Abstract
Mycobacterium marinum causes tuberculosis-like disease in fish and amphibians and has been used as a model mycobacterial species because of its rapid growth and less stringent containment requirements relative to other mycobacterial species. We demonstrate here that M. marinum grows within Dictyostelium discoideum cells, allowing the genetic analysis of host factors that may modulate the replication of mycobacterial species. Intracellular growth of M. marinum was shown to mimic the properties previously observed for growth within cultured phagocytes. A defined bacterial mutant defective for growth within phagocytic cells was shown to be similarly defective for growth within D. discoideum. To test the role of host coronin, which was previously hypothesized to positively modulate mycobacterial growth within mouse macrophages, a defined D. discoideum coronin mutant was analyzed. Surprisingly, the absence of coronin resulted in enhanced intracellular replication of M. marinum relative to the control wild-type strain. Consistent with previous observations, some phagosomes showed persistence of coronin about the surface of the compartment, but colocalization of the protein was far from uniform. We conclude that in D. discoideum factors other than coronin support intracellular replication of M. marinum.
Host factors that affect resistance or susceptibility to infection have been successfully identified using genetic analyses of model organisms. Genetic studies with Drosophila demonstrated that the Toll signal transduction pathway plays a role in innate immunity, and mutations that eliminate components in this path result in increased susceptibility to bacterial or fungal pathogens (27). Similar work has uncovered a mitogen-activated protein kinase pathway encoded by nematodes that is involved in pathogen resistance (25). These findings have been extended to studies in mammals (32, 36, 55) and have rekindled interest in the study of innate immunity. Similarly, searches for mutants in Arabidopsis have revealed many genes and pathways involved in plant disease (5, 52).
Some bacterial pathogens, including chlamydiae, mycobacteria, and legionellae, obtain nutrients and evade the immune system by growing within host cells (1). This has spawned interest in using host cells to perform genetic screens for host factors involved in intracellular pathogen infections. Here, we extended this analysis to the free-living unicellular soil amoebae Dictyostelium discoideum as a host for the intracellular bacterial pathogen Mycobacterium marinum.
M. marinum is closely related to Mycobacterium tuberculosis, one of the leading causes of infectious disease-related death (54). M. marinum causes systemic tuberculosis-like infections in fish, amphibians, and other ectotherms, involving persistent growth within macrophages (15, 40). M. marinum also causes human skin infections such as fish tank granuloma (20). The organism has several attractive features. It has a relatively short doubling time; its growth is limited by high temperature, reducing the risk of systemic infection in humans; and construction of mutations by homologous recombination is relatively easy (39). Like M. tuberculosis, M. marinum can evade the endocytic pathway after uptake by host cells (3), and factors associated with intracellular growth of this organism also appear to play a role in M. tuberculosis pathogenesis (10, 38, 48). Long-term infections of leopard frogs by M. marinum produce granulomas that have similarities to those seen in human tuberculosis infections (7). These data suggest that M. marinum is an excellent model for mycobacterial diseases.
Intracellular growth of mycobacterial species involves a unique trafficking pathway that blocks maturation of the phagolysosome and establishes a replicative compartment that maintains vesicular contact with the plasma membrane surface (23, 49). Thus far, few host factors that are necessary to support the establishment of this compartment have been identified. One host protein suggested to play a role in maintenance of this compartment is the phagosome-associated protein TACO, also known as coronin 1 in the mouse (16, 35, 44). Coronin isoforms are believed to be involved in promoting cytoskeletal remodeling of plasma membrane surfaces and phagosomal biogenesis in mammalian cells (13, 33). Mutants lacking the only characterized D. discoideum isoform of coronin have been isolated, and these strains show defects in particle phagocytosis, fluid phase uptake, migration, and cytokinesis (29, 41).
D. discoideum is a free-living soil amoeba which feeds on bacteria. The cells are easily maintained in the laboratory, and axenic strains of D. discoideum can be grown in the absence of bacteria in a rich medium (6). D. discoideum is highly motile and phagocytic, and many aspects of cytoskeletal function have been characterized (34, 53). Recently, D. discoideum has been established as a host cell for infection with several bacterial pathogens, including Legionella pneumophila (22, 46), Pseudomonas aeruginosa (37), and Mycobacterium avium (45). The attractions of the organism are that it is amenable to genetic analysis and the genome is being sequenced (26, 28, 30).
In this report, we demonstrate that M. marinum can infect and grow within D. discoideum cells. We show that M. marinum mutants that are defective in intracellular growth in macrophages are also defective for growth in D. discoideum cells. We also show that M. marinum growth is stimulated by the absence of the only characterized D. discoideum isoform of coronin.
MATERIALS AND METHODS
Cells and media.
D. discoideum strain AX3 was a kind gift from D. Knecht (University of Connecticut, Storrs). The coronin deletion mutant (strain HG1569), the coronin-green fluorescent protein (GFP) strain (18), and the parental strain AX2-214 were kind gifts from M. Maniak (Kassel, Germany). Cells were routinely grown axenically in HL-5 liquid medium (50) supplemented with penicillin and streptomycin (100 U/ml; GibcoBRL) in 100-mm dishes at 21°C. Healthy logarithmically growing cells were prepared for all experiments by plating cells 2 days prior to use in dishes containing fresh HL-5 medium at a concentration of 1 × 105 to 1.25 × 105 cells/ml.
M. marinum strains were kind gifts from L. Ramakrishnan (University of Washington Medical School). The msp12::gfp strain constitutively expresses GFP and grows efficiently in macrophages (10). GFP expression was maintained by adding the antibiotic apramycin to 20 μg/ml. Strains L1D and P59D contain kanamycin resistance insertions in adjacent genes (named mag24-1 and mag24-2, respectively) that encode members of the PE-PGRS family (38). The growth rate of P59D in macrophages is indistinguishable from that of isogenic wild-type controls, whereas strain L1D is impaired for growth in macrophages due to inactivation of mag24-1 (38). Both strains were grown in liquid medium and on plates containing kanamycin (20 μg/ml). All M. marinum strains were grown in Middlebrook 7H9 medium supplemented with Middlebrook OADC (10% oleic acid-albumin-dextrose complex; Becton Dickinson, Cockeysville, Md.) and 0.5% glycerol. Plates were solidified with 2% agar. Cultures of M. marinum were prepared by inoculating 10 ml of medium in a tissue culture flask from the freezer stock, incubating at 30°C for 1 week, and storing at 4°C for several weeks. To produce rapidly growing M. marinum for all experiments, the stored culture was diluted 1/10 in fresh medium and incubated at 30°C for 3 days prior to infections.
