Abstract
Fas-mediated apoptosis is an important regulator of cell survival, and abnormalities in this system have been shown to result in a number of human pathological conditions. A secreted member of the tumor necrosis factor receptor superfamily, DcR3, was recently reported to be amplified in human lung and colon cancers as a negative regulator of Fas-mediated apoptosis. We identified this gene, which we call M68. M68 genomic DNA, mRNA, and protein levels were examined in a series of human gastrointestinal tract tumors. Using M68 immunohistochemistry and a scoring system similar to that used for HER-2/neu, we found that M68 protein was overexpressed in 30 of 68 (44%) human adenocarcinomas of the esophagus, stomach, colon, and rectum. Tumors examined by Northern blot revealed M68 mRNA highly elevated in a similar fraction of primary tumors from the same gastrointestinal tract regions, as well as in the colon adenocarcinoma cell lines SW480 and SW1116. Further, we found M68 protein to be overexpressed in a substantial number of tumors in which gene amplification could not be detected by fluorescence in situ hybridization or quantitative genomic PCR, suggesting that overexpression of M68 may precede amplification in tumors. Finally, we find that M68 lies within a four-gene cluster that includes a novel helicase-like gene (NHL) related to RAD3/ERCC2, a plasma membrane Ras-related GTPase and a member of the stathmin family, amplification or overexpression of which may also contribute to cell growth and tumor progression.
Members of the tumor necrosis factor receptor (TNFR) superfamily mediate pleiotropic biological processes ranging from cell proliferation and differentiation to cell survival and programmed cell death. They have been shown to play critical roles in tumorigenesis, tissue remodeling, and the regulation of immune/inflammatory processes (1–3); modulation of TNFR signaling has shown therapeutic promise for a growing list of malignant, autoimmune, inflammatory, and bone disorders (4, 5). A subgroup of TNFR family members function predominantly in the induction of apoptosis: ligand binding to this group results in receptor oligomerization, formation of an adaptor complex, activation of the caspase cascade, and ultimately apoptotic cell death (6). A prototypic TNFR family member is Fas (CD95), which plays a critical effector role in cytotoxic immune responses and cytotoxic T lymphocyte-mediated autoimmune diseases. Fas activation is under tight regulatory control to prevent adventitious apoptosis induction. The apoptosis-inducing TNF family member TRAIL, for example, has several membrane-bound “decoy receptors” (DcR1/TRID/TRAIL-R3/LIT and DcR2/TRAIL-R4/TRUNDD), which are capable of binding TRAIL but are defective in transducing the death signal (reviewed in ref. 7). Recently, a secreted decoy receptor, DcR3, was reported (8). DcR3 binds not to TRAIL but to another apoptosis-inducing ligand, FasL. Pitti et al. (8, 9) found that the DcR3 gene was amplified in approximately half of a variety of human lung tumors and human colon adenocarcinomas by using quantitative PCR and suggested that DcR3 receptor overexpression may also occur, conferring growth advantage on tumor cells by blockade of FasL-induced cell death; however, DcR3 protein levels were not directly measured.
We report here the independent identification of DcR3, which we term M68, as part of a larger screen to identify novel secreted proteins. M68 mapped to chromosome 20q13.3, a region known to be associated with gene amplification and rearrangement in human cancer (10, 11). We examined a variety of human cancers for M68 overexpression by Northern blot and immunohistochemistry (IHC) and M68 gene amplification by quantitative genomic PCR and fluorescence in situ hybridization (FISH). We report that M68 protein and mRNA are overexpressed in a substantial percentage of tumors from all levels of the GI tract, and that this overexpression can occur in the absence of detectable M68 gene amplification. Lastly, we sequenced the 115 kb of genomic DNA surrounding M68 and found that M68 exists in a gene cluster that includes a novel gene, novel helicase-like gene (NHL), which belongs to the RAD3/ERCC2 subfamily, SCLIP, a SCG10-like protein of the stathmin/oncoprotein 18 family, and ARP, a plasma membrane-associated Ras-related GTPase. These findings suggest that mechanisms in addition to gene amplification of M68 may be responsible for the putative tumor-promoting actions of M68/DcR3 and have direct diagnostic and therapeutic implications for the management of human GI-tract tumors, which remain a major unsolved health problem throughout the world (12, 13).
Materials and Methods
Cloning, Sequencing, and Mapping.
