Abstract
Cattle infected with Neospora caninum readily experience transplacental parasite transmission, presumably after maternal parasitemia, leading to abortion or birth of congenitally infected calves. Cytotoxic T lymphocytes (CTL) are important mediators of protective immunity against Toxoplasma gondii, an intracellular apicomplexan protozoan closely related to N. caninum. In this study, N. caninum-specific CTL expanded from peripheral blood mononuclear cells of two major histocompatibility complex-mismatched, experimentally infected cattle were identified by using a 51Cr release cytotoxicity assay. Enrichment and blocking of CD4+- and CD8+-T-lymphocyte effector subsets indicated that CD4+ CTL killed N. caninum-infected, autologous target cells and that killing was mediated through a perforin/granzyme pathway. Detection and characterization of CTL responses to N. caninum in the natural, outbred, bovine host will facilitate identification of immunogens and design of immunization strategies to induce parasite-specific CTL against transplacental N. caninum transmission in cattle.
Neosporosis, caused by the apicomplexan protozoan parasite Neospora caninum, is a major cause of infectious abortion and congenital disease in cattle worldwide (16). Unlike other infectious causes of abortion in cattle, vaccines proven effective at limiting outbreaks or preventing future abortions are not available; thus, vaccine development to prevent N. caninum-induced abortion is a high research priority. The protective immune responses and antigens to be targeted for vaccine development against neosporosis in cattle are unknown.
Some degree of protective immunity to N. caninum transplacental transmission, a major mode of natural transmission (14, 41), develops in both experimentally and naturally infected cattle. Cattle experimentally infected with N. caninum before pregnancy and challenged with tachyzoites during mid-gestation developed sufficient immunity to protect against vertical transmission (21). In natural herd outbreaks, epidemiologic evidence that supports the development of protective immunity includes the following. (i) Cows with chronic N. caninum infection, as indicated by avidity enzyme-linked immunosorbent assay (ELISA), were less likely to abort N. caninum infected fetuses than cows with acute infection (31). (ii) Epidemic N. caninum-associated abortions did not occur in chronically infected dams (40). (iii) Finally, only 5 to 10% of infected cattle that aborted an infected fetus subsequently aborted a second N. caninum-infected fetus (1, 32). Because some immune protection develops in natural and experimental infections, priming protective immune responses and stimulating memory responses via targeted immunization during critical periods before or during gestation are realistic strategies for limiting congenital neosporosis.
CD8+ T lymphocytes that function as cytotoxic-T-lymphocytes (CTL) are important in immune control of murine and human infections with Toxoplasma gondii, an intracellular protozoan closely related to N. caninum. This has been demonstrated by adoptive transfer (7, 35) and depletion of CD8+-T-lymphocyte populations (18, 34). CTL are present in cattle infected with other intracellular pathogens, including viral (8), bacterial (27), and protozoal (9) agents. CTL are considered major antiparasite effectors in bovine theileriosis, for which the generation of CTL is closely related to control, and macroschizont-infected cells are killed in a major histocompatibility complex (MHC) class I-restricted manner (20). CTL are therefore expected to contribute to immune control of bovine N. caninum infections.
Our overall hypothesis is that induction of N. caninum-specific CTL through immunization will limit transplacental N. caninum transmission in cattle. The purpose of the present study is to demonstrate N. caninum-specific CTL in the peripheral blood of cattle experimentally infected with the parasite and to determine the cell type mediating CTL activity. We describe here a 51Cr release cytotoxicity assay for identifying N. caninum specific CTL in cattle and provide evidence that killing is mediated by CD4+ CTL through a perforin/granzyme secretory pathway. These data lay the foundation for future studies to determine whether N. caninum-specific CTL induced prior to and during gestation will limit transplacental parasite transmission in the outbred, bovine host.
MATERIALS AND METHODS
Cattle.
Two, female, nonpregnant, 5- to 7-year-old Friesian-Holstein cattle (Bos taurus) with disparate MHC haplotypes (Table 1) and seronegative for antibodies to T. gondii and N. caninum were purchased from the Washington State University dairy, Pullman. The cow bovine lymphocyte antigen (BoLA)-A class I alleles were defined by serological typing (13), and their DRB3 alleles were characterized by exon 2 PCR-restriction fragment length polymorphism (RFLP) analysis (47). The DRB3 and DQA alleles associated with these haplotypes in American Holstein cattle have been confirmed by exon 2 cloning and sequencing (J. Y. Park, J. Norimine, and C. J. Davies, unpublished results). Three of the class II haplotypes carried by these cattle (DH8A, DH16A, and DH22C) have duplicated DQA and DQB genes; the fourth haplotype (DH24A) has only one DQA and one DQB gene (3, 12). The BoLA-DQ haplotypes of the cows were inferred from BoLA-A and DRB3 typing on the basis of haplotypes defined in the Fifth and Seventh International BoLA Workshops (11, 12, 26, 38) (BoLA nomenclature website [http://www.projects.roslin.ac.uk/bolahome.html]).
TABLE 1.
MHC haplotypes of N. caninum-infected cattle
| Cow no. | BoLA-A serotype | Class II haplotype | DRB3 PCR-RFLP | DRB3 allele | DQA allele(s) | DQB allele(s) |
|---|---|---|---|---|---|---|
| 495 | A15(A8) | DH22C | 22 | 1101 | 12022 | NDa |
| WSU2-2b | ND | |||||
| A20 | DH8A | 8 | 1201 | 12011 | 1005 | |
| 2201 | 1201 | |||||
| 562 | A11 | DH24A | 24 | 0101 | 0101 | 0101 |
| A12(A30) | DH16A | 16 | 1501 | 10011 | 0102 | |
| 22021 | 1101 |
ND, not determined.
DQA WSU2-2 is a new allele that does not yet have an official name from the BoLA Nomenclature Committee of the International Society for Animal Genetics.
Cattle were infected with an initial dose of 107 N. caninum Nc-1 strain tachyzoites split between intravenous and intramuscular routes. They were reinfected intramuscularly at monthly intervals with 5 × 106 Nc-1 tachyzoites and monitored periodically for persistent N. caninum infection by using a competitive inhibition ELISA specific for N. caninum (5) available through the Washington Animal Disease Diagnostic Laboratory, Pullman.
Parasites.
The Nc-1 strain of N. caninum tachyzoites were maintained by regular serial passage in Vero cells in RPMI 1640 supplemented with 2% fetal bovine serum (HyClone, Logan, Uah), 2 mM glutamine, 10 mM HEPES buffer, and 10 μg of gentamicin/ml. Tachyzoites were isolated from the cells between passages 5 and 25 (6). Vero cell debris was removed by centrifugation at 500 × g for 5 min, and then the supernatant containing tachyzoites was filtered through a 10-μm (pore-size) Magna nylon filter (Osmonics, Inc., Kent, Wash.) to remove additional Vero cell debris. Tachyzoites were washed twice in Hanks balanced salt solution, counted with a hemocytometer by using trypan blue exclusion, and used for infection of experimental cattle and for infection of stimulator and target cells in 51Cr release cytotoxicity assays.
