptides with anti-C. pecorum sera (P < 0.05; analysis of variance, Bonferroni).
FIG. 4.
Seroreactivity of synthetic peptides. Fifty-two dodecapeptides spanning the N-terminal part of POMP90 were tested with six serum pools from flocks with ovine enzootic abortion and five individual serum samples from SPF lambs infected with C. pecorum. The data for the six most strongly reacting peptides with A405/Abackground values of ≥1.50 are shown as the mean values plus the standard deviation for the six serum pools (dark bars) and the five C. pecorum serum samples (open bars).
Surface exposure and BETAWRAP.
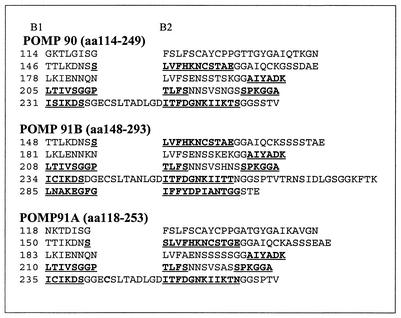
In this study, the antigenic sites of the anti-POMP MAbs were mapped to address the discrepant results of the two reports dealing with the exposure of epitopes on the surface of the EB (2, 14). The mapping data of the present study have placed the inaccessible epitopes of MAbs EB3G2, IC4C7, and JA6C7 within residues 41 to 73 and the epitopes of MAbs FC1A2 and DA4E8 at aa 281 to 294. The surface-accessible binding sites of MAbs 181 and 73/0040 were localized at aa 199 to 216 and 289 to 300, respectively (Table 2). The disagreement in the EM experiments was, thus, due to the different locations of the epitopes within the primary amino acid sequence. This, in turn, indicates that the choice of the MAb under assay was important for determining the accessibility of the POMPs. However, inaccessible epitopes 7 (LRADGFGIFFY, MAb FC1A2) and 7a-7b (DGFGIFFYD, MAb DA4E8) were in close proximity to accessible epitope 8 of MAb 73/0040 (IFFYDPITGGGS), suggesting a turn within the 20-mer peptide. A program named BETAWRAP has been recently developed that can predict parallel beta-helices from the amino acid sequence (1). Many chlamydial Pmp proteins, including POMP98B of C. abortus, were among the 200 best-scoring predictions (1). Therefore, the program was used to assess the potential for POMP90, -91A, and -91B to fold into a beta-helical structure. BETAWRAP predicts a packet of five rungs (one wrap) and generates 10 possible overlapping wraps, which are statistically evaluated in sum. A single rung has three strands (B) separated by three turns (T). The lengths of turns T1 and T3 vary, whereas turn T2 is almost always 2 residues long. Two positions in the sequence, the starts of B1 and B2, can thus describe the position of the rung. Aliphatic residues or “an asparagine ladder” in T2 facilitate the stacking of the rungs. The results of our search are presented in Fig. 5. The best wraps were predicted within residues 114 to 249, 118 to 253, and 148 to 293 for POMP90, -91A, and -91B, respectively. Each line represents one rung. Six epitopes were located within the wrap of 91B and are shown in bold and underlined. Epitopes 3, 6, and 8 were located in the region B2/T2/B3. Interestingly, the epitope of MAb 181 (epitope 4) spanned three-quarters of a rung, from T3 to B2, consistent with its surface exposure. Epitopes 5 and 7 were in turns T3/B1/T1 and B1/T1/B2, respectively.
FIG. 5.
Predicted best beta-helix fold (wrap) in POMP90, -91A, and -91B with the program BETAWRAP. The positions of the epitopes, as determined by epitope mapping, are shown in bold and underlined. Each line represents one rung. A single rung has three strands (B1 to -3) and three turns (T1 to -3). The start positions of strands B1 and B2 are shown.
Figure 6 illustrates the 10 epitopes in a schematic drawing of the N-terminal part of POMP91B. The six epitopes within the beta-helix fold were placed in accordance with their predicted positions in the rungs in Fig. 5. The positions of the epitopes right and left of the beta wrap are not to scale. All three sites of the beta-helix pyramid bear multiple epitopes in a compact antigenic structure.
FIG. 6.