Two expressed sequence tag (EST) sequences (GenBank accession nos. AA155701 and AA025672) were identified that showed sequence similarities to the cysteine repeats of osteoprotegerin. These EST sequences were then used to identify additional EST sequences. Two full-length cDNA clones were recovered and sequenced. Genomic clone was obtained from a bacterial artificial chromosome (BAC) library by using two sets of M68-specific PCR primers. One of the positive BACs was subjected to shotgun sequencing as described (14). Radiation hybrid mapping was performed by using both the Genebridge4 and Stanford G3 radiation hybrid panels (15). The M68 BAC clone was labeled with SpectrumGreen dUTP by nick translation (Vysis, Downers Grove, IL) for FISH mapping.
Northern Analysis.
mRNA isolated from human tumor samples was obtained from BioChain Institute, San Leandro, CA. The cDNA probe was labeled by random priming by using 32P dCTP (Ambion, Austin, TX) and hybridized in ExpressHyb (CLONTECH) with the final wash at 55°C. Human cancer cell line blots and human normal tissue blots were obtained from CLONTECH. A human ubiquitin or a human β-actin control probe was used to verify equal mRNA loading in each lane.
Antibody Production and Characterization.
Four M68-specific peptides were used for antibody production in rabbits (Covance, Denver, PA). Stable Chinese hamster ovary cell lines that express M68-Fc fusion protein were established. The conditioned media were subjected to immunoblot analysis with M68 antibodies (1:5,000–10,000 dilutions), followed by HRP-conjugated anti-rabbit IgG (rabbit-specific) secondary antibody (Amersham, 1:5,000) and enhanced chemiluminescence detection. One antibody generated with NH2-CRMPGLERSVRERFLPVH-COOH, corresponding to the C terminus of M68 with KLH coupling, was used for most of the study.
IHC.
Tissues were obtained from the Cooperative Human Tissue Network and the National Disease Research Interchange (Philadelphia, PA). Formalin-fixed paraffin-embedded sections were sequentially incubated in M68 antibody (1:7,000), biotinylated rabbit IgG (1:200; Boehringer Mannheim), and HRP-conjugated avidin–biotin complex (Vector Laboratories). Sections were processed by using the catalyzed reporter deposition method (Renaissance Tyramide Signal Amplification, NEN), and the end product was detected with diaminobenzidine/NiCl2. The slides were counterstained with nuclear fast red (Vector Laboratories). The slides were graded according to HER-2/neu criteria (16). Immunodetection of Fas (CD95) was accomplished by using monoclonal anti-CD95 antibody (Dako).
FISH.
The M68 BAC clone was directly labeled with SpectrumGreen dUTP by nick translation (Vysis). Four micrometer-thick formalin-fixed paraffin-embedded sections were treated in a pretreatment solution (Vysis) for 30 min at 45°C, followed by Proteinase K (0.25 mg/ml) for 25 to 90 min at 45°C. After dehydration, slides were added with probe mixture, denatured in at 90°C for 12 min, and hybridized overnight at 37°C. Slides were subsequently washed in 50% formamide/2× SSC (pH 7.0), 2× SSC, and 2× SSC/0.1%Nonidet P-40 at 45°C and counterstained with propidium iodide.
Quantitative Genomic PCR.
Genomic DNA (Qiagen) was isolated from frozen tissues. Quantitative PCR was carried out by using a TaqMan (Applied Biosystems 7700) instrument. Primers for M68 were designed by using an intron sequence to avoid amplification from M68 mRNA. The M68-specific primers were 5′-CCAGCACGGCTCACTGC-3′ and 5′-TTTCTGGGCCCCACTCG-3′, and the fluorogenic probe was 5′-CAGGGATTTCTCTCTCCTGCAAACCCC-3′. The β-globin primers were 5′-ACCCTTAGGCTGCTGGTGG-3′and 5′-GGAGTGGACAGATCCCCAAA-3′, and the fluorogenic probe was 5′-CTACCCTTGGACCCAGAGGTTCTTTGAGTC-3′. For each run, DNA isolated from normal matched tissues was used for comparison. A second set of M68 and β-globin primers and probes gave similar results.
Results
Isolation of the M68 cDNA and BAC and Chromosomal Localization of M68 to 20q13.3.
