Short abstract
Quantitative trait locus analysis of two inbred mouse strains has revealed that cell survival is most highly correlated with neurogenesis.
Abstract
Adult neurogenesis in the hippocampus is under complex genetic control. A recent comparative study of two inbred mouse strains using quantitative trait locus analysis has revealed that cell survival is most highly correlated with neurogenesis and identified candidate genes for further investigation.
Neurogenesis - the production of new neurons - is an ongoing process that persists in the adult brain of several species, including humans. It has been most intensively studied in the mouse in two discrete brain regions: the subventricular zone (SVZ) lining the lateral wall of the lateral ventricles; and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus [1] (Figure 1). These regions harbor relatively quiescent astrocyte-like stem cells, which divide and give rise to multipotential, rapidly dividing transit-amplifying cells that will eventually differentiate into neuroblasts. These later generate neuroblasts that are believed to have limited further mitotic potential [2,3]. Neuroblasts from the SVZ and SGZ migrate and eventually mature into functional neurons within the olfactory bulb and dentate gyrus, respectively. Most recent evidence suggests that the stem cells in these regions can also give rise to astrocytes and oligodendrocytes of the glial lineage, indicating that in vivo, as in vitro, these cells are multipotent [4]. A recent study by Kempermann et al. [5] in the Proceedings of the National Academy of Sciences of the USA sheds interesting new light on the genetic complexity of the regulation of neurogenesis.
Figure 1.
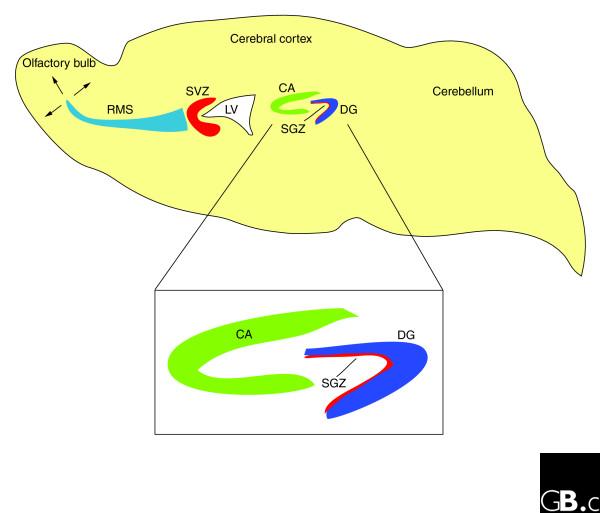
Neurogenic zones in the adult mouse brain. Adult neurogenesis is best characterized in two zones in the adult mouse brain: the subventricular zone (SVZ) adjacent to the lateral ventricle (LV), where neurons are produced that subsequently migrate to the olfactory bulb via the rostral migratory stream (RMS); and the dentate gyrus (DG) of the hippocampus. The hippocampus (shown enlarged in the inset) consists of two interleaved layers of cells - the pyramidal cell layer (CA) and the dentate gyrus. Proliferating neural precursors and quiescent neural stem cells are found in a zone immediately adjacent to the dentate gyrus called the subgranular zone (SGZ).
Genetic differences in adult dentate gyrus neurogenesis
Several groups have found significant differences in proliferation and neuronal survival in the dentate gyrus between several common mouse strains, suggesting a strong degree of genetic regulation of this process. In an earlier study, Kempermann and colleagues [6] used stereology, the quantitative analysis of neurological parameters, in combination with sequential labeling of S-phase cells by bromodeoxyuridine (BrdU) injections to show that genetic variation among strains accounted for differences in all aspects of hippocampal neurogenesis, proliferation, survival and differentiation, as well as overall hippocampal volume and total cell numbers. Proliferation was found to be the highest in the C57BL/6 strain, for instance, whereas CD1 mice displayed the greatest survival of new cells and the 129/SvJ strain produced more astrocytes than any other, as detected by the glial marker glial fibrillary acid protein (GFAP). Using cumulative BrdU labeling at closely spaced intervals, Hayes and Nowakowski [7] attempted to label all of the proliferating cells in the dentate gyrus to estimate the size of the dividing population. These authors compared proliferation, cell-cycle length, and cell survival between C57BL/6 and BALB/cByJ mouse strains and found that, although the size of the proliferating population of cells in the dentate gyrus was twofold greater in the C57BL/6 strain, there were no significant differences between strains in the length of the cell cycle or the amount of cell death. Together, these studies show that although the environmental and molecular influences are significant, there is also a very strong genetic influence on a complex quantitative trait like hippocampal neurogenesis. The molecular basis of these genetic influences remains relatively obscure. Several groups are therefore attempting to unravel some of the genetic determinants that influence phenotypic changes in the hippocampus.