Live infection of D. discoideum with GFP-expressing M. marinum.
To prepare D. discoideum for M. marinum infections, amoebae grown axenically in tissue culture dishes containing HL-5 medium (46) were harvested and washed in phosphate-buffered saline (PBS) as follows. Cells were pelleted by a 5-min spin at 600 × g, the medium was aspirated and replaced with an equal volume of PBS, the cells were pelleted again by a 5-min spin at 600 × g, and the PBS was aspirated. The cells were resuspended to 106 cells/ml in HL-5 medium containing 20 μg of apramycin/ml, disbursed to a glass-bottom 96-well plate (Corning), and allowed to equilibrate for at least 1 h at 25.5°C. M. marinum for infections was prepared by transferring the 3-day culture to a 50-ml conical tube and vortexing for 2 min at top speed with a tabletop vortexer. The approximate concentration of bacteria was determined at A600 by using a 1/10 dilution of culture, assuming that an A600 of 1.0 is equivalent to 7.7 × 107 bacterial/ml. M. marinum clump extensively, so the actual multiplicity of infection (MOI) can vary up to fivefold from this approximation. Viable counts were then performed on the dispersed culture.
D. discoideum were infected at a MOI of approximately 0.5. Infection was initiated by aspirating the medium from the wells and replacing it with HL-5 medium containing 20 μg of apramycin per ml and approximately 5 × 105 bacteria of the msp12::gfp strain per ml. The cells and bacteria were incubated for 3 h at 25.5°C. Non-cell-associated bacteria were removed by washing the cells twice with HL-5. Growth of extracellular bacteria was inhibited by addition of 5 μg of streptomycin per ml plus 20 μg of apramycin per ml in the final growth medium.
At various times the live cells were observed with a Nikon TE300 inverted phase microscope. The number of bacteria per cell was determined by direct observation, and images were captured by a Princeton Micromax slow-scan cooled charge-coupled device camera. The experiment was performed twice, counting 500 amoebae for each time point from multiple wells. No more than 20 cells were counted per field, so more than 25 fields were read for each time point.
Growth of P59D and L1D strains in D. discoideum.
D. discoideum was infected as described above except that cells were plated in 24-well tissue culture plates, the MOI was 0.2, and cells were infected with either M. marinum strain L1D (mag24-1) or strain P59D (mag24-2).
Viable intracellular M. marinum cells were harvested and counted in the following fashion. Medium from a well was aspirated and replaced with 0.5 ml of PBS in order to remove streptomycin from the well. The PBS was aspirated, and the cells were lysed by adding 0.5 ml of PBS containing 0.1% Triton X-100 to release the intracellular bacteria. Dilutions of M. marinum were also performed in PBS containing 0.1% Triton X-100 to minimize bacterial clumping. The number of CFU of M. marinum was determined by plating dilutions of harvested bacteria on 7H9 containing 20 μg of kanamycin per ml. Plates were incubated for 7 days at 30°C before counting.
Transmission electron microscopy.
D. discoideum amoebae were infected with M. marinum strains P59D and L1D as described above (MOI = 0.2). On day 8, cells from six wells of a 24-well plate were harvested and processed for electron microscopy exactly as described previously (46). Samples were analyzed on a Philips CM-10 transmission electron microscope.
Growth of M. marinum in AX2 and a D. discoideum strain lacking coronin.
D. discoideum was infected as described above except that the MOI was 0.02. Both strains of D. discoideum were infected with the same mixture of M. marinum strain P59D and medium and thus were exposed to identical numbers of bacteria.
Viable intracellular M. marinum cells were counted by harvesting infected cells, using repetitive pipetting with a 1,000-μl automatic pipettor, and transferring to a microcentrifuge tube. Cells were pelleted in an Eppendorf microcentrifuge for 3.5 min at 8,000 rpm. The supernatant was aspirated and the pellet resuspended in 0.5 ml of PBS containing 0.1% Triton X-100. Dilutions and plating were as described above.
Polyclonal antibody directed against M. marinum.
Exponentially growing M. marinum cells were harvested, pelleted, and washed twice with PBS. Cells were resuspended in PBS and fixed overnight at 4°C by adding paraformaldehyde to a final concentration of 3%. Fixed bacteria were washed three times in PBS and concentrated fivefold (approximately 3.5 × 108 bacteria/ml). Rats were immunized (Pocono Rabbit Farm and Laboratory, Canadensis, Pa.) with 40 μl of fixed M. marinum and complete Freund's adjuvant on day 0, 20 μl of fixed M. marinum and incomplete Freund's adjuvant on day 14, and 5 μl of fixed M. marinum and incomplete Freund's adjuvant on day 28. The rats were given boosters of 5 μl of fixed M. marinum every 28 days until a robust signal was seen by immunofluorescence, which took approximately 6 months of immunization.
Association of coronin-GFP with M. marinum phagosomes.
The D. discoideum coronin-GFP strain expressing a coronin-GFP fusion was plated on poly-l-lysine-coated coverslips and infected with wild-type M. marinum at an MOI of two bacteria per amoeba as described above. After an overnight incubation (19 h), the cells were fixed for 1 h at room temperature in freshly made 4% paraformaldehyde containing 40 mM HEPES (pH = 7.4) and 6.5% sucrose. Samples were permeabilized by incubation with ice-cold methanol for 30 s, washed five times in PBS, and blocked in 4% goat serum (Gibco-BRL)-PBS for 1 h. M. marinum was visualized with a 1/100 dilution of polyclonal rat anti-M. marinum. After 1 h of incubation with primary antibody, the samples were washed five times in PBS, and anti-rat immunoglobulin G-Texas red was added at a 1/500 dilution (Molecular Probes). Samples were washed and processed as described elsewhere (51). Fixed samples were analyzed with a Nikon TE300 microscope, and images were captured by a Princeton Micromax slow-scan cooled charge-coupled device camera.
Analysis of sequence data.