The full-length M68 cDNA was cloned by recovering EST clones and PCR cloning from a normal human lung cDNA library. Two alternatively sliced forms of the M68 cDNA were identified in human pancreatic tumor and germ cell tumor libraries (M68C and M68E). The M68 cDNA encodes a protein of 300 aa with a putative signal peptide cleavage site (between aa 29 and 30) and the four tandem cysteine-rich repeats that are the hallmark of the TNFR superfamily. Unlike most of the other TNFR family members, however, no transmembrane domain could be identified, suggesting that the M68 protein is secreted.
M68/DcR3 was mapped by using the Genebridge4 radiation hybrid (RH) panel to the extreme telomere of chromosome 20 at 20q13.3, 28cR from D20S173 with a logarithm of odds score of 13. To confirm the RH data and identify additional genes in the region, a BAC clone was isolated and used to independently map M68/DcR3 by FISH to the telomeric end of the q arm of chromosome 20 (data not shown).
Overexpression of M68 mRNA Level in GI Cancers.
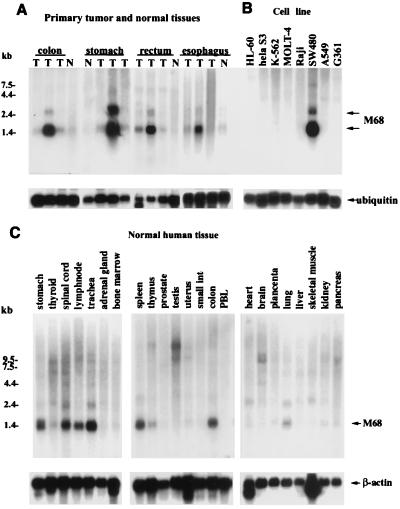
Numerous reports have shown genomic amplifications of 20q13 in breast (17), gastric (18), colon, and lung (10) tumors and in neuroblastomas (19). This chromosomal localization and the high incidence of cDNA clones derived from cancer libraries prompted us to examine M68/DcR3 expression in tumor tissues. Overexpression of M68 mRNA of up to 20-fold, compared to normal controls, was observed in tumors from every level of the GI tract, including esophagus (1/3 tumors), stomach (1/3), rectum (2/3), and colon (1/3) (Fig. 1A). However, no M68/DcR3 mRNA overexpression was seen in any of three for breast, lung, or uterus tumors (data not shown). The human colonic adenocarcinoma cell lines SW480 (Fig. 1B) and SW1116, but not SW403, HT29, or SW948, also showed M68 overexpression (data not shown). M68 mRNA was also detected at a low level in normal human stomach, spinal cord, lymph node, trachea, spleen, colon, and lung as a predominant 1.4-kb message and a minor form at 2.4 kb (Fig. 1C). The 1.4-kb transcript is equal in size to the longest cDNA clone we obtained.
Figure 1.
Expression pattern of M68 mRNA in human tissues and tumors. (A) For each tissue type, mRNA was isolated from tumors from three different donors and from a normal control tissue. T, tumor tissues; N, normal tissues. The same blot was hybridized to a human ubiquitin probe to verify RNA loading in each lane. (B) Human cancer cell lines (PBL, peripheral blood leukocytes). (C) Normal human tissues.
Overexpression of M68 Protein in GI Cancers.
As the level of M68 mRNA appeared to be elevated in a subset of human-GI tract cancers, we next asked whether the same was true for M68 at the protein level. Polyclonal antisera were generated and characterized by ELISA and by Western blot analysis with cells expressing M68 fusion protein. Antisera that recognized a single band at the predicted molecular mass by Western blot (data not shown) were used to carry out IHC. Specificity of the M68 antibodies was demonstrated by (i) absence of immunoreactivity by using preimmune serum from the same rabbit (Fig. 2C) and irrelevant antisera (Fig. 2D); (ii) reduction in immunoreactivity by preincubating antibodies with antigen peptide (Fig. 2B); and (iii) a similar pattern of immunoreactivity seen with two antibodies generated with different M68 antigen peptides (data not shown).
Figure 2.