Schematic 3D drawing of the epitope map of the N-terminal part of POMP91B containing a beta-helical domain with six rungs. Five rungs have been placed in accordance with the predicted positions of strands B1 and B2 in each rung calculated by BETAWRAP (shown in Fig. 5). The positions of the epitopes were determined by peptide mapping (Table 2). The parts left and right of the beta-helical domain are not to scale. B1, B2, and B3, beta strands; L, leader peptide.
Cross-reactivity range of the MAbs.
Seven strains representing the genus Chlamydophila and two strains of C. trachomatis were screened against the 11 anti-C. abortus and 9 anti-psittacine MAbs by immunoperoxidase staining on methanol-fixed inclusions grown in McCoy cell monolayers. The results are summarized in Table 3. The MAbs are listed in the order of their epitopes in the N-terminal part of POMP90 (columns 1 and 2). MAb JA6C7 was the only antibody specific for C. abortus. The other 19 MAbs also recognized psittacine strain 6BC, including MAbs such as EB3G2, IC4C7, 181, 191, and 192, which had been reported to be specific for C. abortus (former serotype 1; 21, 29). Six anti-C. abortus MAbs, namely, G11, 192, DA4E8, FC1A2, H11, and LB4B4, and all of the anti-psittacine MAbs recognized strain MN. Antibodies G11, FC1A2, and DA4E8 and anti-psittacine MAbs 73/04, 73/0200, and 73/0040 bound to inclusions of C. abortus, C. psittaci, C. caviae, and C. felis, all of which belong to the former C. psittaci group. This reconfirmed previous data concerning MAbs FC1A2 and DA4E8 (21) that suggested the existence of an epitope(s) common to all of the members of the former C. psittaci group. The epitopes of these two MAbs were identified in the present study as the dodecapeptide LRAKDGFGIFFYD (epitopes 7 and 7a-7b). Anti-psittacine MAbs 73/0248 and 73/053, binding FFYDPIT (epitope 8a), and MAbs 76/073, 76/049, and 76/030, reacting with DLGTTL (epitope 10), also recognized inclusions from strain T13, an enteric strain of C. pecorum. These MAbs and anti-C. abortus MAb LB4B4, with the core antigenic site GKIVFSGE (epitope 9), also bound to inclusions of C. pneumoniae strain Twar 183. MAbs 73/0248 and 73/053 reacted equally well with the inclusions of all species of the genus Chlamydophila that were tested. None of the 20 MAbs under study stained inclusions of C. trachomatis strain D or L2.
TABLE 3.
Cross-reactivity range of the MAbs
| MAb | Epitope | Reciprocal dilution of ascites or hybridoma supernatanta
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. abortus B577T VR656 |
C. psittaci
|
C. caviae GPICT VR813 | C. felis FePnT VR120 | C. pneumoniae TW-183T VR2282 | C. pecorum T13 |
C. trachomatis
|
||||
| 6BCT VR125 | MNT VR122 | L2/434 VR920B | D/UW-3 VR885 | |||||||
| JA6C7 | 2 | 20,000 | ||||||||
| EB3G2 | 1 | 20,000 | 20,000 | |||||||
| IC4C7 | 1 | 40,000 | 40,000 | |||||||
| G11 | 3 | 800 | 800 | 800 | 800 | 800 | ND | ND | ||
| 181 | 4 | 400 | 400 | |||||||
| 191 | 5 | 6 | 6 | |||||||
| 84/019 | rOMP3/4 | 120 | 120 | 120 | 120 | |||||
| 192 | 6 | 6 | 6 | 6 | ||||||
| 73/04 | rOMP5 | 400 | 400 | 400 | 400 | 400 | ||||
| FC1A2 | 7 | 5,000 | 1,000 | 5,000 | 1,000 | 1,000 | ||||
| 73/0200 | 7a | 400 | 400 | 400 | 400 | 400 | ||||
| DA4E8 | 7a-7b | 20,000 | 20,000 | 20,000 | 1,000 | 20,000 | ||||
| H11 | 8 | 400 | 400 | 400 | ND | ND | ||||
| 73/0040 | 8 | 400 | 400 | 400 | 400 | 400 | ||||
| 73/0248 | 8a | 400 | 400 | 400 | 400 | 400 | 400 | 400 | ||
| 73/053 | 8a | 120 | 120 | 120 | 120 | 120 | 200 | 120 | ||
| LB4B4 | 9 | 20,000 | 20,000 | 20,000 | 1,000 | 20,000 | 5,000 | ND | ND | |
| 76/073 | 10 | 400 | 400 | 400 | 400 | 400 | 100 | 200 | ||
| 76/049 | 10 | 400 | 400 | 400 | 400 | 400 | 100 | 200 | ||
| 76/030 | 10 | 400 | 400 | 400 | 400 | 400 | 100 | 200 | ||
ND, not determined.