Comparison of ORFs with previously identified genes was performed by using the Basic Local Alignment Search Tool (BLASTP or tBLASTN) in the public National Center for Biotechnology Information databases. Searches for paralogs to the D. discoideum coronin protein were performed with the BLAST server for the unfinished D. discoideum sequencing project (http://dicty.sdsc.edu/). Sequence data for D. discoideum were obtained from the Genome Sequencing Centre Jena website at http://genome.imb-jena.de/dictyostelium/. These sequence data were also produced by the D. discoideum Sequencing Group at the Sanger Institute.
RESULTS
Microscopic observations of a live infection of D. discoideum with M. marinum.
To demonstrate intracellular growth of M. marinum, a clinical isolate that constitutively expresses GFP was introduced onto D. discoideum monolayers plated in glass-bottom 96-well plates, and live infections were monitored over time (see Materials and Methods). After various temperatures had been tried, infections were performed at 25.5°C, which is within the optimal range for M. marinum growth and is near the upper limits for D. discoideum thermotolerance (46). To suppress the growth of extracellular bacteria during an infection, streptomycin was added to the medium after an initial incubation of M. marinum with the cells in the absence of antibiotic. In addition, the MOI was set below 1.0, because we found that adding M. marinum to D. discoideum at MOIs greater than 5.0 led to rounding and lysis of the amoebae (data not shown).
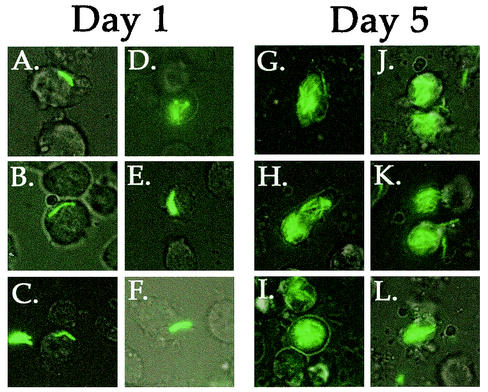
When bacteria were introduced onto D. discoideum monolayers at an MOI of 0.5 and 25.5°C, luxuriant intracellular replication could be observed (Fig. 1). On day 1, the infected D. discoideum amoebae had few bacteria, with most cells having fewer than two bacteria per cell (Fig. 1A to C). The amoebae having more than one M. marinum cell probably internalized small clumps of two to eight bacteria (Fig. 1D to F). In contrast, at day 5, many of the infected amoebae appeared to be filled with M. marinum, and there were far too many bacteria in each cell to obtain an accurate count of intracellular bacteria (Fig. 1G to L). M. marinum did not grow in HL-5 medium with 5 μg of streptomycin per ml in the absence of cells (data not shown), indicating that the growth of M. marinum observed in this experiment was intracellular growth within D. discoideum.
FIG. 1.
Intracellular growth of GFP-expressing M. marinum within D. discoideum amoebae during a 5-day infection period. D. discoideum Ax3 was plated in glass-bottom 96-well tissue culture plates and infected at an MOI of 0.5 with M. marinum msp12::gfp (see Materials and Methods). (A to F) Typical infected cells 24 h after incubation with bacteria show amoebae with one or two bacteria (A to C) or small groups of bacteria (D to F). (G to L) Five days after infection, most of the infected amoebae are filled with M. marinum. Images of live cells were obtained with simultaneous bright-field and UV illumination, and images were processed as described in Materials and Methods.
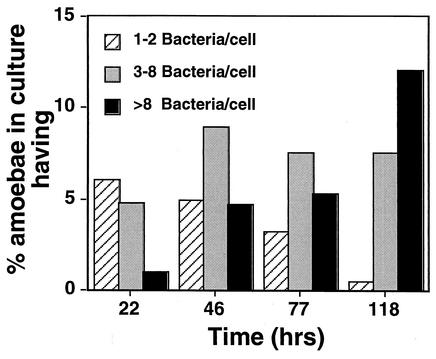
The number of uninfected cells over the course of the experiment remained nearly constant at 80% (Fig. 2). This indicates that the antibiotic in the medium killed the extracellular bacteria, so that there was very little reinfection of cells during the course of the experiment. The large decrease in the number of D. discoideum cells having one or two M. marinum cells that occurred between 22 and 118 h after initial infection also indicates that there was little reinfection (Fig. 2). Furthermore, intracellular growth clearly took place, as the percentage of cells with eight or more bacteria increased. It is important to note that 40 to 50% of the infected cells maintained three to eight bacteria per cell throughout the experiment. This suggests that there may have been a pool of cells infected by small clumps of bacteria in which the M. marinum organisms did not grow. It is possible that an infection of a large bolus of bacteria poisons the cell or that the bacteria that initiate infection in this fashion may become dormant shortly after internalization by the target cell.
FIG. 2.
The proportion of D. discoideum harboring large numbers of bacteria increases during incubation with M. marinum. Amoebae were incubated with M. marinum msp12::gfp (see Materials and Methods) and fixed at the indicated times, and the numbers of intracellular M. marinum were determined microscopically by GFP fluorescence. At each time point, the percentage of total amoebae in the culture with one or two, three to eight, and eight or more bacteria/cell were quantitated and displayed. Over 500 cells were examined at each time point. The data from one representative experiment are shown, and the experiment was performed three times in total, with similar results.
An M. marinum mutant with reduced growth in macrophages also has reduced growth in D. discoideum.
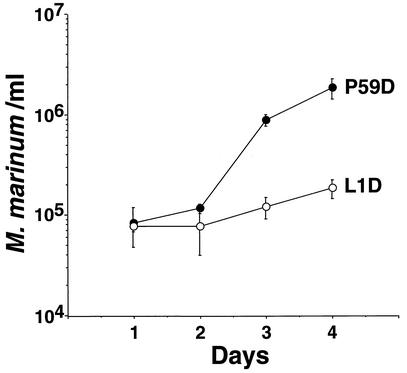
To test whether the intracellular growth of M. marinum in D. discoideum was similar to intracellular growth in macrophages, a bacterial mutant with reduced intracellular growth in macrophages was analyzed to determine if it also had reduced growth in D. discoideum. To this end, a M. marinum mutant with a mutation in mag24-1, which encodes a protein in the glycine-rich PE-PGRS family (8), was analyzed (38). A mutant having an insertion in this gene (strain L1D) was found to have reduced growth in D. discoideum as well (Fig. 3). For the growth-proficient control strain (P59D), which has an insertion mutation in the downstream mag24-2 gene, there was more than a 20-fold increase in viable counts during 4 days of incubation with D. discoideum. In contrast, the growth of L1D increased just over twofold during this incubation period. Although no titer was obtained at day 0, in numerous experiments there was no difference between the number of viable intracellular bacteria on day 0 and day 1 (see Fig. 5).
