Short abstract
Recent studies of the protein Dicer, a key enzyme in the RNA interference process, have started to reveal how this single enzyme is targeted to different RNA-silencing pathways.
Abstract
Research into the mechanism of RNA interference has seen immense progress over the past few years. Recent studies of the protein Dicer, a key enzyme in the process, have started to reveal how this single enzyme is targeted to different RNA-silencing pathways.
The nuclease Dicer was identified as the protein responsible for cutting double-stranded RNA (dsRNA) into small interfering RNAs (siRNAs) of approximately 21 bp during the process of RNA interference (RNAi) [1]. These siRNAs were first identified in RNAi-like silencing processes in plants [2], and were later also found associated with RNAi in animals [3,4]. siRNAs are incorporated into ribonuclease complexes called RNA-induced silencing complexes (RISCs), where their role is to confer specificity for the cleavage of a homologous mRNA. Within a RISC, the siRNA is bound by RDE-1, a protein of the Argonaute family [5], which initiates the destruction of the targeted mRNA by introducing a single-strand nick in the mRNA in the region base-paired to the siRNA [6,7]. Even early on, however, it became clear that Dicer produces other kinds of small RNAs apart from siRNAs and that these were bound by similar, but different, complexes, resulting in multiple parallel silencing pathways mediated by a variety of small RNAs [8]. Some of these pathways, such as those involving siRNAs, lead to mRNA degradation, some to translation inhibition (those involving microRNAs, or miRNAs) [9], and yet others to the remodeling of chromatin structure [10]. Despite the existence of so many different small RNA pathways, animal genomes encode only one Dicer enzyme. So how is this enzyme targeted in a regulated fashion to these various pathways? A study published in Cell earlier this year by Duchaine et al. [11] starts to shed light on this problem by showing the physical interaction of Dicer with a variety of proteins involved in these pathways.
Finding the partners
Reasoning that proteins involved in guiding Dicer to the different pathways should physically interact with the Dicer protein, the team led by Craig Mello [11] used a proteomics approach to identify proteins that co-purify in immunoprecipitation (IP) procedures. Using antibodies directed against the Dicer protein from Caenorhabditis elegans, protein complexes were pulled out of extracts, and analyzed in bulk using a multidimensional protein identification technology (MudPIT) approach [12]. This mass-spectrometry-based technology is capable of recognizing and identifying relatively small amounts of a protein in complex mixtures and is a powerful way of identifying proteins in a given sample. Repeating this procedure several times and comparing samples from animals with or without Dicer protein led to the robust identification of 20 proteins that interact with Dicer. Another 88 proteins were identified in a less reproducible way - that is, they were identified as Dicer interactors in only one of the purifications - and were not studied further. Nevertheless, given the fact that a well-known Dicer interactor, RDE-1 [13,14], barely made it to the top-20 list, many relevant interactors may well be represented in this 'lower confidence' list. Confining themselves to the 'high-confidence' list of 20, Duchaine et al. [11] used reverse genetics to elucidate the roles of these proteins by identifying deletion alleles of the genes and studying their role in Dicer-related pathways.
On the basis of the predicted functions of the 20 candidate proteins, Duchaine et al. [11] could divide them into five groups. Two groups could have been expected from previous work: these are known proteins involved in the siRNA pathway, which the authors call the 'RNAi group', and known proteins involved in the miRNA pathway - the 'miRNA group'. These two groups can be regarded as a positive control for the approach, as most of them have been associated with Dicer in previous studies, and they will not be discussed here. A third group is composed of six proteins with unknown functions. This group was not studied further by Duchiane et al. [11] - not because these proteins would be uninteresting, but because the phenotypes associated with the available deletion alleles are very pleiotropic, and it will take significant extra effort to reach meaningful conclusions on their precise roles in RNAi-related pathways. The most interesting findings of Duchaine et al. [11] come from the remaining two groups of proteins.