Summarized, the data showed that epitopes 7 to 10 were conserved among members of the former C. psittaci group, whereas epitopes 8a to 10 were common among the species of the new genus Chlamydophila. To substantiate this finding, the genomes of C. caviae and C. pneumoniae were searched for identical or similar peptides. Table 4 shows alignments of the 10 epitopes in POMP90 with the corresponding sites of POMP91A, -91B, and -98B, with the related sites of Pmp2 as a representative of the C. pneumonia Pmp proteins, and with the most-homologous peptides resulting from a genomic search of C. caviae. The hexapeptide FYDPIT (epitope 8a) was found in the deduced amino acid sequences of Pmp1, -2, -3, -4, -5, -6, -7, -8, -9, -10, -11, -13, and -14 but not in Pmp20 and -21 of C. pneumoniae. Pmp12, -15, -16, -17, -18, and -19 contained the pentapeptide FYDPI. The octapeptide IFFYDPIT was found in Pmp10 and -11. Similarly, the hexapeptide IVFSGE (epitope 9) was observed in Pmp2, -4, -7, -8, -9, -10, and -13. Pmp2 was the only Pmp containing a peptide similar to the hexapeptide DLGTTL. The dodecapeptide LRAKDGFGIFFY (epitope 7) was conserved in C. caviae as LRARDGFGVFFY. Only two similarities were scored in POMP98B, namely, the peptides FYDPIT (epitope 8a) and IIFSGE (epitope 9), suggesting that interspecies orthologues are more conserved than intraspecies paralogues. Besides the differences within epitopes 1 and 2, described in detail at the beginning of this section, the few amino acid substitutions within the other epitopes among C. abortus POMP90, -91A, and -91B were tolerated by the MAbs, as judged by 1D and 2D immunoblot assays (not shown).
TABLE 4.
Alignment of the 10 epitopes among Chlamydophila spp.
| Protein or strain | Epitope 1 Epitope 2 | Epitope 3 | Epitope 4 | |
|---|---|---|---|---|
| POMP91B | GNTNSEPFNPLSTSN-SNGTIYTCTGNICIAYAGLDGSGLSSSCF | DNSSLVFHKNCSTAEGGAI | KGGAIYADKLTIVSGGPTLFSNN | |
| POMP91B | GNVTSDEFEVKETTS---GAIYTCEGNVCISYAGKD-SPLNKSCF | DNSSLVFHKNCSTGEGGAI | SGGAIYADKLTIVSGGPTLFSNN | |
| POMP90 | GNVTSEEFQVKETSS---GTTYTCEGNVCISFAGKD-SGLKKSCF | DNSSLVFHKNCSTAEGGAI | KGGAIYADKLTIVSGGPTLFSNN | |
| GPIC | GNTTTTPFVPKETST---GAEYTCNGNVCITYAGKT-TPLTKSCF | NDFSVLFKKNCSTAAGGAI | EGGAIYAKKLSIISGGPTLFSNN | |
| Pmp2 | GTTSTTSFSSKTSSA-TDGTNYVFKDSVVIENVPKTGETQSTSCF | DNDKVLIQDNFSTGDGGAI | RGGAIHTKNLTLSSGGETLFQGN | |
| POMP98B | LDANG-AFSPQS-TSTAGGTIYNVESDISIVDV-GQTAALASSAF | VAFSNNAVSGSSDGCGGAI | KGGAIYTDKLILTSGGPTAFINN/PICK> | |
| Epitope 5 | Epitope 6 | Epitope 7 Epitope 8 | Epitope 9 | Epitope 10 |
| SVS-ASSPKGGAICIKDS | GDITFDGNKIITTNGGSP | GKFTKLNAKEGFGIFFYDPIANTG-G | YTGKIVFSGEKL | TQTEGATVVMDLGTTLQ |
| SVS-ASSPKGGAICIKDS | GDITFDGNKIIKTNGGSP | GKFTKLNAKEGFGIFFYDPITG-G-G | YTGKIVFSGERL | TQTKGSTVVMDLGTTLQ |
| SVSNGSSPKGGAISIKDS | GDITFDGNKIIKTSGGSS | GKFTKLRAKDGFGIFFYDPITG-G-G | YTGKIVFSGEKL | TQEAGSTVVMDLGTTLQ |
| STSKAADPKGGAICIADA | GDIIFDGNKIITT--GTP | GKFSQLRARDGFGVFFYDPIAN-N-G | YSGRIVFSGEKL | TQTAGSAVVMDAGTTLQ |
| TA-PTAAGKGGAIAIADS | GDIIFEGNTI----GATG | AKITALRAAQGHTIYFYDPITVTGST | YTGTIVFSGEKL | SQDANSKLIMDLGTSLV |
| KVT-HATPKGGAIGIAAN | GDITFDNN-LMATQDN-A | GKFVNLRAASGKTISFYDPITVEGNA | YNGRIIFSGEKL | VQTAGSLILMDAGTKLS |
DISCUSSION
In this study, we have used 20 MAbs that react with the three POMPs of C. abortus to produce a detailed antigenic map of the N-terminal domain of the molecules. In addition, we have used the program BETAWRAP to predict the folding of this domain, thereby creating a 3D antigenic map. The results have antigenic, structural, phylogenetic, and serological implications.