51Cr release cytotoxicity assay.
51Cr release cytotoxicity assays were performed to detect the cytotoxic activity of stimulated lymphocytes from peripheral blood mononuclear cells (PBMC) of N. caninum-infected cattle. PBMC were collected by jugular venipuncture into 1/3 volume acid citrate dextrose and separated over Lymphoprep 1.077 (Axis-Shield PoCAS, Oslo, Norway) according to standard procedures.
(i) Target cells.
Peripheral blood adherent cells (PBAC) were isolated from PBMC by adherence to polystyrene T-75 culture flasks. The PBAC were lifted with 0.5 mM EDTA in cold Hanks balanced salt solution by gentle tapping and scraping with a rubber cell scraper and then were counted on a hemocytometer with trypan blue exclusion and dispensed at 3 × 104 targets/well into 96-well plates in RPMI 1640 supplemented with 10% fetal bovine serum (HyClone), 2 mM glutamine, 10 mM HEPES buffer, and 10 μg of gentamicin/ml with 5 × 10−5 M 2-mercaptoethanol (complete RPMI). Target cells were infected with N. caninum tachyzoites at a multiplicity of infection (MOI) of 3:1 in 100 μl of complete RPMI for 18 h. Immunohistochemical staining of tachyzoite-infected target cells with hyperimmune anti-N. caninum goat serum (VMRD, Inc., Pullman, Wash.) showed that more than 40% of the target cells were infected at this MOI in preliminary experiments (data not shown). Target cells were labeled with 1.25 μCi of 51Cr (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.)/well in 50 μl of complete RPMI for 18 h at 37°C in 5% CO2 and 5% humidity and then washed four times with 200 μl of complete RPMI prior to the killing assay. Initially, primary cultures of autologous dermal fibroblasts established from dermal punch biopsies were used as target cells; however, demonstration of parasite-specific CTL that killed in a manner consistent with MHC restriction by using dermal fibroblasts as targets was unsuccessful.
(ii) Stimulator cells.
Approximately 107 PBAC per T-75 flask were infected with N. caninum tachyzoites at an MOI of 2:1 and, after 18 h, irradiated at 3,000 rads by using a fixed cobalt beam for use as antigen-presenting stimulator cells.
(iii) Effector cells.
For expansion of parasite-specific effector cells from the PBMC of infected cattle, approximately 108 γδ-depleted T lymphocytes were cultured with 107 PBAC stimulator cells in T-75 polystyrene culture flasks for 5 to 7 days in complete RPMI 1640 at 37°C in 5% CO2 and 5% humidity. After the first round of stimulation, the lymphocytes were pelleted, resuspended in complete RPMI with 2 ng of recombinant human interleukin-2 (rhIL-2; R&D Systems, Minneapolis, Minn.)/ml, and expanded for 5 to 7 days. An additional 2 ng of rhIL-2/ml was added to the effector cultures on day 3 of stimulation with rhIL-2.
γδ T lymphocytes were depleted from PBMC prior to stimulation by using complement-mediated lysis. Freshly isolated PBMC were incubated with a murine monoclonal antibody against the bovine δ chain (GB21A) at 1 μg/106 target cells for 60 min in Hanks balanced salt solution at 4°C with tilting and rotation and then washed once in Hanks balanced salt solution. The antibody-labeled PBMC were incubated with rabbit complement (Pel-Freez Clinical Systems, LLC, Brown Deer, Wis.) at a 1:16 dilution in complete RPMI for 30 min at 37°C and then pelleted, incubated with fresh complement at a 1:16 dilution for an additional 30 min, separated over Lymphoprep 1.077 (Axis-Shield PoCAS, Oslo, Norway), and washed twice with complete RPMI. PBMC phenotypes were evaluated by flow cytometry. γδ T lymphocytes commonly comprised 20% of the initial lymphocytes from peripheral blood and were depleted to less than 4% of total lymphocytes by using complement-mediated lysis.
(iv) 51Cr release assay.
Test groups consisted of (i) effectors plus N. caninum infected, autologous, PBAC targets to demonstrate parasite-specific killing consistent with MHC-restriction; (ii) effectors plus uninfected, autologous, PBAC targets as a control for non-parasite-specific killing consistent with MHC restriction; (iii) effectors plus heterologous, infected targets as a control for parasite-specific, non-MHC-restricted killing; and (iv) effectors plus uninfected, heterologous targets to control for non-parasite-specific, non-MHC-restricted killing. Test groups were plated in replicates of three to five at various effector/target cell ratios. After the addition of effector cells, the plates were centrifuged at 500 × g for 30 s and then incubated at 37°C in 5% CO2 and 5% humidity for 4 to 6 h. At the end of incubation, 25 μl of supernatant was harvested and combined with 150 μl of OptiPhase HiSafe 3 scintillation fluid (Fisher Chemicals, Loughborough Leics, United Kingdom). The counts per minute (cpm) were determined in a MicroBeta TriLux beta emission counter (Perkin-Elmer Wallac, Inc., Gaithersburg, Md.) windowed for 51Cr and transformed into the percent specific lysis by using the following calculation: % specific lysis = [(mean cpm of the test sample − mean cpm of the spontaneous release)/(mean cpm of the maximal release − mean cpm of the spontaneous release)] × 100. The percent spontaneous lysis (i.e., mean spontaneous cpm/mean maximal cpm) was <20% in every experiment. The standard error of the mean (SEM) was calculated by using a formula that takes individual variances within maximal, spontaneous, and experimental release wells into account (42). A significant difference in the percent specific lysis between the experimental groups was defined as >2 SEM.
Immunofluorescence flow cytometry.
PBMC and stimulated T lymphocytes were analyzed for cell phenotype by two-color flow cytometry. Lymphocytes (107/ml) were suspended in phosphate-buffered saline (PBS)-1% gamma globulin free horse serum-0.02% sodium azide, and 50 μl of the suspension was incubated with bovine monoclonal antibodies to CD4 (ILA11A), CD8 (CACT 80C or 7C2B), γδ (GB21A), CD3 (MM1A), and CD2 (16-1E10) at 15 μg/ml for 25 min at 4°C, washed three times, and pelleted at 1,500 × g for 2 min. Lymphocytes were resuspended in PBS-0.02% sodium azide and incubated with fluorescein- and phycoerythrin-conjugated murine secondary antibodies (Caltag Laboratories, Burlingame, Calif.) at a 1/100 dilution in PBS-0.02% sodium azide for 25 min at 4°C. The cells were washed twice in PBS-0.02% sodium azide, fixed in 2% formaldehyde in PBS, and stored cold for flow cytometry. The fluorescein-labeled cells were counted and analyzed on a Becton Dickinson FACSscan with CellQuest analysis software (Becton Dickinson, Franklin Lakes, N.J.).
CD8+-T-lymphocyte enrichment.