Quantitative trait locus analysis of hippocampal neurogenesis
In the follow-up study to their earlier work [6], Kempermann et al. [5] use a systems-genetics approach to identify phenotypic variance in proliferation, survival and neurogenesis within the hippocampus of two adult mouse inbred strains - BXD and AXB/BXA - using quantitative trait locus (QTL) analysis. A QTL is identified when there is a strong association between a genotype and the quantitative trait phenotype; the association may result either from the interaction of several QTLs or from an interaction between a QTL and the environment that results in phenotypic consequences [8]. Expanding on the authors' previous observations [6], the rates of cell proliferation, survival and neural differentiation were quantified for each strain (in other words, these were treated as quantitative traits). Using these numbers and the WebQTL database [9], Kempermann et al. [5] showed a significant correlation between cell survival and neurogenesis, indicating that 85% of the variance in neurogenesis between strains could be accounted for by different cell-survival rates. Interestingly, proliferation is only a mild predictor of neurogenesis, which agrees with an earlier report [7] that concluded that differences in proliferation had little effect on neurogenesis in the mouse hippocampus.
By examining a web-based transcriptome database [10] and looking for transcripts whose abundance correlated with at least two of the possible phenotypes (proliferation, survival, neurogenesis, or astrocyte differentiation), Kempermann et al. [5] generated a list of 190 candidates for genes involved in these traits. This list was further subdivided into cis- or trans-acting genes on the basis of linkage-analysis criteria, and 21 genes were found to be cis-acting - that is, acting directly at a locus controlling the trait. A number of transcripts correlated with proliferation, survival and neurogenesis, including musashi (Msi1h), a gene with a known function in stem-cell self-renewal and asymmetric cell division [11,12]. Future studies using in vitro functional studies and/or knock-in strategies should be performed to confirm the genes that determine a quantitative trait [8]. This study [5] sheds some light on the complexity of the genetic control of neurogenesis in the adult brain, but it leaves the elucidation of exactly how the identified genes contribute to neurogenesis to future studies.
Because neurogenesis is a complex quantitative trait that probably involves genes at several loci, a common strategy is to begin the QTL analysis by finding correlations between alleles at known chromosomal locations and differences in a simpler quantitative trait, such as hippocampal size or structure [8,13]. As genetic variation is highly heritable (around 50%), recombinant inbred (RI) mouse strains have been used to identify the genetic basis of variation in gene expression. For example, QTL analysis of the BXD recombinant inbred and parental mouse strains has mapped two genetic loci, Hipp1a and Hipp5a, that modulate both neuron number in the dentate gyrus and hippocampal weight [13]. Two candidate genes for the control of neurogenesis within these loci include those encoding retinoic acid receptor γ (Rxrg) and fibroblast growth factor receptor 3 (Fgfr3), but it remains to be determined whether either of these genes are involved in controlling neuron number or hippocampal weight. More recently, Chesler et al. [10] examined gene-expression microarrays of the BXD inbred strain and used information about transcript abundance to map QTLs that modulate gene expression. By combining these two techniques (gene expression and QTL analysis), these authors were able to identify QTLs that modulate single-gene transcription and to identify gene networks in the brain.
The biology of neurogenesis
To make progress in understanding how genetic variation might control dentate gyrus neurogenesis, it is important to consider the available information on the genetic control of neural precursor proliferation and neuronal survival in the setting of the evolving understanding of the biology of the system. Neurogenesis in the adult brain is a dynamic process, involving asymmetric division of a stem or progenitor cell balanced by naturally occurring cell death, which selects a subset of cells that will survive and integrate as functional neurons. Several investigators have successfully quantified the amount of proliferation, cell death and differentiation in the dentate gyrus by examining the 'life cycle' of dentate gyrus granule neurons, believed to participate in learning and memory, finding correlations between the numbers of dividing cells and the stage-specific expression of markers as well as the ultimate percentage of surviving cells [14,15]. These studies [14,15] were performed using rodents kept in typical housing conditions, but many other studies have revealed a range of physiological and environmental factors influencing adult hippocampal neurogenesis - including age [16], how enriched the environment of the animals is [17], and the level of physical activity [18-21]. In particular, an enriched environment or voluntary exercise significantly increased the proliferation and survival of cells in the dentate gyrus, and this was accompanied by enhanced long-term potentiation, defined as the strengthening of the connection between neurons [20].
It has been suggested that the regulation of proliferation of neural precursor cells in the dentate gyrus is controlled separately from the ability of these cells to survive to maturity. Gould and colleagues [22] found that participation in learning trials had a large effect on the percentage survival of newly born dentate gyrus granule neurons with little effect on proliferation of precursors. In contrast, Gage and colleagues [21] found that when mice were running in a wheel (in an otherwise non-enriched environment) the most dramatic effect was on the number of proliferating cells in the dentate gyrus, and that in this case the increased number of mature neurons was due primarily to an increased rate of birth of new neurons without any change in the percentage survival. Thus, even with these rather global approaches, there seem to be at least two distinct control nodes for neurogenesis - proliferation of precursors, and survival of the resulting newborn cells (Figure 2).
Figure 2.