All 20 of the MAbs used in this study bound in Western blot assays after denaturing SDS-PAGE of either solubilized whole EBs or recombinant fusion peptides. They were therefore expected to recognize sequential epitopes. However, the epitopes defined by MAbs JA6C7, 84/019, and 73/04 were shown to be conformational since they were lost when fragmented from approximately 70-residue-long fusion peptides to 12-mer peptides (Table 2). Linear (or sequential or continuous) epitopes contain four to six adjacent residues of the primary sequence (18), indicating that identified epitopes 7a, 8a, 9, and 10 are true sequential epitopes. The majority of the antigenic sites, epitopes 1, 3, 4, 5, 6, 7, 8, and 7a-7b, although continuous in their primary sequences, must retain a considerable conformational element since these epitopes were lost when downsized from 12-mers to hexapeptides (Table 2). The program BETAWRAP predicted most of these epitopes (the binding sites of 13 MAbs) to be located within a compact beta-helix fold (Fig. 5). Experimental data support the beta-helix folding in this domain. In the beta-helix structure, segments widely separated in their primary sequences are brought together in adjacent positions. Some MAbs, such as G11, 84/019, 191, and 73/0040, bound more than one fragment (Table 2). Furthermore, most of the epitopes within the putative beta wrap (epitopes 3, 4, 5, 6, 7, 8, and 7a-7b) had a strong conformational element whereas the linear epitopes, especially epitope 8a, were at the end of the beta-helix. Moreover, epitope 4, which has been shown in EM studies to be accessible to antibodies on the EB surface, was within an external active site in the beta-helix fold. In a previous study, we had speculated that a possible role for the N-linked oligosaccharides identified in the POMPs may be promotion of the proper folding of these proteins (30). According to the beta-helix model, the three GGAI motifs that are preceded by the three potentially glycosylated FXXN motifs are located within a fold (Fig. 5). The structural elements in the POMPs right and left of the beta-helix are unknown. The conformation of the N-terminal part to the left may be of high segmental mobility since epitope 1 is highly accessible in RBs and inaccessible in EBs (2). The more C-terminal part to the right that contains two sequential epitopes (9 and 10) could be a random coil. The overall antigenic organization of the N-terminal part of the POMPs is very compact, since epitopes are not only adjacent in their primary sequence (epitopes 1 and 2 and epitopes 7 and 8) but are brought into close proximity by folding.