FIG. 3.
Replication of growth-proficient P59D (mag24-2) and intracellular growth-defective mutant L1D (mag24-1) in the presence of D. discoideum. D. discoideum was plated in tissue culture wells and incubated with M. marinum at an MOI of 0.2 (see Materials and Methods). After the initial incubation period, streptomycin was added to each well to eliminate extracellular growth (see Materials and Methods). On each day following the initial incubation, intracellular bacteria were harvested and counted by plating for CFU. The experiment was performed in triplicate; each point represents the mean, and error bars indicate standard deviations for (n − 1)-weighted samples.
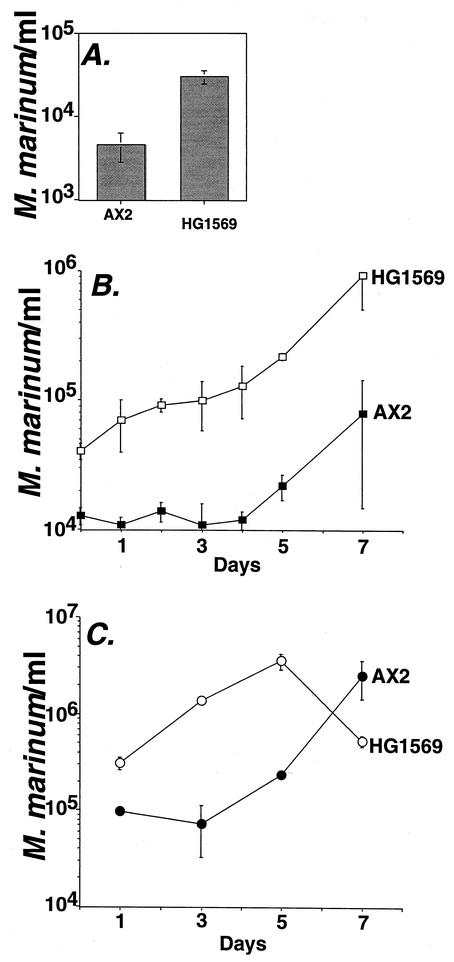
FIG. 5.
Uptake and growth of M. marinum in D. discoideum AX2 (wild type) and HG1569 (coronin deletion mutant). D. discoideum was plated in tissue culture plates and incubated with M. marinum P59D (MOI = 0.02). At 3 h postinfection, the monolayers were washed and incubated with antibiotic to kill extracellular bacteria (see Materials and Methods) On each day following the initial incubation, intracellular bacteria were harvested and counted by plating for CFU. The experiment was repeated three times, and each point represents the mean of triplicate determinations from a single experiment. Error bars indicate standard deviations for (n − 1)-weighted samples. (A) Uptake and initial survival of M. marinum P59D after 3 h of incubation with D. discoideum. (B) Enhanced initial rate of replication of M. marinum P59D during the first 3 days of bacterial incubation with HG1569 (coronin deletion). (C) Early climax of M. marinum P59D replication cycle within HG1569.
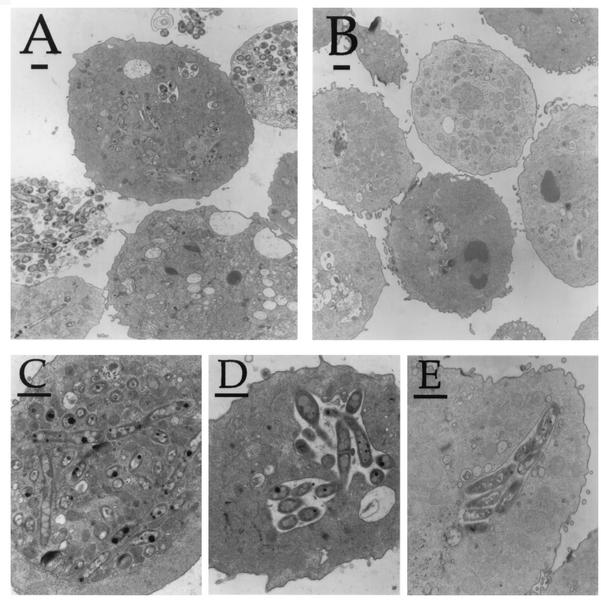
D. discoideum AX3 infected with the growth-proficient M. marinum strain P59D or the intracellular-growth-defective strain L1D was analyzed by electron microscopy after extended incubation (Fig. 4). Figure 4A shows a typical D. discoideum cell infected with the P59D. Many of the amoebae had numerous intracellular bacteria. Lysed cells were occasionally observed, in which there appeared to be ghosts of amoebae filled with M. marinum (Fig. 4A). It was also apparent that growth-proficient M. marinum was found in two types of phagosomal compartments within these cells. In one form (Fig. 4C), the bacteria were spread throughout the amoebic cell, with each bacterial cell presumably in an individual phagosomal compartment. The other phagosomal form (Fig. 4D) had several bacteria clustered in single phagosomes. In contrast, most of the cells infected with L1D were free of intracellular bacteria and had intact cell membranes (Fig. 4B). Occasionally, small phagosomes filled with L1D were observed (Fig. 4E). These pockets of bacteria are consistent with growth curves in both macrophages (38) and D. discoideum, indicating that L1D is able to promote small amounts of intracellular replication, although they could represent internalized clumps of bacteria that failed to replicate, as noted in Fig. 2.
FIG. 4.
Transmission electron micrographs of D. discoideum infected with M. marinum after 8 days of incubation. (A) D. discoideum incubated with growth-proficient P59D (mag24-2). Shown are uninfected, lightly infected, and heavily infected cells. (B) D. discoideum incubated with growth-defective mutant L1D (mag24-1). Cells rarely have intact bacteria. D. discoideum cells incubated with P59D show M. marinum, spread throughout the cell in individual phagosomes (C) or clustered together in single phagosomes (D). The intracellular-growth-deficient mutant L1D occasionally yields clusters of bacteria in single phagosomes within D. discoideum (E). Bar, 1 μm.