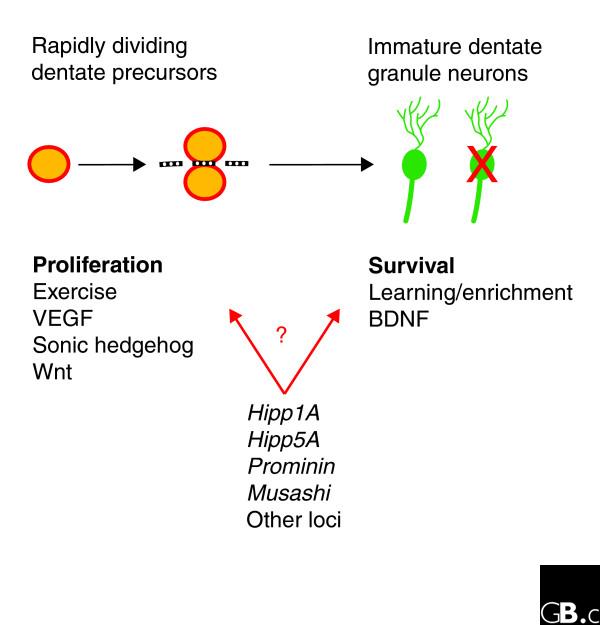
Neurogenesis is regulated in part by distinct groups of factors. Two discrete steps in dentate gyrus neurogenesis appear to be under separate genetic and biological controls. Proliferation of neural precursor cells is directly regulated by a set of physiological and biological factors, some of which are listed here, whereas survival of newly produced neurons is controlled by a separate group of factors. Several genetic loci and candidate genes that have been suggested by QTL studies to regulate neurogenesis are shown; whether these directly regulate neuronal proliferation or survival is still unclear (as indicated by the question mark). VEGF, vascular endothelial growth factor; BDNF, brain-derived neurotrophic factor.
Recent studies have begun to shed light on the specific molecular signals that control dentate gyrus neurogenesis molecular factors that might be regulated by physiological and environmental stimuli like those described above. Signaling by two primary developmental signaling networks those stimulated by the extracellular signaling proteins Sonic hedgehog (Shh) and Wnts has been shown to regulate dentate gyrus neurogenesis. Preliminary indications are that Shh primarily regulates precursor proliferation, whereas Wnts regulate multiple steps in neurogenesis [23]. Interestingly, neurotrophins (for example, brain-derived neurotrophic factor (BDNF)) primarily regulate the survival of immature neurons [22,24], whereas vascular endothelial growth factor (VEGF) appears to selectively control precursor proliferation without affecting the percentage of surviving neurons [25] (Figure 2). In addition, a number of stress hormones and related neurotransmitters produced by afferent neurons to the dentate gyrus have selective effects on precursor proliferation, neuronal survival, or both [26,27]. Given the complex variety of molecular and physiological influences on these two major indices of neurogenesis - proliferation and survival - it seems likely that understanding the regulation of neurogenesis under physiological conditions in behaving animals will further the study of the regulation of complex networks controlling biological processes. One approach for such studies is the use of QTL mapping to define the roles of multiple genes that contribute to the regulation of physiological events.
In summary, the recent study by Kempermann et al. [5] found correlations between a number of transcripts across several loci and phenotypes associated with adult neurogenesis. The authors conclude that a complex phenomenon such as adult neurogenesis is likely to be controlled by the interaction of several regulatory loci involving many genes, and not one master regulatory locus acting as a switch to turn neurogenesis 'on' or 'off'. Future studies will require large sample sizes to precisely map QTLs; they should not be focused on the contribution of an individual gene, but instead should be aimed at understanding how loci behave within regulatory genetic networks. It will also be interesting to determine to what degree these regulatory genetic networks intersect with the known molecular controls on dentate gyrus neurogenesis.
References
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2006;103:780–785. doi: 10.1073/pnas.0510291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/S0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK, Bennett B, Blankenhorn EP, Blizard DA, Bolivar V, Brockmann GA, et al. The nature and identification of quantitative trait loci: a community's view. Nat Rev Genet. 2003;4:911–916. doi: 10.1038/nrg1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Wang J, Williams RW, Manly KF. WebQTL: rapid exploratory analysis of gene expression and genetic networks for brain and behavior. Nat Neurosci. 2004;7:485–486. doi: 10.1038/nn0504-485. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Shou S, Qu Y, Gu J, Wang J, Hsu HC, Mountz JD, Baldwin NE, Langston MA, et al. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet. 2005;37:233–242. doi: 10.1038/ng1518. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T. Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res. 2005;306:349–356. doi: 10.1016/j.yexcr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Lu L, Airey DC, Williams RW. Complex trait analysis of the hippocampus: mapping and biometric analysis of two novel gene loci with specific effects on hippocampal structure in mice. J Neurosci. 2001;21:3503–3514. doi: 10.1523/JNEUROSCI.21-10-03503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/S0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Pozniak CD, Pleasure SJ. A tale of two signals: Wnt and Hedgehog in dentate neurogenesis. Sci STKE. 2006;2006:pe5. doi: 10.1126/stke.3192006pe5. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]