The common elements in the primary sequence of the POMP/Pmp family are the repeated FXXN-GGAI motifs. Surprisingly, these motifs were not detected as antigenically related in the phylogenetic studies. MAb 191 binds within a GGAI motif (epitope 5). Alignment of this epitope with the C. pneumoniae Pmp proteins (Table 4; only Pmp2 is shown) showed considerable variation in the amino acid residues before and after GGAI, although the lysine before and the isoleucine after the motif were conserved in some of the Pmp proteins. This suggested that, although orthologous Pmp proteins may have the same structure, the parallel beta-helices, they generally do not have the same antigenic sites within this domain. However, antibodies with a strong conformational character, such as G11 and 84/019, may recognize structural homologies among the species C. abortus, C. psittaci, C. caviae, and C. felis (Table 3). One of the important findings of this study is the identification of the binding sites of seven MAbs within a 20-aa stretch, defining the sequence LRAKDGFGIFFYDPITGGGS as a major epitope of the POMPs. Most interestingly, this major epitope is conserved in the members of the former C. psittaci group, together with epitopes 9 and 10. Such a large antigenic homology has not been recognized previously. Moreover, linear epitopes 8a, 9, and 10 were found in all of the species of the genus Chlamydophila that were tested but were absent in C. trachomatis. Therefore, these are the first known linear epitopes to be identified in the C. pneumoniae and C. pecorum Pmp proteins. Further studies with more strains will prove whether these epitopes represent Chlamydophila genus-specific epitopes. Epitopes FYDPIT and IVFSGE were not only conserved among the different Chlamydophila nonhuman animal species but were also present in the majority of the C. pneumoniae Pmp proteins. The hexapeptide FYDPIT was present in 13 Pmp proteins, while the pentapeptide FYDPI was present in 6 of them. Following a preliminary analysis of the C. abortus genome, peptide FYDPIT or FYDPI has been identified in 10 POMPs: 5 of the 6 originally identified POMPs contained FYDPIT, while the other contained the pentapeptide FYDPI (Longbottom, unpublished). It is worth mentioning that, in addition to MAb 76/073 (7), POMP105kDa also bound MAbs 73/053 and 73/0040, which react with FFYDPIT and IFFYDPITGGGS, respectively (E. Vretou and P. Giannikopoulou, unpublished results). The large conservation between the proteins and throughout the species of the genus Chlamydophila indicates that these sites may have an as yet unknown important function. FYDPIT may signify the end of the putative beta-helix folding, or the signal for the subsequent fragmentation, according to the similarity of the POMPs with type V autotransporters. FYDPIT preceded a cleavage site in Pmp6, as identified recently (28). It is interesting that a 20-mer peptide following FYDPIT was selected for the generation of specific polyclonal antibodies on the basis of its variation between the Pmp proteins (9). In C. abortus, the peptide IFFYDPITGGGS was more reactive with postabortion ewe sera than most of the other peptides, although its serological importance was diminished by its cross-reaction with the anti-C. pecorum sera.
Autotransporters consist of a C-terminal domain that is incorporated in the outer membrane as a beta-barrel, and an N-terminal domain, named the passenger, that is translocated through the C-terminal domain (10). Once the passenger domain is exported, it may be cleaved or remain attached. The function of the passenger domain in the chlamydial POMPs is unknown, and we have no indication of its possible secretion. In the present study, we have provided some evidence that supports the folding of the passenger domain into a beta-helix fold. Beta-helices are found in a series of human and animal pathogens that are involved in surface recognition (1). The active site of the passenger domain in the POMPs may be located along the axis of the beta-helix, as shown for the phage P22 tailspike protein (22). The predicted beta-helix model opens the way for possible speculation. The cysteine residues in the two first rungs of POMP90 and -91A, brought together by stacking, may, under appropriate conditions, oxidize to form intramolecular disulfide bridges. Possible intramolecular disulfide bridges have been presumed previously, on the basis of the unfolding of the polypeptides at 90 kDa in the presence of iodoacetamide (E. Vretou, P. Yannikopoulou, E. Psarrou, P. Papavassiliou, V. Pallini, and L. Bini, Proc. 3rd Meet. Eur. Soc. Chlamydia Res., p. 52, 1996). Further, when the POMPs are cleaved, the proteolytic fragments could be held together by these intramolecular disulfide bridges and only released or transferred to other molecules within a reducing environment. 2D immunoblotting with MAb 76/073 has previously indicated fragmentation of the POMPs. This MAb, which binds at the end of the N-terminal domain (epitope 10), recognized the three POMPs and reacted repeatedly with several spots at 75.8, 72, 67, 60.5, and 58.7 kDa, whereas other MAbs did not (7, 30). These results were difficult to interpret, since the spots may have originated from different POMPs that have not been identified yet. After the C. abortus genome has been completely mapped, MAbs with precisely located epitopes may prove to be useful molecular tools, together with mass spectrometry techniques, with which to trace the processing of the POMPs and to help elucidate their role in chlamydial pathogenesis.
Acknowledgments
We thank A. Rodolakis (Institut National de la Recherche Agronomique, Nouzilly, France) for the gift of antibodies and Erifily Nicolacopoulou for the drawings.