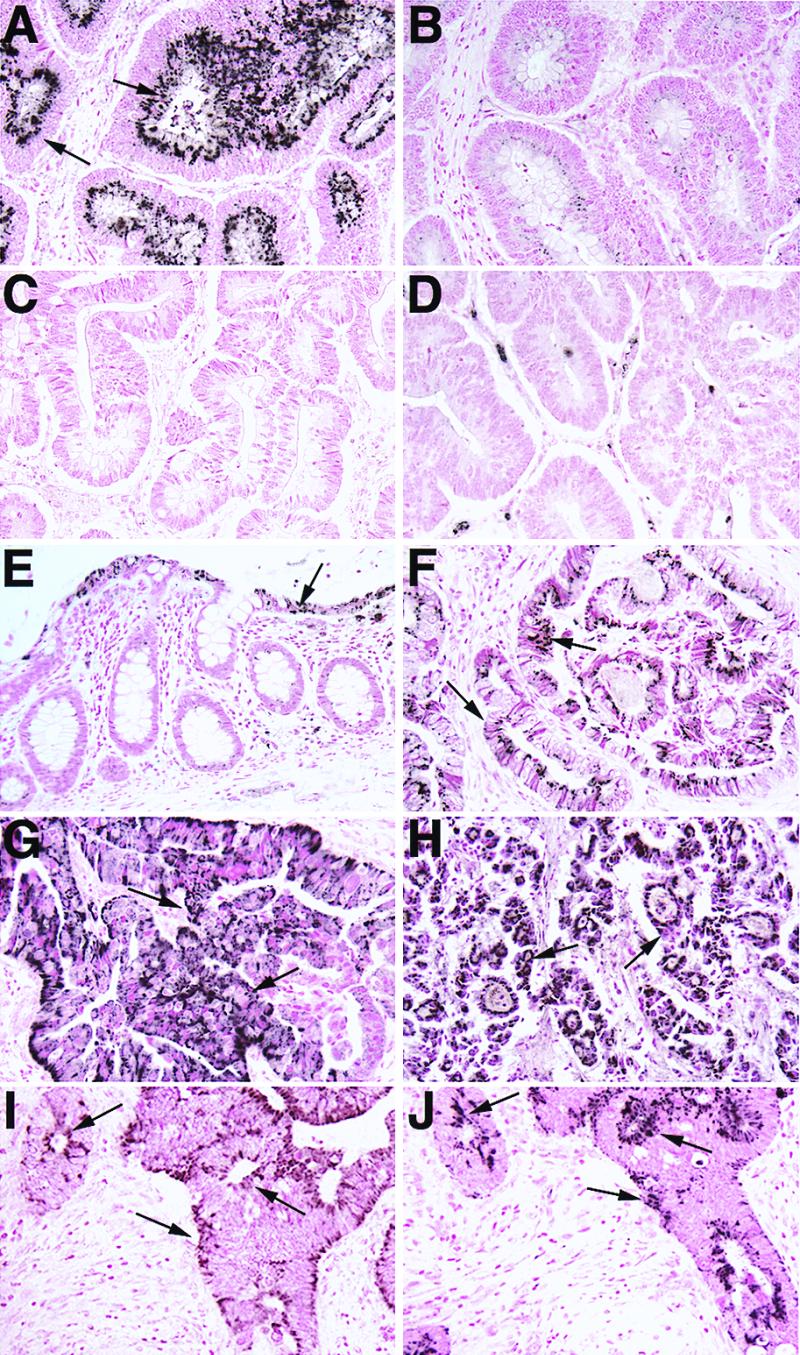
IHC of M68 and FAS in normal and tumor tissues of GI tract. Note strong positive staining in malignant epithelial cells (arrows) in tumor samples (A, colon; F, esophagus; G, stomach; and H, rectum). In normal adjacent colon (E), weak M68 expression was detected in epithelial cells (arrows) lining the lumen and was generally absent in the glandular epithelium. A significant decrease in staining was observed when M68 antibody was preincubated with the immunizing peptide (B), and no tumor epithelial cell staining was observed in tumor tissues with preimmune serum (C) or a nonimmune rabbit serum (D). IHC of Fas (CD95) (I) and M68 (J) in a colon adenocarcinoma. Note the similar staining pattern and coexpression of CD95 and M68 in the tumor epithelial cells (arrows).
In normal GI tissues, M68 immunoreactivity was very weak or undetectable. When M68 immunoreactivity was detectable, it was seen in epithelial cells in a punctate staining pattern suggestive of endoplasmic reticulum, a common location for immunostaining of secreted proteins. Of interest, in some normal colon samples, expression of M68 appeared to be most prevalent in the luminal portions of the crypt epithelium (Fig. 2E); these cells are maturing and subsequently undergo apoptosis and phagocytosis or are shed into the lumen of the gut when they reach the surface epithelium. Apoptotic regulation is thought to be a dominant factor controlling epithelial cell turnover, yet the underlying mechanism remains unclear (20, 21). This raises the possibility that M68 may normally function to regulate apoptosis of maturing colonic epithelial cells.