CD8+ T lymphocytes were enriched by positive selection with magnetic beads. γδ-Depleted lymphocytes that had been cultured with stimulator cells for 5 to 7 days were incubated with a monoclonal antibody to bovine CD8 (CACT 80C and/or 7C2B) at 1 μg/106 cells for 30 min at 4°C with rotation. The cells were then washed, incubated with magnetic beads coated with antibody to murine immunoglobulin G (IgG; Dynal, Oslo, Norway), eight beads/target cell in Hanks balanced salt solution for 30 min at 4°C with rotation, and separated with a magnet. The CD8+ T lymphocytes adhered to beads were placed into complete RPMI with 2 ng of rhIL-2 (R&D Systems)/ml for an additional 5- to 7-day expansion period. An additional 2 ng of rhIL-2/ml was added to the cultures on day 3 of this expansion. After expansion with rhIL-2, the cells were pipetted with moderate vigor to release the cells from the beads and then evaluated for purity the day before the 51Cr release assay by flow cytometry. CD8+-enriched effector populations were >99% CD8+, <1% CD4+, and <1% γδ T lymphocytes.
CD4+-T-lymphocyte enrichment.
CD4 T lymphocytes were enriched by negative selection of CD8 and γδ T lymphocytes with magnetic beads. γδ-Depleted T lymphocytes that had been cultured with stimulator cells for 5 to 7 days were incubated with monoclonal antibodies to bovine CD8 (CACT 80C and/or 7C2B) and bovine γδ T lymphocytes at 1 μg/106 cells for 30 min at 4°C with rotation. The cells were then washed, incubated with magnetic beads coated with antibody to murine IgG (Dynal, Oslo, Norway) at eight beads/target cell in Hanks balanced salt solution for 30 min at 4°C with rotation, and separated with a magnet. The CD8 and γδ T lymphocytes that adhered to beads were discarded, and the remaining lymphocytes were placed into complete RPMI with 2 ng of rhIL-2 (R&D Systems)/ml for an additional 5- to 7-day expansion period. An additional 2 ng of rhIL-2/ml was added to the cultures on day 3 of this expansion. The purity was assessed by flow cytometry, and CD4+ T lymphocytes generally comprised 80 to 99% of the lymphocytes. When necessary, increased purity of CD4+-enriched effector populations was attained with additional negative selection of γδ T lymphocytes and CD8+ T lymphocytes by using magnetic beads and the appropriate antibodies the day before the 51Cr release assay. In several cases, CD3− CD2+ bovine NK-like cells (19) composed ca. 10% of the lymphocyte-gated population, and these cultures were discarded.
Blocking of effector subsets with monoclonal antibodies to bovine CD4 and CD8 T lymphocytes and concanamycin A.
To determine the lymphocyte type mediating CTL activity, aliquots from the expanded, γδ-depleted effector cultures were incubated with monoclonal antibodies to bovine CD4 (30 μg of ILA11A/ml) and/or a cocktail of monoclonal antibodies to bovine CD8 (15 μg of CACT80C/ml plus 15 μg of 7C2B/ml) in complete RPMI-10% fetal bovine serum at 4°C for 1 to 2 h before and throughout the 51Cr release assay. To determine whether the CD8 T lymphocyte subset (blocked with antibodies to CD4) and the CD4-T-lymphocyte subset (blocked with antibodies to CD8) mediated killing via a perforin/granzyme pathway, 10 nM concanamycin A (Sigma) was added to CD4-blocked and CD8-blocked effector groups for 1 to 2 h before and throughout the 51Cr release assay. In another experiment with cow 495 effectors (data not shown), CD8+ T lymphocytes were depleted from the bulk-expanded effector population, and the effectors were 95% CD4+ T lymphocytes, <1% CD8+ T lymphocytes, and <1% γδ T lymphocytes. Effectors were incubated with 10 nM concanamycin A for 3 h at 4°C and then washed twice prior to use in a 4.5-h 51Cr release cytotoxicity assay. To control for possible nonspecific drug toxicity of concanamycin A on the effector population, a simultaneous 3H proliferation assay was performed. For the proliferation assay, the same effector groups used in the 51Cr release assay were incubated with or without 10 nM concanamycin A, washed after a 3-h incubation at 4°C, and placed in a 3-day 3H proliferation assay. For the proliferation assay, 3 × 105 autologous PBMC irradiated at 3,000 rads as antigen-presenting cells and 2 × 104 effectors per well were combined with medium alone, 10 μg of sonicated N. caninum tachyzoite antigen/ml, or 5 μg of the mitogen concanavalin A/ml. All antibodies for flow cytometry, magnetic bead sorting, and effector subset blocking were purchased from VMRD.
RESULTS
N. caninum-infected cattle developed parasite-specific CTL.
Two Holstein cattle, 495 and 562, with disparate MHC haplotypes were infected with N. caninum tachyzoites. At 2 weeks after the initial N. caninum inoculation, a competitive inhibition ELISA specific for N. caninum (5) was positive for both cows. Cattle were reinfected at monthly intervals, and both cattle remained seropositive for antibodies to N. caninum by competitive inhibition ELISA throughout the study.
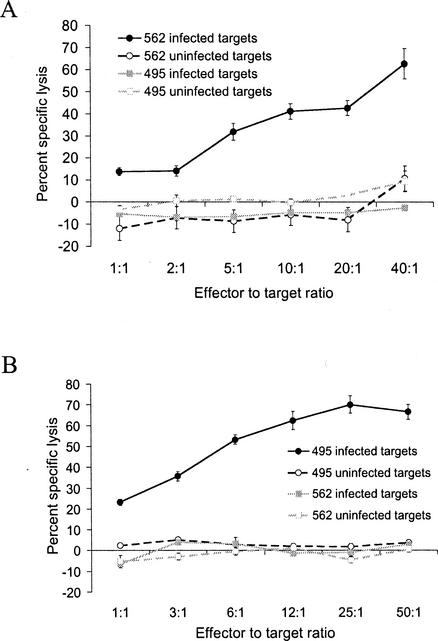
γδ-T-lymphocyte-depleted PBMC from the infected cattle were cultured in the presence of tachyzoite-infected, irradiated PBAC for 5 to 7 days and expanded with rhIL-2 for 5 to 7 days. Results representative of three 51Cr release cytotoxicity assays per cow obtained with expanded, γδ-depleted effectors and N. caninum-infected and uninfected autologous and heterologous targets for both cows are shown in Fig. 1. Killing of autologous, infected targets by γδ-T-lymphocyte-depleted effectors from both cattle was significantly higher (>2 SEM) than killing of autologous uninfected targets, heterologous infected targets, and heterologous uninfected targets. These data show dose-dependent, parasite-specific killing by in vitro-stimulated T-lymphocyte effectors from two cattle with different MHC haplotypes. The effectors from both MHC-mismatched cattle failed to kill heterologous, infected, and uninfected targets, and the killing is therefore consistent with MHC restriction.
FIG. 1.