M. marinum grows more efficiently in coronin mutant D. discoideum.
Ferrari and colleagues have shown that Mycobacterium bovis sequesters TACO/coronin 1 on its vacuole in mouse macrophages (16). The persistence of TACO/coronin 1 on the vacuole correlates well with mycobacterial evasion of the endocytic pathway, and the protein potentially plays a role in intracellular survival of the microorganism (16). To evaluate the contribution of coronin to M. marinum survival, we analyzed intracellular growth after infection of a D. discoideum coronin mutant. The characterized coronin isoform is the most similar D. discoideum ortholog to mouse TACO/coronin 1 (see Discussion), and mutations affecting production of this protein result in well-defined defects in a variety of cytoskeleton-associated functions (13, 14, 21, 29).
M. marinum was introduced into cultures of the wild-type control strain AX2 and the coronin mutant HG1569, by using procedures identical to those described above, and viable counts were determined at various times after infection (Fig. 5). The most striking phenotype was that within 3 h after infection, the absence of coronin enhanced the yield of intracellular bacteria compared to the wild-type control (Fig. 5A). This could be due to either increased uptake of the bacteria or enhanced survival of M. marinum after phagocytosis by the coronin mutant. Furthermore, the rate of intracellular replication also appeared to be enhanced (Fig. 5B), particularly during the first 3 days after initial infection. Both phenotypes were entirely reproducible, with some experiments showing that the rate of replication within the coronin mutant was sufficiently enhanced that by 7 days after initial infection, amoebae lysed and exposed the bacteria to the streptomycin in the medium, as visualized by a decrease in viable counts between 5 and 7 days postinfection (Fig. 5C). Coronin appears to limit the intracellular growth of M. marinum.
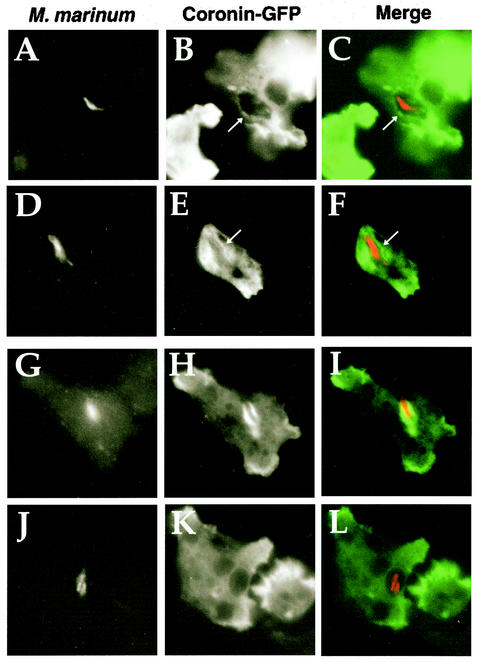
As the loss of coronin appeared to have the opposite effect of that predicted from previous work (16), we wanted to verify that the behavior of coronin in D. discoideum was similar to the behavior of TACO/coronin 1 during M. bovis BCG infections of mouse macrophages. Furthermore, although M. marinum is similar to M. bovis, the determinants of intracellular survival of these two organisms could be quite different. One key observation of previous work is that TACO/coronin 1 is sequestered and retained around the M. bovis BCG vacuole in mouse macrophages for extended periods (16), although in another study this persistence was not observed after infection of human macrophages (44). To analyze coronin sequestration in D. discoideum, a strain that expresses a coronin-GFP fusion as its only functional copy of coronin was challenged with M. marinum for 20 h, and the samples were fixed and immunoprobed for the bacteria. At this time point, the heat-killed control M. marinum was completely digested by the D. discoideum amoebae (data not shown). Analysis of amoebae challenged with the live bacteria showed that a fraction had associated coronin-GFP staining about the vacuole harboring M. marinum, although amoeba showing dense coronin staining about the phagosome were difficult to find. Figure 6 displays four typical examples of coronin-GFP sequestration around M. marinum phagosomes, showing the range of morphologies observed. Most of the phagosomes had faintly enhanced staining of coronin-GFP relative to the pools of protein found in the cell, making it difficult to quantitate the phagosomes having associated coronin (Fig. 6A to F). Occasionally, intense association of coronin-GFP with the compartment harboring the bacteria could be observed; however, the staining did not appear to be entirely circumferential (Fig. 6G to I). In addition, examples of compartments in which there was no clear association of coronin-GFP were found (Fig. 6J to L). We conclude that the D. discoideum compartment harboring M. marinum is heterogeneous with regard to coronin localization, with clear colocalization of the protein found in interspersed areas about the phagosome.
FIG. 6.
Association of coronin-GFP protein fusion with M. marinum phagosomes in D. discoideum. D. discoideum cells expressing coronin-GFP fusion protein were incubated with M. marinum (MOI = 2.0). After a 20-h incubation, cells were fixed for immunofluorescence analysis. Four different examples of coronin-GFP association are shown in the four rows. The left column shows staining of bacteria with rat anti-M. marinum, the middle column shows coronin-GFP staining, and the right column shows a superimposition of the two images, with coronin-GFP staining in green and anti-M. marinum staining in red. (A to F) Weak staining of phagosome with coronin-GFP; (G to I) intense staining of phagosome with coronin; (J to L) no apparent coronin staining. Arrows point to phagosome regions stained with coronin-GFP.
DISCUSSION
An impressive amount of information is available regarding the cellular biology of mycobacterial targeting within host cells (17, 43), but genetic analysis of the growth of Mycobacteria within host cells has been difficult, both in the host and in the microorganism. Recent studies have identified mutations within biosynthetic genes and genes encoding selected secreted and surface proteins required for persistence and survival within host cells (4, 9, 12, 19, 31, 38, 42). No bacterial loci, however, that are directly responsible for formation of the unique mycobacterial compartment have yet been identified by genetic analysis, although some of mutants have quite strong phenotypes and may potentially affect trafficking of the phagosome (4). Analysis of host factors has also been limited. It is clear that the presence of the NRAMP-1 transporter on the phagosome restricts mycobacterial growth, presumably due to limitation of divalent cations within this compartment (24). On the other hand, the only host factor for which a strong case has been made for playing a positive role in formation of the replication compartment is the mouse TACO/coronin 1 protein (16). Therefore, the development of systems that facilitate genetic analysis of both host and bacterial factors involved in formation of the replication compartment is desirable. In this report, we demonstrate that growth of M. marinum within D. discoideum represents an opportunity for just such detailed analyses and has allowed a test of the model in which coronin isoforms contribute to mycobacterial growth.