In many tumor samples, both the number of cells expressing M68 protein and the intensity of the M68 immunoreactivity were greatly elevated. To quantify M68 protein expression in tumors, a scoring system was developed based on the validated system for HER-2/neu, a protein overexpressed in a subset of human breast and ovarian tumors (16). Slides were scored on a four-point scale (Fig. 3). Overall, 44% (30 of 68) of tumors scored 2+ or 3+ on this scale and were therefore designated as overexpressing M68. Analysis by location of tumor within the GI tract indicated overexpression in all GI segments: 5 of 8 (63%) of primary esophageal tumors, 8 of 28 (29%) primary gastric tumors, 17 of 25 (68%) primary colon tumors, and 2 of 6 (33%) primary rectal tumors showed M68 overexpression (Fig. 2 A–H). Analysis of 30 normal tissues adjacent to these tumors showed 0 or occasionally 1+ expression, indicating that M68 protein overexpression is not a result of variability among individuals.
Figure 3.
IHC scoring of M68 overexpression in formalin-fixed paraffin-embedded sections from GI-tract cancer tissues. (A) No or minimal staining, in <10% of the tumor cells; score, 0. (B) Faint barely visible staining in >10% of the tumor cells; score, 1+. (C) Weak to moderate staining in >10% of the tumor cells; score, 2+. (D) Strong staining in >10% of the tumor cells; score, 3+. 2+ and 3+ are considered positive for M68 overexpression.
M68 Is Coexpressed with Fas in Colonic Epithelial Cells and May Exhibit a Tissue-Protective Role.
If M68 plays the role of a “decoy” receptor, competing with Fas to bind FasL, M68 might be expressed in GI-tract epithelial cells close to those expressing Fas. To test this hypothesis, Fas IHC was carried out on adjacent sections of the colon tumors in which M68 overexpression was seen. Fas was found to be expressed in colonic epithelium in a pattern similar to that of M68 (Fig. 2 I and J). Interestingly, although M68 protein expression was elevated in tumors, Fas protein expression in tumors was unchanged or diminished compared with control tissues.
If coexpressed with Fas and FasL in normal tissue, M68 might protect against apoptosis caused by Fas stimulation. Fas-induced apoptosis plays a role in the hepatic pathology of Wilson's disease, in which FasL is induced together with Fas in hepatocytes overloaded with copper (22, 23). We used an in vitro model of copper-induced hepatocellular injury to test whether expression of M68 could protect cells from apoptosis. HepG2 hepatocytes were treated with 100 μM copper chloride for 48 h and after infection with an adenovirus vector expressing M68 (V.S. and C.B., unpublished work) or with cultured medium from M68-Ad-infected cells. Cells infected with M68-Ad or treated with M68-Ad-cultured medium showed approximately half the level of apoptosis as did cells infected with a control vector. The extent of protection from apoptosis with M68 was similar to that seen with z-FAD-FMK (data not shown). These data suggest that M68 may indeed serve a physiologically important role in the regulation of apoptosis.
M68 Overexpression May Occur Without M68 Gene Amplification.
M68/DcR3 gene amplification was proposed to be the mechanism for M68/DcR3 to promote tumor survival in lung and colon cancer (8). To determine whether M68/DcR3 overexpression was always the consequence of gene amplification, quantitative PCR was carried out on genomic DNA from tumors that had shown 3+ overexpression of M68 by IHC and their matching normal tissue control, with relative M68 gene copy number determined by normalization to the β-globin gene. Unexpectedly, only one of six tumors showed a greater than 2-fold amplification (Fig. 4). Failure to detect significant M68 gene amplification in the tumor samples was not caused by PCR inhibitory factors present in our tumor DNA preparation, because when M68 BAC DNA at comparable or double molar ratios to genomic DNA was added to the tumor DNA samples, M68 gene amplification was detected at the expected level (data not shown).
Figure 4.