Killing of N. caninum-infected adherent cell targets by stimulated T-lymphocyte effectors from N. caninum-infected cow 562 (A) and cow 495 (B) was dose dependent, parasite specific, and consistent with MHC restriction. Graphs shown are from single chromium release cytotoxicity assays representative of three assays per cow. The percent specific lysis of infected autologous targets is significant (>3 SEM) compared to uninfected autologous targets and infected and uninfected heterologous target cells. Cow 495 effectors were determined to be CD4+ 99%, CD8+ <1%, and <1% γδ T lymphocytes by flow cytometry. Cow 562 effectors were determined to be CD4+ 86%, CD8+ 12%, and <1% γδ T lymphocytes by flow cytometry. Error bars indicate the SEM.
Effect of monoclonal antibodies to CD4 and CD8 T lymphocytes on cytolytic activity of effectors.
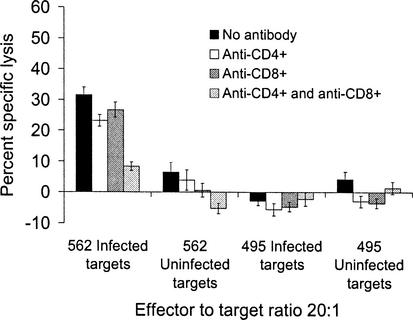
Murine CD8+ CTL kill target cells infected with T. gondii (34), and human CD4+ CTL kill T. gondii-infected targets (33). Because CD4+ T lymphocytes frequently comprised more than 80% of our expanded, γδ-depleted, bulk effector cultures, blocking experiments were performed to determine whether killing was due to CD8+- and/or CD4+-T-lymphocyte effector subsets. When monoclonal antibodies to bovine CD8 were used to block killing by the CD8+-T-lymphocyte subset, the unblocked, CD4+-T-lymphocyte effector population killed infected, autologous targets (Fig. 2 and 4). When CD4+-T-lymphocyte effectors were blocked with monoclonal antibodies to CD4+, the unblocked, CD8+-T-lymphocyte effector population killed infected, autologous targets in some (Fig. 2) but not all (Fig. 4) experiments. When subsets were concurrently blocked with monoclonal antibodies to both CD4 and CD8 in four replicate experiments, killing of autologous, infected targets was reduced to near-background levels (<10% specific lysis), suggesting that the combined antibodies effectively blocked killing by both CTL subsets (Fig. 2 and 4). The results of subset blocking in Fig. 4 suggested that only CD4+ T lymphocytes in this expanded effector population functioned as CTL.
FIG. 2.
Blocking of T-lymphocyte effectors with monoclonal antibodies to CD4 and CD8 suggested that both CD4+ and CD8+ T lymphocytes were parasite-specific, MHC-restricted CTL. Killing was present without antibody blocking, when CD4+-T-lymphocyte effectors were blocked and when CD8+-T-lymphocyte effectors were blocked. When subsets were concurrently blocked, killing was reduced by 75%. Results are from a single experiment with cow 562 effectors, with findings representative of two experiments per cow. The effectors were 95% CD4+, 1% CD8+, and <1% γδ T lymphocytes by flow cytometry. Error bars indicate the SEM.
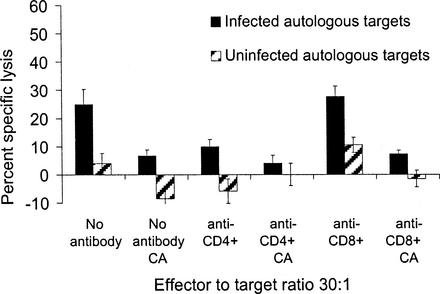
FIG. 4.
Concanamycin A, a potent inhibitor of the perforin/granzyme pathway, blocked CTL killing of N. caninum-infected targets. Mixed effectors were incubated with or without monoclonal antibodies to CD4 or CD8 with or without concanamycin A (CA) in the cytotoxicity assay. In all groups treated with concanamycin A, killing was blocked by at least 72%. The percent specific lysis of CD4+ effectors was reduced by 74% when the perforin pathway was blocked (anti-CD8+ plus concanamycin A group compared to anti-CD8+ alone group). Effectors were 86% CD4+, 11% CD8+, and <1% γδ T lymphocytes as determined by flow cytometry. The results are one of two similar experiments. Error bars indicate the SEM.
N. caninum-specific CD4+ T lymphocytes are CTL.
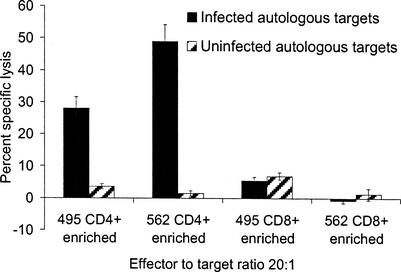
Blocking of effector subsets by antibodies suggested that in some cases CD8+ T lymphocytes functioned as CTL and, in other instances, CD8+ T lymphocytes did not contribute to killing. It was not possible to consistently expand the same relative percentages of CD4+- and CD8+-T-lymphocyte effectors from the bulk, γδ-depleted, stimulated effector populations, and CD8+ T lymphocytes generally comprised 1 to 11% of the total mixed effector population. Because CD8+ T lymphocytes were often a small percentage of the effectors and antibody blocking experiments yielded equivocal results, immunomagnetic selection of subsets was undertaken to further clarify whether both the CD4+ and CD8+ subsets functioned as CTL. When CD8+ T lymphocytes were depleted from bulk-cultured effectors to <1% of the effector population in each of five replicate experiments per cow, the CD4+-T-lymphocyte-enriched subset killed autologous targets infected with N. caninum (Fig. 3). The results indicated that N. caninum-specific CD4+ CTL were expanded from the peripheral blood of experimentally infected cattle. For five replicate experiments with CD4+-enriched effectors, cultures consisted of 90 to 100% CD4+ T lymphocytes, <1% CD8+ T lymphocytes, <1% γδ T lymphocytes, 90 to 100% CD3+ CD2+ T lymphocytes, and <1% CD3− CD2+ T lymphocytes by flow cytometry. Bovine NK-like cells (CD33− CD2+) (19) and γδ T lymphocytes were each <1% of the effector populations. When CD8+ T lymphocytes were positively selected with magnetic beads from expanded effector cultures, parasite-specific killing by the CD8+-enriched subset was not demonstrated (Fig. 3). Enriched CD8+ effector populations in all replicate experiments were 99 to 100% CD8+ T lymphocytes, <1% CD4+ T lymphocytes, <1% γδ T lymphocytes, 90 to 100% CD3+ CD2+ T lymphocytes, and <1% CD3− CD2+ T lymphocytes by flow cytometry. Replicate experiments were performed by using effector to target ratios of 10:1 or 20:1.
FIG. 3.
CD4+-enriched, but not CD8+-enriched T-lymphocyte effectors killed autologous, N. caninum-infected targets. For all experiments with CD4+-enriched effectors, cultures were 90 to 100% CD4+ T lymphocytes, <1% CD8+ T lymphocytes, <1% γδ T lymphocytes, 90 to 100% CD3+ CD2+ T lymphocytes, and <1% CD3− CD2+ T lymphocytes as determined by flow cytometry. CD8+-enriched effector populations in all replicate experiments were 99 to 100% CD8+, <1% CD4+, <1% γδ, 90 to 100% CD3+ CD2+, and <1% CD3− CD2+ T lymphocytes. Results for CD4+-enriched effectors are representative of five similar assays per cow (effector/target ratios of 20:1 and 10:1). Results for CD8+-enriched effectors are one of two replicate experiments for cow 492 and one of five similar replicates for cow 562. Error bars indicate the SEM.