We think it likely that our studies have underestimated the efficiency of intracellular replication of M. marinum, at least in a population of infected cells. Examination of infected D. discoideum cells by fluorescence microscopy indicated that many amoebae supporting intracellular growth had far too many bacteria to count, although we rarely observed more than a 10- to 20-fold increase in viable counts of the entire population over a 7-day incubation period. There are two explanations for this discordance. First, all incubations were performed in the presence of antibiotics to suppress extracellular growth, and heavily infected cells may have become permeable to the antibiotic. Second, there may be some heterogeneity and asynchrony in the replication of the bacteria within the amoebae (Fig. 2), with some D. discoideum cells lysing and exposing bacteria to the antibiotic long before the replication cycle is completed in other amoebae. This complication is also reflected in the fact that in these studies, the lag between initial infection of the amoebae and initiation of intracellular growth varied considerably between experiments, indicating there may have been some variation in the physiological states of the bacterial or amoebic cultures prior to infection.
Two other examples of replication center heterogeneity within D. discoideum were found. First, a pool of amoebae containing three to eight bacteria was observed in which the number of intracellular M. marinum cells did not appear to change over the course of the experiment (Fig. 2). It is possible that small clumps of bacteria internalized by a single amoeba are incapable of initiating intracellular growth and remain in either a dormant or an undigested state. Alternatively, infection of a single amoeba with multiple bacteria may be toxic for D. discoideum. M. marinum was found to cause considerable cytotoxicity to D. discoideum, even at relatively low MOIs (4:1). Perhaps the uptake of several M. marinum cells is enough to irreparably injure the host cell. Whatever the explanation, the presence of such heterogeneity was to be expected, as this has been observed with other mycobacteria in a variety of cell types and found to be difficult to eliminate (2, 11). Second, once replication was established within D. discoideum, we observed that the morphology of the phagosomes was heterogeneous. In some cases, phagosomes with large groups of bacteria could be observed, whereas in other cases, bacteria were found in their own individual phagosomes. Similar heterogeneity was observed during M. marinum granuloma formation in the frog (7). It is possible that M. marinum may use more than one tactic to establish and maintain replication within host cells and that this property is reflected in the different phagosome morphologies that we observed. This explanation implies that there may be more than one set of bacterial proteins capable of promoting intracellular growth, each set leading to formation of a compartment having a distinct morphology. Alternatively, it may be that there is one trafficking pattern for all vacuoles, but one of the morphologically distinct compartments is a precursor for the other.
The M. marinum mag24-1 mutant (strain L1D) has reduced growth in both macrophages and D. discoideum, indicating that intracellular replication in the two hosts may proceed via similar mechanisms. The mag24-1 mutant showed modest intracellular growth in the plating assay for viable bacteria (Fig. 3). When such infections were analyzed in more detail by electron microscopy, it appeared that most of the D. discoideum cells were infected with one or a few bacteria, with occasional cells showing small clusters of bacteria. Examples of luxuriant intracellular growth of this mutant, on the other hand, could not be found. This suggests that the mag24-1 mutation causes an early block in bacterial replication that can occasionally be bypassed. As the occasional bypass does not appear to result in efficient intracellular growth, the mutation may cause defects in both establishment and maintenance of replication within host cells.
The most surprising result from these studies was that the absence of coronin enhanced the intracellular growth of M. marinum. Previous work with M. bovis BCG strains has shown that mouse TACO/coronin 1 persists on the phagosome, and phagocytes that fail to sequester the protein cannot support intracellular survival of the organism (16). In a conflicting study in human macrophages, TACO/coronin 1 was reported to associate with the BCG-containing phagosome, but continued persistence was not observed (44). Those authors found persistence of TACO/coronin 1 about phagosomes only in cells that internalized clumps of bacteria. The latter study proposed that TACO/coronin 1 plays a role in the early steps involved in establishing the mycobacterial vacuole. We found a similar ambiguity in the localization of D. discoideum coronin about mycobacterial vacuoles, with intense colocalization being only rarely observed. Coronin in D. discoideum, however, cannot play a role in supporting either establishment or maintenance of a replication vacuole in D. discoideum. In fact, the improved survival and growth of bacteria in such a mutant indicates that the protein interferes with mycobacterial growth in D. discoideum.
That D. discoideum coronin mutants exhibit a variety of defects in phagocytosis and motility seems inconsistent with the observed increase in intracellular growth (29, 41), but we have observed similar stimulation of intracellular growth of L. pneumophila in D. discoideum mutants defective for phagocytosis (46). The simplest explanation for this phenomenon is that for many pathogens that grow within vacuoles of host cells, there is a unique uptake pathway that leads to formation of a replication compartment distinct from the default pathway of phagocytosis. As the default pathway traffics the microorganism into a compartment that is either degradative or incapable of supporting intracellular growth, it potentially competes with the route that leads to intracellular growth. Mutations that eliminate proteins necessary for default phagocytosis should remove this competition and enhance the efficiency of establishing a replication compartment. Since earlier work showed that D. discoideum coronin clearly is involved in the default pathway (29), it might have been predicted based on this model that the coronin mutant would be more proficient than wild-type strains at promoting intracellular growth of M. marinum. It should be noted that previous results indicating that the absence of coronin causes defective phagocytosis were based on assays using D. discoideum incubated in suspension culture. Many mutations affecting phagocytosis in suspension culture have little effect when analyzed with D. discoideum grown on solid supports, and all the assays performed with M. marinum in this study were done with amoebic monolayers, which may suppress the phagocytosis defect that is caused by the absence of coronin.