This work was supported by the Greek General Secretary for Research and Technology and by the Scottish Executive Environment and Rural Affairs Department.
Editor: F. C. Fang
REFERENCES
- 1.Bradley, P., L. Cowen, M. Menke, J. King, and B. Berger. 2001. BETAWRAP: successful prediction of parallel β-helices from primary sequence reveals an association with many microbial pathogens. Proc. Natl. Acad. Sci. USA 98:4819-14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buendia, A. J., J. Salinas, J. Sanchez, M. C. Gallego, A. Rodolakis, and F. Cuello. 1997. Localization by immunoelectron microscopy of antigens of Chlamydia psittaci suitable for diagnosis or vaccine development. FEMS Microbiol. Lett. 150:113-119. [DOI] [PubMed] [Google Scholar]
- 3.Cevenini, R., A. Moroni, V. Sambri, S. Perini, and M. La Placa. 1989. Serological response to chlamydial infection in sheep, studied by enzyme-linked immunosorbent assay and immunoblotting. FEMS Microbiol. Immunol. 47:459-464. [DOI] [PubMed] [Google Scholar]
- 4.Cevenini, R., M. Donati, E. Brocchi, F. de Simone, and M. La Placa. 1991. Partial characterization of an 89-kDa highly immunoreactive protein from Chlamydia psittaci A/22 causing ovine abortion. FEMS Microbiol. Lett. 65:111-115. [DOI] [PubMed] [Google Scholar]
- 5.Everett, K. D. E., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simcaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 6.Geysen, M. H., R. H. Meloen, and S. J. Barteling. 1984. Use of peptide synthesis to probe viral antigens for epitopes to resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 81:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannikopoulou, P., L. Bini, P. D. Simitsek, V. Pallini, and E. Vretou. 1997. Two-dimensional electrophoretic analysis of the protein family at 90 kDa of abortifacient Chlamydia psittaci. Electrophoresis 18:2104-2108. [DOI] [PubMed] [Google Scholar]
- 8.Grimwood, J., and R. S. Stephens. 1999. Computational analysis of the polymorphic membrane protein superfamily of Chlamydia trachomatis and Chlamydia pneumoniae. Microb. Comp. Genomics 4:187-201. [DOI] [PubMed] [Google Scholar]
- 9.Grimwood, J., L. Olinger, and R. S. Stephens. 2001. Expression of Chlamydia pneumoniae polymorphic membrane protein family genes. Infect. Immun. 69:2383-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson, I. R., and A. C. Lam. 2001. Polymorphic proteins of Chlamydia spp.—autotransporters beyond the Proteobacteria. Trends Microbiol. 9:573-578. [DOI] [PubMed] [Google Scholar]
- 11.Kalman, S., W. Mitchell, R. Marathe, C. Lammel, J. Fan, R. W. Hyman, L. Olinger, J. Grimwood, R. W. Davis, and R. S. Stephens. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 21:385-389. [DOI] [PubMed] [Google Scholar]
- 12.Longbottom, D., M. Russell, G. E. Jones, F. A. Lainson, and A. J. Herring. 1996. Identification of a multigene family coding for the 90 kDa proteins of the ovine abortion subtype of Chlamydia psittaci. FEMS Microbiol. Lett. 142:277-281. [DOI] [PubMed] [Google Scholar]
- 13.Longbottom, D., M. Russell, S. M. Dunbar, G. E. Jones, and A. J. Herring. 1998. Molecular cloning and characterization of the genes coding for the highly immunogenic cluster of 90-kilodalton envelope proteins from the Chlamydia psittaci subtype that causes abortion in sheep. Infect. Immun. 66:1317-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longbottom, D., J. Findlay, E. Vretou, and S. M. Dunbar. 1998. Immunoelectron microscopic localization of the OMP90 family on the outer membrane surface of Chlamydia psittaci. FEMS Microbiol. Lett. 164:111-117. [DOI] [PubMed] [Google Scholar]
- 15.Longbottom, D., E. Psarrou, M. Livingstone, and E. Vretou. 2001. Diagnosis of ovine enzootic abortion using an indirect ELISA (rOMP91BiELISA) based on a recombinant protein fragment of the polymorphic outer membrane protein POMP91B of Chlamydophila abortus. FEMS Microbiol. Lett. 195:157-161. [DOI] [PubMed] [Google Scholar]
- 16.Longbottom, D., S. Fairley, S. Chapman, E. Psarrou, E. Vretou, and M. Livingstone. 2002. Serological diagnosis of ovine enzootic abortion by enzyme-linked immunosorbent assay with a recombinant protein fragment of the polymorphic outer membrane protein POMP90 of Chlamydophila abortus. J. Clin. Microbiol. 40:4235-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montigiani, S., F. Falugi, M. Scarselli, M. O. Finco, R. Petracca, G. Galli, M. Mariani, R. Manetti, M. Agnusdei, R. Cevenini, M. Donati, R. Nogarotto, N. Norais, I. Garaguso, S. Nuti, G. Saletti, D. Rosa, G. Ratti, and G. Grandi. 2002. Genomic approach for analysis of surface proteins in Chlamydia pneumoniae. Infect. Immun. 70:368-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris, G. E. (ed.). 1996. Epitope mapping protocols. Humana Press, Totowa, N.J.