Depletion and enrichment of T-lymphocyte subsets indicated that CD4+ CTL were expanded from PBMC of N. caninum-infected cattle, whereas CD8+ CTL at effector/target ratios of 10:1 and 20:1 were not detected in 51Cr release assays.
Killing by N. caninum-specific, CD4+ CTL was reduced when the perforin/granzyme pathway was blocked in vitro.
To determine a potential mechanism of cell killing, effector populations were treated with concanamycin A, a metabolic inhibitor of the perforin/granzyme pathway (22, 52). Aliquots from the mixed effector population were incubated with or without monoclonal antibodies to CD4 or CD8 with or without concanamycin A in the following combinations: (i) no antibodies, (ii) no antibodies and concanamycin A, (iii) anti-CD4, (iv) anti-CD4 and concanamycin A, (v) anti-CD8, and (vi) anti-CD8 and concanamycin A. Figure 4 is a single representative of two replicate experiments. Effectors from cow 495 were 86% CD4+, 11% CD8+, <1% γδ, and <1% CD3− CD2+ T lymphocytes. In all treatment groups blocked with concanamycin A, the percent specific lysis was reduced by at least 72%. The percent specific lysis was reduced by 60% with blocking of the CD4+ effector population and by 74% when both CD4+ and the perforin pathway were blocked. The percent specific lysis was not reduced with blocking of the CD8+ effector population but was reduced by 72% when both CD8+ and the perforin pathway were blocked. Similar results were obtained by using purified CD4+ effector cells obtained by negative selection (effectors were 95% CD4+, <1% CD8+, and <1% γδ T lymphocytes). Effectors were incubated with 10 nM concanamycin A for 3 h and then washed twice prior to use in a 4.5-h 51Cr release cytotoxicity assay. A simultaneous 3H proliferation assay was performed with the same effector population to control for nonspecific drug toxicity. When the perforin/granzyme pathway was blocked with 10 nM concanamycin prior to the 51Cr release assay, killing by the purified CD4+-T-lymphocyte effectors was reduced by 40%, a statistically significant reduction compared to control cells not treated with concanamycin A. In a simultaneous 3H proliferation assay, the effectors exposed to media alone or concanamycin A had an equally potent proliferative response to mitogen (stimulation indices of 25 and 30, respectively), indicating that treatment with concanamycin A did not affect effector cell viability in the 51Cr release assay. Inhibition of the perforin/granzyme pathway of either bulk-cultured CD4+-T-lymphocyte effectors blocked with anti-CD8+ antibody or purified CD4+ T lymphocytes with magnetic beads reduced target cell lysis. Therefore, one mechanism of in vitro killing by CD4+ CTL expanded in this experimental system was via perforin-granzyme release.
DISCUSSION
We showed here that two, nonpregnant, adult, female, Holstein cattle with disparate MHC haplotypes and experimentally infected with tachyzoites of N. caninum developed parasite-specific CD4+ T lymphocytes that killed autologous, infected targets, but not heterologous, infected targets. Killing mediated by the CD4+ T lymphocytes was therefore consistent with parasite specificity and MHC restriction. Furthermore, blocking of the perforin/granzyme pathway significantly reduced killing mediated by CD4+ CTL, suggesting that N. caninum-specific, bovine CD4+ CTL could kill through the perforin/granzyme pathway.
Studies investigating the mammalian immune response to infection with N. caninum, primarily performed in mice, indicate that both CD4+ and CD8+ T lymphocytes (6, 23, 36) and increased levels of IFN-γ (28, 36) are important for immune control. In cattle infected with N. caninum, T lymphocytes proliferate and produce gamma interferon (IFN-γ) in response to stimulation with parasite antigens (30), suggesting that a cell-mediated immune response and increased levels of IFN-γ could also be important in resistance to infection in cattle. The finding of N. caninum-specific CTL in the peripheral blood of experimentally infected cows is consistent with the paradigm of immune control of intracellular protozoa: that infection induces a protective immune response mediated by T lymphocytes. Indeed, it is well established that IFN-γ and CTL are important in immune control of the closely related protozoan parasite T. gondii (15); however, the relative importance of CTL in the cell-mediated immune response of cattle to N. caninum infection, and the role of CTL in controlling transplacental transmission requires further investigation.
In cattle chronically infected with N. caninum, data consistent with a recrudescence of parasitemia and increased maternal parasite numbers during mid-gestation include (i) IFN-γ secretion and T-lymphocyte proliferative capacity are decreased in mid-gestation regardless of N. caninum infection status (21), (ii) N. caninum antibody titers rise in mid to late gestation irrespective of time of year or parity (43) as an indicator of increased parasite numbers, and (iii) fetal infection is associated with a marked increase in maternal N. caninum antibody (17). Decreased immune resistance in pregnant cattle infected with N. caninum may result in greater numbers of parasites crossing the placenta and increased severity of fetal lesions, culminating in abortion. Because transplacental parasite transmission is a primary method of acquisition and parasite persistence within a herd (2, 14, 41), decreasing vertical transmission by efficacious maternal immunization could provide control of bovine neosporosis.
Our hypothesis is that induction of N. caninum-specific CTL through targeted immunization will limit transplacental N. caninum transmission in cattle. We assume that N. caninum transplacental transmission in cattle is dependent upon maternal parasitemia, an assumption supported by data from a murine congenital transmission model in which an increased maternal parasite dose correlated with an increased rate of vertical parasite transmission (Timothy Baszler, Washington State University, unpublished data). The development of a 51Cr release cytotoxicity assay for in vitro characterization of N. caninum-specific bovine CTL was a crucial step toward investigating the role of CTL in limiting transplacental parasite transmission and neosporosis abortion in cattle.
The cattle used in these experiments had disparate BoLA-A and DRB alleles as determined by serotyping and PCR-RFLP analysis, respectively. Furthermore, the inferred DQA and DQB alleles carried by the two experimentally infected cattle were also mismatched. The MHC typing data are consistent with the observed lack of allogeneic killing by CTL in 51Cr release assays. The CD4+-T-lymphocyte-mediated cytotoxicity identified in the present study was consistent with MHC-restricted killing and was likely restricted by the MHC class II molecules as seen in other examples of CD4+ CTL (44, 51, 53).
T. gondii-specific CD4+ CTL have been identified in humans with toxoplasmosis (10, 33, 37, 51); however, the relative importance of CD4+ and CD8+ CTL and the biologic significance of CD4+ CTL in toxoplasmosis are not known. Whether CTL exert protective effects by killing the intracellular parasites is a subject of debate, since one study indicated that lysis of T. gondii-infected cells did not lead to parasite death (50). However, lysis of infected cells could facilitate phagocytosis of parasites and parasite antigens released from the parasitophorous vacuole by activated macrophages and result in increased presentation of parasite antigens in the context of MHC class II molecules (15, 29). This would likely result in T-lymphocyte secretion of IFN-γ (45), a cytokine that enhances CTL activity in T. gondii-specific, human CD4+ CTL (51) and that has parasite antiproliferative effects (46, 49).