The involvement of the D. discoideum coronin protein in the default pathway of phagocytosis appears to contradict the proposal that establishment of an M. bovis BCG replication compartment requires an isoform of this protein. One explanation for these conflicting observations is that among the multiple members of the coronin protein family in mammals, some isoforms participate in phagocytosis of nonpathogens, whereas others are involved in establishment of intravacuolar growth. For instance, coronin 2 in mouse macrophages has been found on phagosomes harboring particles coated with complement component C3, indicating a role for coronin 2 in phagocytosis of nonpathogens, whereas there has been no report of TACO/coronin 1 on similar phagosomes harboring inert particles (33). The function of mammalian coronin 2 may be closely related to that of D. discoideum coronin, whereas TACO/coronin 1 may play a role in cellular physiology that is promoted by some other D. discoideum protein. The biochemical functions of members of this family are relatively obscure, and different isoforms could have very different activities or interacting partners. Comparison of the D. discoideum coronin sequence with its most closely related orthologs in mammalian cells does not give any hints as to whether it would be predicted to have a function corresponding to a particular mammalian isoform. Most of the amino acid changes that differentiate the mouse coronin isoforms are localized in the carboxyl termini of these proteins (47). Even though this particular region shows low sequence similarity among isoforms, the different mouse isoforms appear to be more highly similar to each other than to the D. discoideum coronin in this region. The other notable feature is that there are residues present in several isoforms that are missing in both TACO/coronin 1 and the D. discoideum coronin. This is the only link observed between these two proteins that potentially differentiates them from other family members.
Our results show that if TACO/coronin 1 plays a critical role in mycobacterial intracellular growth, its function can be replaced by factors other than coronin in D. discoideum, or else M. marinum and M. bovis BCG have very different requirements for establishing and maintaining a replication compartment within host cells. It is possible that the corresponding factors could be other uncharacterized coronin isoforms. D. discoideum has at least two other predicted ORFs that appear to be part of the coronin family, although they are rather divergent from mammalian coronins and are predicted to encode significantly larger polypeptides than other known members of the coronin family (Materials and Methods). Future work on the details of the molecular interactions promoted by different isoforms of coronin in both mammalian cells and D. discoideum should clarify this issue.
Acknowledgments
We thank L. Ramakrishnan for strains, suggestions regarding use of antibiotics in the culture media, and review of the text and D. Knecht for continued aid and support. We thank Isabelle Derré, Zhao-Qing Luo, Marion Shonn, and Susan VanRheenen for review of the text.
J.J.S. was supported by an NRSA fellowship. R.R.I. is an investigator of the Howard Hughes Medical Institute.
Editor: F. C. Fang
REFERENCES
- 1.Amer, A. O., and M. S. Swanson. 2002. A phagosome of one's own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 5:56-61. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, J. A., and P. D. Hart. 1975. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 142:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker, L. P., K. M. George, S. Falkow, and P. L. Small. 1997. Differential trafficking of live and dead Mycobacterium marinum organisms in macrophages. Infect. Immun. 65:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthet, F. X., M. Lagranderie, P. Gounon, C. Laurent-Winter, D. Ensergueix, P. Chavarot, F. Thouron, E. Maranghi, V. Pelicic, D. Portnoi, G. Marchal, and B. Gicquel. 1998. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erp gene. Science 282:759-762. [DOI] [PubMed] [Google Scholar]
- 5.Bonas, U., and T. Lahaye. 2002. Plant disease resistance triggered by pathogen-derived molecules: refined models of specific recognition. Curr. Opin. Microbiol. 5:44-50. [DOI] [PubMed] [Google Scholar]
- 6.Bonner, J. T. 1944. A descriptive study of the development of the slime mold Dictyostelium discoideum. Am. J. Bot. 31:175-182. [Google Scholar]
- 7.Bouley, D. M., N. Ghori, K. L. Mercer, S. Falkow, and L. Ramakrishnan. 2001. Dynamic nature of host-pathogen interactions in Mycobacterium marinum granulomas. Infect. Immun. 69:7820-7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan, M. J., G. Delogu, Y. Chen, S. Bardarov, J. Kriakov, M. Alavi, and W. R. Jacobs, Jr. 2001. Evidence that mycobacterial PE-PGRS proteins are cell surface constituents that influence interactions with other cells. Infect. Immun. 69:7326-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camacho, L. R., D. Ensergueix, E. Perez, B. Gicquel, and C. Guilhot. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34:257-267. [DOI] [PubMed] [Google Scholar]
- 10.Chan, K., T. Knaak, L. Satkamp, O. Humbert, S. Falkow, and L. Ramakrishnan. 2002. Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proc. Natl. Acad. Sci. USA 99:3920-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens, D. L., and M. A. Horwitz. 1996. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 184:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox, J. S., B. Chen, M. McNeil, and W. R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79-83. [DOI] [PubMed] [Google Scholar]
- 13.de Hostos, E. L. 1999. The coronin family of actin-associated proteins. Trends Cell Biol. 9:345-350. [DOI] [PubMed] [Google Scholar]
- 14.de Hostos, E. L., C. Rehfuess, B. Bradtke, D. R. Waddell, R. Albrecht, J. Murphy, and G. Gerisch. 1993. Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. J. Cell Biol. 120:163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulin, M. P. 1979. A review of tuberculosis (mycobacteriosis) in fish. Vet. Med. Small Anim. Clin. 74:731-735. [PubMed] [Google Scholar]
- 16.Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 17.Fratti, R. A., J. Chua, and V. Deretic. 2002. Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J. Biol. Chem. 277:17320-17326. [DOI] [PubMed] [Google Scholar]
- 18.Gerisch, G., R. Albrecht, C. Heizer, S. Hodgkinson, and M. Maniak. 1995. Chemoattractant-controlled accumulation of coronin at the leading edge of Dictyostelium cells monitored using green fluorescent protein-coronin fusion protein. Curr. Biol. 1:1280-1285. [DOI] [PubMed] [Google Scholar]
- 19.Glickman, M. S., J. S. Cox, and W. R. Jacobs, Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 20.Gluckman, S. J. 1995. Mycobacterium marinum. Clin. Dermatol. 13:273-276. [DOI] [PubMed] [Google Scholar]
- 21.Hacker, U., R. Albrecht, and M. Maniak. 1997. Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci. 110:105-112. [DOI] [PubMed] [Google Scholar]
- 22.Hagele, S., R. Kohler, H. Merkert, M. Schleicher, J. Hacker, and M. Steinert. 2000. Dictyostelium discoideum: a new host model system for intracellular pathogens of the genus Legionella. Cell. Microbiol. 2:165-171. [DOI] [PubMed] [Google Scholar]
- 23.Honer zu Bentrup, K., and D. G. Russell. 2001. Mycobacterial persistence: adaptation to a changing environment. Trends Microbiol. 9:597-605. [DOI] [PubMed] [Google Scholar]
- 24.Jabado, N., A. Jankowski, S. Dougaparsad, V. Picard, S. Grinstein, and P. Gros. 2000. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192:1237-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, D. H., R. Feinbaum, G. Alloing, F. E. Emerson, D. A. Garsin, H. Inoue, M. Tanaka-Hino, N. Hisamoto, K. Matsumoto, M. W. Tan, and F. M. Ausubel. 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297:623-626. [DOI] [PubMed] [Google Scholar]
- 26.Kreppel, L., and A. R. Kimmel. 2002. Genomic database resources for Dictyostelium discoideum. Nucleic Acids Res. 30:84-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 28.Loomis, W. F., D. Welker, J. Hughes, D. Maghakian, and A. Kuspa. 1995. Integrated maps of the chromosomes in Dictyostelium discoideum. Genetics 141:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maniak, M., R. Rauchenberger, R. Albrecht, J. Murphy, and G. Gerisch. 1995. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein tag. Cell 83:915-924. [DOI] [PubMed] [Google Scholar]
- 30.Mann, S. K. O., P. N. Devreotes, S. Elliott, K. Jermyn, A. Kuspa, M. Fechheimer, R. Furukawa, C. A. Parent, J. Segall, G. Shaulsky, P. H. Vardy, J. Williams, K. L. Williams, and R. A. Firtel. 1998. Cell biological, molecular genetic, and biochemical methods used to examine Dictyostelium, p. 431-465. In J. E. Celis (ed.), Cell biology, a laboratory handbook, vol. 1. Academic Press, San Diego, Calif.