- 19.Salti-Montesanto, V., E. Tsoli, P. Papavassiliou, E. Psarrou, B. K. Markey, G. E. Jones, and E. Vretou. 1997. Diagnosis of ovine enzootic abortion, using a competitive ELISA based on monoclonal antibodies against variable segments 1 and 2 of the major outer membrane protein of Chlamydia psittaci serotype 1. Am. J. Vet. Res. 58:228-235. [PubMed] [Google Scholar]
- 20.Shaw, A. C., K. Gevaert, H. Demol, B. Hoorelbeke, J. Vandekerckhove, M. R. Larsen, P. Roepstorff, A. Holm, G. Christiansen, and S. Birkelund. 2002. Comparative proteome analysis of Chlamydia trachomatis serovar A, D and L2. Proteomics 2:164-186. [DOI] [PubMed] [Google Scholar]
- 21.Souriau, A., J. Salinas, C. De Sa, K. Layashi, and A. Rodolakis. 1994. Identification of subspecies and serotype 1-specific epitopes on the 80- to 90-kilodalton protein region of Chlamydia psittaci that may be useful for diagnosis of chlamydial induced abortion. Am. J. Vet. Res. 55:510-514. [PubMed] [Google Scholar]
- 22.Steinbacher, S., S. Miller, U. Baxa, A. Weintraub, R. Seckler, and R. Huber. 1996. Crystal structure of phage P22 tailspike protein complexed with Salmonella sp. O-antigen receptors. Proc. Natl. Acad. Sci. USA 93:10584-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 24.Stephens, R. S. (ed.). 1999. Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 25.Storz, J. 1971. Chlamydia and chlamydia-induced diseases. Charles C Thomas, Springfield, Ill.
- 26.Tanzer, R. J., D. Longbottom, and T. P. Hatch. 2001. Identification of polymorphic outer membrane proteins of Chlamydia psittaci 6BC. Infect. Immun. 69:2428-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandahl, B. B., S. Birkelund, H. Demol, B. Hoorelbeke, G. Christiansen, J. Vandekerckhove, and K. Gevaert. 2001. Proteome analysis of the Chlamydia pneumoniae elementary body. Electrophoresis 22:1204-1223. [DOI] [PubMed] [Google Scholar]
- 28.Vandahl, B. B., A. S. Pedersen, K. Gevaert, A. Holm, J. Vandekerckhove, G. Christiansen, and S. Birkelund. November 2002, posting date. The expression, processing and localization of polymorphic membrane proteins in Chlamydia pneumoniae strain CWL029. BMC Microbiol. 2:36. [Online.] http://www.biomedcentral.com. [DOI] [PMC free article] [PubMed]
- 29.Vretou, E., E. Loutrari, L. Mariani, K. Costelidou, P. Eliades, G. Conidou, S. Karamanou, O. Mangana, V. Siarkou, and O. Papadopoulos. 1996. Diversity among abortion strains of Chlamydia psittaci demonstrated by inclusion morphology, polypeptide profiles and monoclonal antibodies. Vet. Microbiol. 51:275-289. [DOI] [PubMed] [Google Scholar]
- 30.Vretou, E., P. Giannikopoulou, and E. Psarrou. 2001. Polymorphic outer membrane proteins of Chlamydophila abortus are glycosylated. Microbiology 147:3303-3310. [DOI] [PubMed] [Google Scholar]