Our findings are consistent with studies in which human T. gondii-specific CD4+ CTL were expanded in vitro by using similar culture conditions (10, 33, 37, 51) and concur with depletion and enrichment experiments in which the human CD8+ T lymphocyte subset also failed to kill infected targets (37). The expansion of CTL in vitro by stimulating T lymphocytes with N. caninum tachyzoite-infected PBAC may not be representative of in vivo subset expansion, and the use of PBAC as stimulators may preferentially expand CD4+ CTL in vitro. The PBAC used as stimulators adhered to polystyrene culture flasks, had the morphology of macrophages, and were likely primarily mononuclear phagocytes that expressed both MHC class I and class II molecules. In our stimulator cultures, the tachyzoites might actively invade PBAC and reside in parasitophorous vacuoles. In the case of active invasion, some intracellular parasite antigens from the parasitophorous vacuoles could be processed through the endogenous antigen-processing pathways for presentation in the context of MHC class I molecules and recognition by CD8+ CTL. On the other hand, many tachyzoites and tachyzoite antigens in the cultures could also be phagocytosed and enter the exogenous phagolysosomal antigen-processing pathway for presentation in the context of MHC class II molecules and recognition by CD4+ CTL. Antigen processing and presentation primarily through the exogenous pathway might preferentially expand CD4+ CTL. The percentage of CD8+ T lymphocytes from the peripheral blood of the infected cattle was commonly 20%, whereas after a 1-week stimulation, the CD8+-T-lymphocyte population generally dropped to 1 to 11% of the total effector population. Stimulation of effectors by parasite antigens primarily processed through the exogenous antigen-processing pathway and presented in the context of MHC class II molecules may be one explanation for selective expansion of CD4+ CTL in this culture system.
In the present study, inhibition of the perforin/granzyme pathway abrogated killing by N. caninum-specific CD4+ CTL. Other instances of killing by CD4+ CTL through perforin/granzyme pathways are found in intracellular infections of humans (4, 24, 52) and mice (48). In a murine toxoplasmosis model, when the Fas/FasL pathway of T. gondii-specific CD8+ T lymphocytes was inhibited, killing of infected cells by CTL and parasite proliferation within cells was not altered, whereas inhibition of the granule exocytosis pathway resulted in a significant reduction in the cytotoxic and parasite antiproliferative effects of CD8+ T lymphocytes (34). Granulysin, a protein released from intracellular compartments during granule exocytosis, has demonstrated antimicrobial activity (39) and may contribute to the antiproliferative effects of CTL in human toxoplasmosis. These observations lend support for a protective role mediated by CTL using granule exocytosis pathways in resistance to infections caused by apicomplexan protozoa. Recent studies suggest a link between the perforin/granzyme and Fas/FasL killing pathways. FasL has been localized to cytoplasmic granules that stored perforin and granzyme and may participate in granule-mediated cytotoxicity (25). The killing pathways used by CTL may not be mutually exclusive, and the relative importance of Fas/FasL and perforin/granzyme-mediated killing by CTL in N. caninum infection remains to be elucidated.
Blocking with antibody to CD4+ significantly reduced killing by the effector population in some experiments (Fig. 4), suggesting that the unblocked CD8+ effector subset did not contribute to killing. In other instances of blocking with antibody to CD4+, killing, presumably by the unblocked CD8+ effector subset, remained (Fig. 2). One explanation for this apparent discrepancy was incomplete blocking by the anti-CD4+ antibody, since in later experiments with immunomagnetically purified CD4+ T lymphocytes this subset had cytolytic activity (Fig. 3), whereas purified CD8+ T lymphocytes did not kill infected, autologous targets. Another possible explanation is that frequencies of CD4+ and CD8+ CTL varied over the time course of experimental infection. Cattle were reinfected with tachyzoites at monthly intervals to maintain persistent infection, but blood for assays was drawn irrespective of reinfection dates. It is possible that CD4+ and CD8+ CTL memory frequencies and ratios differed throughout the time course of reinfections and that CD8+ CTL could have been present in antibody blocking experiments performed earlier in infection.
The presence of N. caninum-specific CTL that kill infected targets suggests that a parasite-specific immune response can be mediated by CTL in infected Holstein cattle, a natural, outbred host for this infection. CD4+ CTL that kill through perforin-granzyme release may indicate an adaptive, preferential use of secretory pathways due to antiproliferative effects of granule contents such as granulysin. Induction of N. caninum-specific CTL in cattle immediately prior to gestation and in critical periods of suppressed natural immunity during gestation could limit maternal parasitemia either after acute or recrudescent infection. Priming the immune system of adult, naive cattle with N. caninum antigens restricted by specific Holstein MHC haplotypes and induction of sufficient numbers of memory CTL could result in decreased parasite numbers, decreased rate and severity of transplacental parasite transmission, and reductions in abortions due to N. caninum. Experiments that determine N. caninum CTL epitopes presented by specific bovine MHC molecules could also lead to identification of Holstein cattle with MHC haplotypes that are more resistant to N. caninum abortion and aid in the selection of sires used in artificial insemination breeding programs.
The studies presented here will facilitate (i) selection of immunogens that induce parasite-specific CTL in cattle, (ii) mapping of N. caninum epitopes that are presented by common Holstein MHC class II haplotypes, and (iii) testing whether induction of N. caninum specific CTL will limit transplacental N. caninum transmission in the outbred bovine host.
Acknowledgments
This research was supported by National Institutes of Health, U.S. Department of Health and Human Services, Public Health Service grant 1K08 AI49182-01 and by research grant US 2913-97 from BARD, the United States-Israel Binational Agricultural Research Development Fund.