Two new partners for Dicer
One of these groups (the so-called 'PIR-1 group') contains two evolutionarily conserved proteins - DRH-3 and PIR-1 - that are both required for RNAi and for viability [11]. C. elegans PIR-1 is the ortholog of the human PIR1 protein [15], which is an RNA phosphatase that removes the γ- and β-phosphate groups from the 5' ends of RNA molecules [16]. C. elegans mutants that lack PIR-1 develop normally but arrest at the last larval stage and hence never produce progeny. At a molecular level, these animals are resistant to RNAi, and this is because they do not produce any siRNAs from dsRNA [11].
These mutants do, however, accumulate a larger RNA species when attempts are made to trigger RNAi experimentally. This RNA is most probably the product of one of the RNA-dependent RNA polymerases (RdRPs) that have been shown to be essential for RNAi in C. elegans [17,18]. These polymerases are involved in the production of the so-called 'secondary' siRNAs that lie 5' of the region recognized by the dsRNA [18]. They turn the initial single-stranded mRNA target into dsRNA, which is then thought to be cleaved by Dicer to produce these new siRNAs. RdRPs can start de novo RNA synthesis on an RNA template [19], which leads to dsRNA molecules that have a triphosphate group on the 5' end of one strand (the newly synthesized strand), and a methylated cap on the 5' end of the other strand (the original mRNA molecule). These are presumably bad substrates for Dicer, as 5'-monophosphate groups are preferred throughout the RNAi pathway [20]. PIR-1 would therefore seem to be the perfect candidate to convert these dsRNA molecules into better Dicer substrates, enabling siRNA production (Figure 1). This model makes the following two predictions: first, sequences downstream of the original target should not be present in the accumulating RNA intermediates; and second, primary siRNAs (the siRNAs that are produced directly from exogenously added dsRNA) should be formed in pir-1 mutants, as long as one uses dsRNA with 5'-monophosphate ends.
Figure 1.
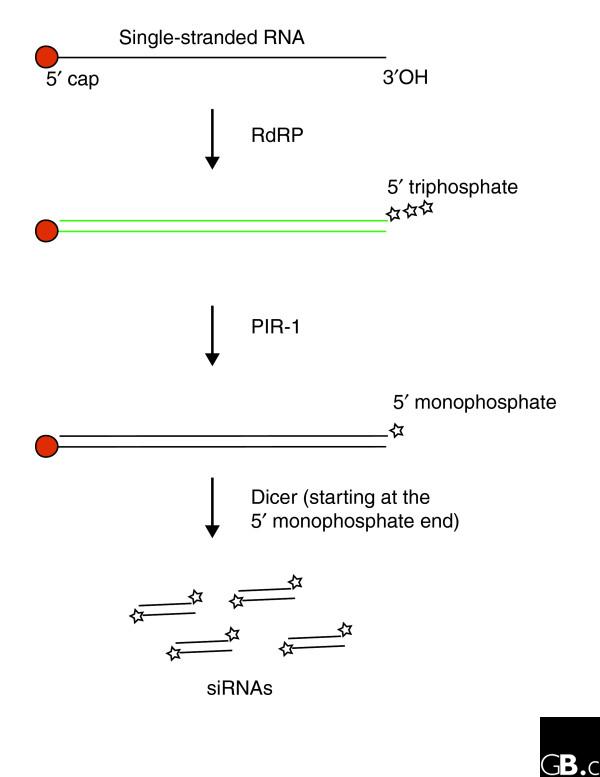
RNA-dependent RNA polymerases (RdRPs) synthesize RNA on RNA templates. During RNAi in C. elegans this process is used to amplify the initial RNAi response, and without it, no effect of RNAi is observed. The precise RNA molecules that serve as templates for RdRPs have not been identified, but it is assumed that they will at least contain the 5' end of the mRNA that is targeted by RNAi, and hence will have a 5'-cap structure. De novo RNA synthesis on this RNA template would result in a 5' end having a triphosphate group (indicated with three stars). PIR-1 may function in trimming such ends to monophosphate groups, allowing Dicer to cleave the dsRNA. The RNA species accumulating when PIR-1 is absent is indicated in green.