- 31.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 32.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 33.Morrissette, N. S., E. S. Gold, J. Guo, J. A. Hamerman, A. Ozinsky, V. Bedian, and A. A. Aderem. 1999. Isolation and characterization of monoclonal antibodies directed against novel components of macrophage phagosomes. J. Cell Sci. 112:4705-4713. [DOI] [PubMed] [Google Scholar]
- 34.Noegel, A. A., and M. Schleicher. 2000. The actin cytoskeleton of Dictyostelium: a story told by mutants. J. Cell Sci. 113:759-766. [DOI] [PubMed] [Google Scholar]
- 35.Okumura, M., C. Kung, S. Wong, M. Rodgers, and M. L. Thomas. 1998. Definition of family of coronin-related proteins conserved between humans and mice: close genetic linkage between coronin-2 and CD45-associated protein DNA. Cell Biol. 17:779-787. [DOI] [PubMed] [Google Scholar]
- 36.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 37.Pukatzki, S., R. H. Kessin, and J. J. Mekalanos. 2002. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 99:3159-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishnan, L., H. T. Tran, N. A. Federspiel, and S. Falkow. 1997. A crtB homolog essential for photochromogenicity in Mycobacterium marinum: isolation, characterization, and gene disruption via homologous recombination. J. Bacteriol. 179:5862-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramakrishnan, L., R. H. Valdivia, J. H. McKerrow, and S. Falkow. 1997. Mycobacterium marinum causes both long-term subclinical infection and acute disease in the leopard frog (Rana pipiens). Infect. Immun. 65:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauchenberger, R., U. Hacker, J. Murphy, J. Niewohner, and M. Maniak. 1997. Coronin and vacuolin identify consecutive stages of a late, actin-coated endocytic compartment in Dictyostelium. Curr. Biol. 7:215-218. [DOI] [PubMed] [Google Scholar]
- 42.Raynaud, C., C. Guilhot, J. Rauzier, Y. Bordat, V. Pelicic, R. Manganelli, I. Smith, B. Gicquel, and M. Jackson. 2002. Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 45:203-217. [DOI] [PubMed] [Google Scholar]
- 43.Russell, D. G. 2001. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell Biol. 2:569-577. [DOI] [PubMed] [Google Scholar]
- 44.Schuller, S., J. Neefjes, T. Ottenhoff, J. Thole, and D. Young. 2001. Coronin is involved in uptake of Mycobacterium bovis BCG in human macrophages but not in phagosome maintenance. Cell. Microbiol. 3:785-793. [DOI] [PubMed] [Google Scholar]
- 45.Skriwan, C., M. Fajardo, S. Hagele, M. Horn, M. Wagner, R. Michel, G. Krohne, M. Schleicher, J. Hacker, and M. Steinert. 2002. Various bacterial pathogens and symbionts infect the amoeba Dictyostelium discoideum. Int. J. Med. Microbiol. 291:615-624. [DOI] [PubMed] [Google Scholar]
- 46.Solomon, J. M., A. Rupper, J. A. Cardelli, and R. R. Isberg. 2000. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 68:2939-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spoerl, Z., M. Stumpf, A. A. Noegel, and A. Hasse. 2002. Oligomerization, F-actin interaction, and membrane association of the ubiquitous mammalian coronin 3 are mediated by its carboxyl terminus. J. Biol. Chem. 277:48858-48867. [DOI] [PubMed] [Google Scholar]
- 48.Steyn, A. J., D. M. Collins, M. K. Hondalus, W. R. Jacobs, Jr., R. P. Kawakami, and B. R. Bloom. 2002. Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc. Natl. Acad. Sci. USA 99:3147-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sturgill-Koszycki, S., U. E. Schaible, and D. G. Russell. 1996. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15:6960-6968. [PMC free article] [PubMed] [Google Scholar]
- 50.Sussman, M. 1987. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 28:9-29. [DOI] [PubMed] [Google Scholar]
- 51.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomma, B. P., I. A. Penninckx, W. F. Broekaert, and B. P. Cammue. 2001. The complexity of disease signaling in Arabidopsis. Curr. Opin. Immunol. 13:63-68. [DOI] [PubMed] [Google Scholar]
- 53.Titus, M. A. 2000. The role of unconventional myosins in Dictyostelium endocytosis. J. Eukaryot. Microbiol. 47:191-196. [DOI] [PubMed] [Google Scholar]
- 54.Tonjum, T., D. B. Welty, E. Jantzen, and P. L. Small. 1998. Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: mapping of their relationships to M. tuberculosis by fatty acid profile analysis, DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J. Clin. Microbiol. 36:918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]