We gratefully acknowledge the excellent technical support of Bruce Mathison and sincerely thank Colleen Olmstead for MHC haplotyping our experimental cattle.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anderson, M. L., C. W. Palmer, M. C. Thurmond, J. P. Picanso, P. C. Blanchard, R. E. Breitmeyer, A. W. Layton, M. McAllister, B. Daft, H. Kinde, et al. 1995. Evaluation of abortions in cattle attributable to neosporosis in selected dairy herds in California. J. Am. Vet. Med. Assoc. 207:1206-1210. [PubMed] [Google Scholar]
- 2.Anderson, M. L., J. P. Reynolds, J. D. Rowe, K. W. Sverlow, A. E. Packham, B. C. Barr, and P. A. Conrad. 1997. Evidence of vertical transmission of Neospora sp. infection in dairy cattle. J. Am. Vet. Med. Assoc. 210:1169-1172. [PubMed] [Google Scholar]
- 3.Andersson, L., and L. Rask. 1988. Characterization of the MHC class II region in cattle: the number of DQ genes varies between haplotypes. Immunogenetics 27:110-120. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., J. J. Zaunders, L. Papagno, J. Sutton, A. Jaramillo, A. Waters, P. Easterbrook, P. Grey, D. Smith, A. J. McMichael, D. A. Cooper, S. L. Rowland-Jones, and A. D. Kelleher. 2002. Characterization of CD4+ CTLs ex vivo. J. Immunol. 168:5954-5958. [DOI] [PubMed] [Google Scholar]
- 5.Baszler, T. V., S. Adams, J. Vander-Schalie, B. A. Mathison, and M. Kostovic. 2001. Validation of a commercially available monoclonal antibody-based competitive-inhibition enzyme-linked immunosorbent assay for detection of serum antibodies to Neospora caninum in cattle. J. Clin. Microbiol. 39:3851-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baszler, T. V., M. T. Long, T. F. McElwain, and B. A. Mathison. 1999. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int. J. Parasitol. 29:1635-1646. [DOI] [PubMed] [Google Scholar]
- 7.Casciotti, L., K. H. Ely, M. E. Williams, and I. A. Khan. 2002. CD8+-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect. Immun. 70:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childerstone, A. J., L. Cedillo-Baron, M. Foster-Cuevas, and R. M. Parkhouse. 1999. Demonstration of bovine CD8+ T-cell responses to foot-and-mouth disease virus. J. Gen. Virol. 80:663-669. [DOI] [PubMed] [Google Scholar]
- 9.Conze, G., J. D. Campbell, A. K. Nichani, E. J. Glass, R. L. Spooner, and J. S. Ahmed. 1998. Evidence for strain specificity in cytotoxic T-lymphocyte-mediated, major histocompatibility complex class I-dependent killing of Theileria annulata-infected cells. Parasitol. Res. 84:593-595. [DOI] [PubMed] [Google Scholar]
- 10.Curiel, T. J., E. C. Krug, M. B. Purner, P. Poignard, and R. L. Berens. 1993. Cloned human CD4+ cytotoxic T lymphocytes specific for Toxoplasma gondii lyse tachyzoite-infected target cells. J. Immunol. 151:2024-2031. [PubMed] [Google Scholar]
- 11.Davies, C. J., L. Anderson, S. A. Ellis, E. J. Hensen, H. A. Lewin, S. Mikko, N. E. Muggli-Cockett, J. J. van der Poel, and G. C. Russell. 1997. Nomenclature for factors of the BoLA system, 1996: report of the ISAG BoLA Nomenclature Committee. Anim. Genet. 28:159-168. [Google Scholar]
- 12.Davies, C. J., I. Joosten, L. Andersson, M. A. Arriens, D. Bernoco, B. Bissumbhar, G. Byrns, M. J. van Eijk, B. Kristensen, H. A. Lewin, et al. 1994. Polymorphism of bovine MHC class II genes. Eur. J. Immunogenet. 21:259-289. [DOI] [PubMed] [Google Scholar]
- 13.Davies, C. J., I. Joosten, D. Bernoco, M. A. Arriens, J. Bester, G. Ceriotti, S. Ellis, E. J. Hensen, H. C. Hines, P. Horin, et al. 1994. Polymorphism of bovine MHC class I genes. Eur. J. Immunogenet. 21:239-258. [DOI] [PubMed] [Google Scholar]
- 14.Davison, H. C., A. Otter, and A. J. Trees. 1999. Estimation of vertical and horizontal transmission parameters of Neospora caninum infections in dairy cattle. Int. J. Parasitol. 29:1683-1689. [DOI] [PubMed] [Google Scholar]
- 15.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1-59. [DOI] [PubMed] [Google Scholar]
- 17.Guy, C. S., D. J. L. Williams, D. F. Kelly, J. W. McGarry, F. Guy, C. Bjorkman, R. F. Smith, and A. J. Trees. 2001. Neospora caninum in persistently infected, pregnant cows: spontaneous transplacental infection is associated with an acute increase in maternal antibody. Vet. Rec. 149:443-449. [DOI] [PubMed] [Google Scholar]
- 18.Hakim, F. T., R. T. Gazzinelli, E. Denkers, S. Hieny, G. M. Shearer, and A. Sher. 1991. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J. Immunol. 147:2310-2316. [PubMed] [Google Scholar]
- 19.Hope, J. C., P. Sopp, and C. J. Howard. 2002. NK-like CD8+ cells in immunologically naive neonatal calves that respond to dendritic cells infected with Mycobacterium bovis BCG. J. Leukoc. Biol. 71:184-194. [PubMed] [Google Scholar]
- 20.Innes, E. A., P. Millar, C. G. Brown, and R. L. Spooner. 1989. The development and specificity of cytotoxic cells in cattle immunized with autologous or allogeneic Theileria annulata-infected lymphoblastoid cell lines. Parasite Immunol. 11:57-68. [DOI] [PubMed] [Google Scholar]
- 21.Innes, E. A., S. E. Wright, S. Maley, A. Rae, A. Schock, E. Kirvar, P. Bartley, C. Hamilton, I. M. Carey, and D. Buxton. 2001. Protection against vertical transmission in bovine neosporosis. Int. J. Parasitol. 31:1523-1534. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka, T., N. Shinohara, H. Takayama, K. Takaku, S. Kondo, S. Yonehara, and K. Nagai. 1996. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156:3678-3686. [PubMed] [Google Scholar]
- 23.Khan, I. A., J. D. Schwartzman, S. Fonseka, and L. H. Kasper. 1997. Neospora caninum: role for immune cytokines in host immunity. Exp. Parasitol. 85:24-34. [DOI] [PubMed] [Google Scholar]
- 24.Khanolkar, A., H. Yagita, and M. J. Cannon. 2001. Preferential utilization of the perforin/granzyme pathway for lysis of Epstein-Barr virus-transformed lymphoblastoid cells by virus-specific CD4+ T cells. Virology 287:79-88. [DOI] [PubMed] [Google Scholar]
- 25.Kojima, Y., A. Kawasaki-Koyanagi, N. Sueyoshi, A. Kanai, H. Yagita, and K. Okumura. 2002. Localization of Fas ligand in cytoplasmic granules of CD8+ cytotoxic T lymphocytes and natural killer cells: participation of Fas ligand in granule exocytosis model of cytotoxicity. Biochem. Biophys. Res. Commun. 296:328-336. [DOI] [PubMed] [Google Scholar]
- 26.Lewin, H. A., G. C. Russell, and E. J. Glass. 1999. Comparative organization and function of the major histocompatibility complex of domesticated cattle. Immunol. Rev. 167:145-158. [DOI] [PubMed] [Google Scholar]
- 27.Liebana, E., R. M. Girvin, M. Welsh, S. D. Neill, and J. M. Pollock. 1999. Generation of CD8+ T-cell responses to Mycobacterium bovis and mycobacterial antigen in experimental bovine tuberculosis. Infect. Immun. 67:1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long, M. T., T. V. Baszler, and B. A. Mathison. 1998. Comparison of intracerebral parasite load, lesion development, and systemic cytokines in mouse strains infected with Neospora caninum. J. Parasitol. 84:316-320. [PubMed] [Google Scholar]
- 29.Luder, C. G., and F. Seeber. 2001. Toxoplasma gondii and MHC-restricted antigen presentation: on degradation, transport, and modulation. Int. J. Parasitol. 31:1355-1369. [DOI] [PubMed] [Google Scholar]
- 30.Lunden, A., J. Marks, S. W. Maley, and E. A. Innes. 1998. Cellular immune responses in cattle experimentally infected with Neospora caninum. Parasite Immunol. 20:519-526. [DOI] [PubMed] [Google Scholar]
- 31.McAllister, M. M., C. Bjorkman, R. Anderson-Sprecher, and D. G. Rogers. 2000. Evidence of point-source exposure to Neospora caninum and protective immunity in a herd of beef cows. J. Am. Vet. Med. Assoc. 217:881-887. [DOI] [PubMed] [Google Scholar]
- 32.Moen, A., W. Wouda, and A. de Gee. 1996. Clinical and serological observations of bovine Neospora abortion in three Dutch dairy herds. World Association for Buiatrics/British Cattle Veterinary Association, Edinburgh, United Kingdom.