Some discrepancies remain, however. If the only role of PIR-1 is to convert RdRP products into suitable Dicer substrates, then why do no RdRP mutants show pir-1-like phenotypes [17,18]? Most probably, PIR-1 is performing additional tasks, perhaps in other small-RNA-mediated silencing pathways or even in unrelated pathways. The fact that PIR1 is also found in human cells could suggest that this protein is indeed doing more than just helping RdRPs, as no involvement of RdRPs in human RNAi has been observed. On the other hand, PIR1 may help in fighting virus infections in human cells: RNA viruses have their own RdRPs, and if human cells are to take the dsRNA intermediates of these viruses through the RNAi pathway, they have to turn them into suitable Dicer substrates, just as C. elegans needs to process its RdRP products to trigger RNAi. This scenario would predict that PIR1-deficient human cells would be more sensitive to RNA virus infections.
The second protein in the 'PIR-1 group' [11] is the RNA helicase DRH-3. This enzyme appears to be required for RNAi only in the germline, and drh-3 mutants display severe germline defects and defects in the first embryonic cell division. DRH-3 is a member of a conserved protein family, members of which are also found in human cells [21]. drh-3 mutant embryos show defects in chromosome segregation and in the production of endogenous siRNAs (see below), suggesting the involvement of a small-RNA-mediated pathway in the establishment of proper chromosome dynamics. The latter function has been clearly demonstrated in the fission yeast Schizosaccharomyces pombe [10], but in animals it has still to be unambiguously established. Interestingly, other C. elegans RNAi mutants also show hints of chromosome-segregation defects [13,22]. Many of these mutants display a so-called Him (high incidence of males) phenotype, which is indicative of X-chromosome mis-segregation. Whether any of these other genes work together with DRH-3 is unknown.
In drh-3 homozygous mutant animals, decreased cell proliferation in the germline is observed; that is, the mitotic phase of germ-cell production is affected [11]. This defect might be related to previously described germline under-proliferation in worms with compromised activity of the Piwi protein, a member of the Argonaute family [23]. It may be that DRH-3 and Piwi function in the germline in the same way that the known Dicer interactors DRH-1, DRH-2 and RDE-1 function in the soma [14].
Competition between small RNA pathways
The second group of novel players identified by Duchaine et al. [11] as interacting with Dicer is called the 'enhanced RNAi (Eri) group' and may constitute a multiprotein complex involved in the endogenous RNAi that is initiated naturally in C. elegans against certain genes at certain times. Small RNAs that are associated with these endogenous RNAi responses are named endo-siRNAs when they match a known C. elegans gene or tiny noncoding RNAs (tncRNAs) when they do not [24]. Duchaine et al. [11] show that the Dicer-interacting proteins that fall into this group have effects on the production of these small RNA molecules (see also [25]), as does DRH-3, as described above. DRH-3 also physically interacts with the proteins of this group. Interestingly, not all mutants in this group affect all types of small RNAs to the same extent, indicating that we are only just beginning to see how these small RNA molecules are produced and how they function.
Duchaine et al. [11] also show that the endogenous RNAi pathway competes with the experimental, or exogenous, RNAi pathway: triggering exogenous RNAi decreases the effect of the endogenous RNAi pathway. Conversely, genetic disruption of the endo-siRNA pathway makes the exo-siRNA pathway more efficient. These pathways probably share a number of components - they share Dicer at least - and so when one pathway is compromised, the other becomes more effective. These results indicate that all these pathways are in delicate balance, and that unexpected side-effects might be observed in RNAi experiments as a result of disturbing this balance.