- 33.Montoya, J. G., K. E. Lowe, C. Clayberger, D. Moody, D. Do, J. S. Remington, S. Talib, and C. S. Subauste. 1996. Human CD4+ and CD8+ T lymphocytes are both cytotoxic to Toxoplasma gondii-infected cells. Infect. Immun. 64:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakano, Y., H. Hisaeda, T. Sakai, M. Zhang, Y. Maekawa, T. Zhang, M. Nishitani, H. Ishikawa, and K. Himeno. 2001. Granule-dependent killing of Toxoplasma gondii by CD8+ T cells. Immunology 104:289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen, H. V., S. L. Lauemoller, L. Christiansen, S. Buus, A. Fomsgaard, and E. Petersen. 1999. Complete protection against lethal Toxoplasma gondii infection in mice immunized with a plasmid encoding the SAG1 gene. Infect. Immun. 67:6358-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishikawa, Y., K. Tragoolpua, N. Inoue, L. Makala, H. Nagasawa, H. Otsuka, and T. Mikami. 2001. In the absence of endogenous gamma interferon, mice acutely infected with Neospora caninum succumb to a lethal immune response characterized by inactivation of peritoneal macrophages. Clin. Diagn. Lab. Immunol. 8:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purner, M. B., R. L. Berens, P. B. Nash, A. van Linden, E. Ross, C. Kruse, E. C. Krug, and T. J. Curiel. 1996. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect. Immun. 64:4330-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell, G. C., C. J. Davies, L. Anderson, S. A. Ellis, E. J. Hensen, H. A. Lewin, S. Mikko, N. E. Muggli-Cockett, and J. J. van der Poel. 1997. BoLA class II nucleotide sequences, 1996: report of the ISAG BoLA Nomenclature Committee. Anim. Genet. 28:169-180.
- 39.Russell, J. H., and T. J. Ley. 2002. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20:323-370. [DOI] [PubMed] [Google Scholar]
- 40.Schares, G., A. Barwald, C. Staubach, P. Sondgen, M. Rauser, R. Schroder, M. Peters, R. Wurm, T. Selhorst, and F. J. Conraths. 2002. p38-avidity-ELISA: examination of herds experiencing epidemic or endemic Neospora caninum-associated bovine abortion. Vet. Parasitol. 106:293-305. [DOI] [PubMed] [Google Scholar]
- 41.Schares, G., M. Peters, R. Wurm, A. Barwald, and F. J. Conraths. 1998. The efficiency of vertical transmission of Neospora caninum in dairy cattle analysed by serological techniques. Vet. Parasitol. 80:87-98. [DOI] [PubMed] [Google Scholar]
- 42.Siliciano, R. F., A. D. Keegan, R. Z. Dintzis, H. M. Dintzis, and H. S. Shin. 1985. The interaction of nominal antigen with T-cell antigen receptors. I. Specific binding of multivalent nominal antigen to cytolytic T-cell clones. J. Immunol. 135:906-914. [PubMed] [Google Scholar]
- 43.Stenlund, S., H. Kindahl, U. Magnusson, A. Uggla, and C. Bjorkman. 1999. Serum antibody profile and reproductive performance during two consecutive pregnancies of cows naturally infected with Neospora caninum. Vet. Parasitol. 85:227-234. [DOI] [PubMed] [Google Scholar]
- 44.Sun, Q., R. L. Burton, and K. G. Lucas. 2002. Cytokine production and cytolytic mechanism of CD4+ cytotoxic T lymphocytes in ex vivo expanded therapeutic Epstein-Barr virus-specific T-cell cultures. Blood 99:3302-3309. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka, T., H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 2000. Growth-inhibitory effects of interferon-gamma on Neospora caninum in murine macrophages by a nitric oxide mechanism. Parasitol. Res. 86:768-771. [DOI] [PubMed] [Google Scholar]
- 47.van Eijk, M. J., J. A. Stewart-Haynes, and H. A. Lewin. 1992. Extensive polymorphism of the BoLA-DRB3 gene distinguished by PCR-RFLP. Anim. Genet. 23:483-496. [DOI] [PubMed] [Google Scholar]
- 48.Williams, N. S., and V. H. Engelhard. 1996. Identification of a population of CD4+ CTL that utilizes a perforin- rather than a Fas ligand-dependent cytotoxic mechanism. J. Immunol. 156:153-159. [PubMed] [Google Scholar]
- 49.Yamane, I., H. Kitani, T. Kokuho, T. Shibahara, M. Haritani, T. Hamaoka, S. Shimizu, M. Koiwai, K. Shimura, and Y. Yokomizo. 2000. The inhibitory effect of interferon gamma and tumor necrosis factor alpha on intracellular multiplication of Neospora caninum in primary bovine brain cells. J. Vet. Med. Sci. 62:347-351. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita, K., K. Yui, M. Ueda, and A. Yano. 1998. Cytotoxic-T-lymphocyte-mediated lysis of Toxoplasma gondii-infected target cells does not lead to death of intracellular parasites. Infect. Immun. 66:4651-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, T. H., F. Aosai, K. Norose, M. Ueda, and A. Yano. 1995. Enhanced cytotoxicity of IFN-gamma-producing CD4+ cytotoxic T lymphocytes specific for T. gondii-infected human melanoma cells. J. Immunol. 154:290-298. [PubMed] [Google Scholar]
- 52.Yasukawa, M., H. Ohminami, J. Arai, Y. Kasahara, Y. Ishida, and S. Fujita. 2000. Granule exocytosis, and not the fas/fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4+ as well as CD8+ cytotoxic T lymphocytes in humans. Blood 95:2352-2355. [PubMed] [Google Scholar]
- 53.Yasukawa, M., H. Ohminami, Y. Yakushijin, J. Arai, A. Hasegawa, Y. Ishida, and S. Fujita. 1999. Fas-independent cytotoxicity mediated by human CD4+ CTL directed against herpes simplex virus-infected cells. J. Immunol. 162:6100-6106. [PubMed] [Google Scholar]