It is interesting that both exo- and endo-RNAi pathways appear to use similar proteins. Both use Dicer, obviously, but Duchaine et al. and other workers find that both pathways also need an RdRP [11,17,18,25], RNA helicases [11,14,26] and (at least to some extent) 3'-5' exonucleases [11,22,25]. The Argonaute protein of the endo-siRNA pathway has yet to be identified. There are apparently many flavors of RNAi machines, all using their own specialized set of proteins. This may be part of the answer to the question raised above: why does the pir-1 mutant have such a severe phenotype? Maybe PIR-1, like the other essential RNAi factor DRH-3, is also required for the production of endo-siRNAs and tncRNAs. Future experiments will tell.
A picture emerges in which Dicer is targeted to many different pathways through interactions with different proteins. To produce the required allocation of Dicer in a given situation, proteins from different pathways compete for it. This may mean that Dicer is the rate-limiting step in many small-RNA-mediated processes. Downstream of Dicer, many variants of the RNAi pathway operate, each of them guiding their effector molecules to specific sequences, either in RNA or in chromosomal DNA.
Acknowledgments
Acknowledgements
I thank Ronald Plasterk and Leonie Kamminga for critical reading of the manuscript.
References
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- Tang G. siRNA and miRNA: an insight into RISCs. Trends Biochem Sci. 2005;30:106–114. doi: 10.1016/j.tibs.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Martienssen RA, Zaratiegui M, Goto DB. RNA interference and heterochromatin in the fission yeast Schizosaccharomyces pombe. Trends Genet. 2005;21:450–456. doi: 10.1016/j.tig.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Duchaine TF, Wohlschlegel JA, Kennedy S, Bei Y, Conte D, Jr, Pang K, Brownell DR, Harding S, Mitani S, Ruvkun G, et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343–354. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/S0092-8674(00)81644-X. [DOI] [PubMed] [Google Scholar]
- Tabara H, Yigit E, Siomi H, Mello CC. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell. 2002;109:861–871. doi: 10.1016/S0092-8674(02)00793-6. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Li DM, Sun H. PIR1, a novel phosphatase that exhibits high affinity to RNA:ribonucleoprotein complexes. J Biol Chem. 1998;273:20347–20353. doi: 10.1074/jbc.273.32.20347. [DOI] [PubMed] [Google Scholar]
- Deshpande T, Takagi T, Hao L, Buratowski S, Charbonneau H. Human PIR1 of the protein-tyrosine phosphatase superfamily has RNA 5'-triphosphatase and diphosphatase activities. J Biol Chem. 1999;274:16590–16594. doi: 10.1074/jbc.274.23.16590. [DOI] [PubMed] [Google Scholar]
- Smardon A, Spoerke JM, Stacey SC, Klein ME, Mackin N, Maine EM. EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C. elegans. Curr Biol. 2000;10:169–178. doi: 10.1016/S0960-9822(00)00323-7. [DOI] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/S0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- Makeyev EV, Bamford DH. Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol Cell. 2002;10:1417–1427. doi: 10.1016/S1097-2765(02)00780-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Ketting RF, Haverkamp TH, van Luenen HG, Plasterk RH. Mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell. 1999;99:133–141. doi: 10.1016/S0092-8674(00)81645-1. [DOI] [PubMed] [Google Scholar]
- Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V, Lee RC, Lavanway A, Williams PT, Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr Biol. 2003;13:807–818. doi: 10.1016/S0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- Lee RC, Hammell CM, Ambros V. Interacting endogenous and exogenous RNAi pathways in Caenorhabditis elegans. RNA. 2006 doi: 10.1261/rna.2231506. doi:10.1261/rna.2231506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsterman M, Ketting RF, Okihara KL, Sijen T, Plasterk RH. RNA helicase MUT-14-dependent gene silencing triggered in C. elegans by short antisense RNAs. Science. 2002;295:694–697. doi: 10.1126/science.1067534. [DOI] [PubMed] [Google Scholar]