M68 gene amplification and overexpression. (A and B) A representative of a M68-overexpressing gastrointestinal tumor without M68 gene amplification. (A) FISH dot count with M68 BAC DNA. (B) IHC of M68 protein expression to the adjacent section of A. (C and D) A representative of M68-overexpressing gastrointestinal tumor with M68 gene amplification. (C) FISH dot count with M68 BAC DNA. (D) IHC of M68 protein expression to the adjacent section of C. (E) TaqMan quantitative PCR analysis of M68 genomic DNA. Samples c and f were from the same individual tumors as shown in A, B and C, D, respectively. Data shown are the average of at least two independent experiments.
To address the possibility that the lack of amplification was caused by noncancerous cells in the tumor samples that may reduce the sensitivity of the PCR-based assay, we carried out FISH analysis (24) by using sections adjacent to those used for IHC from the same tumors used for PCR above. Five counts of one hundred nonoverlapping nuclei in different areas of the sections were used to obtain a percentage of cells with or without amplification. Three of six tumors showed only the normal two and occasionally four dots, indicating the normal M68 gene number, consistent with the PCR results (Fig. 4 A and B). The sixth tumor did show M68 gene amplification by FISH, with approximately half of the cells showing more than five dots (Fig. 4 C and D). The remaining two tumors showed marginal amplification, with 11 and 20 of 500 cells showing 5 or more dots; some of these may be polyploid/aneuploid cells with more than two copies of chromosome 20. The levels of gene amplification observed here are unlikely to account for the dramatic increases in M68 mRNA and protein.
M68 Is Located in a Gene-Rich Cluster with Other Potentially Tumor-Related Genes.
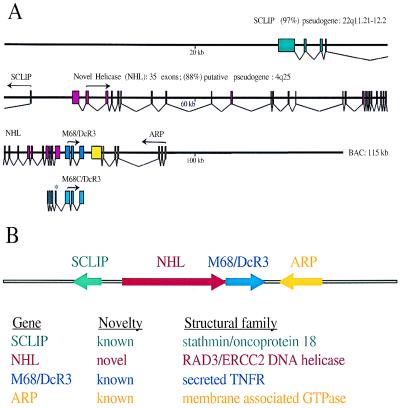
The complete DNA sequence of the BAC clone, hbm168, was completely determined. The coding region for M68/DcR3 consists of three exons that span less than 2 kb of genomic DNA (Fig. 5A). No putative transmembrane domain-encoding sequence was found in this region, indicating that the isolated M68 cDNA is not an alternatively spliced form of a membrane-bound receptor. blast analysis (25) identified two additional known genes in the genomic sequence: SCLIP (26) and ARP (27).
Figure 5.
Genomic structure of M68 and the adjacent genes. (A) Exon/intron organization of human M68 BAC genomic sequence. Exons, which are represented as shaded boxes, were determined by using the crossmatch program and manual editing to conform exon boundaries to standard splice-site consensus sequences. Arrows represent the translation initiation methionine and the direction of protein translation. (B) Schematic representation of the M68 gene cluster and family structure.
Between the SCLIP and the M68/DcR3, exon prediction by using grail2 and sequence alignment to a contiguous 4.5-kb region of chromosome 4 (88% sequence identity) revealed another putative gene structure containing 35 exons (Fig. 5 A and B). The complete exon structure of this gene, termed NHL, was subsequently confirmed by reverse transcription–PCR analysis and recent submissions of two partial cDNA sequences (GenBank accession nos. a1080127 and ab029011), which confirmed exons 9 through 35, with the exception of al080127, which revealed an additional 53 bases on the 5′ end of exon 31. The predicted 1,219-aa sequence shares 26% sequence identity and 48% sequence similarity with the RAD3/ERCC2 gene family of DNA helicases (28–30). The seven domains of known helicases (31) were highly conserved in NHL (Fig. 6). However, the C-terminal end of NHL did not show similarity to any known protein sequences. Of interest, the 5′ untranslated region of one of the M68 clones, M68C, showed sequence overlap with exons 32 through 35 of the putative NHL helicase. Although the significance of this sequence overlap is unclear, coordinate regulation of gene transcripts has been suggested in other examples of overlap (32). Northern blot analysis of ARP mRNA levels in a small number of tumors did not demonstrate overexpression (data not shown).
Figure 6.
Alignment of the putative novel DNA helicase to Rad3/ERCC2 subfamily of DNA helicases. The multiple sequence alignment was performed by using the program clustalw Ver. 1.74 and enhanced to display identical amino acid residues in shaded green and similar residues in shaded blue. In addition, the seven conserved domains of known DNA helicases are shown above the alignment, and the nucleotide- and DNA-binding domains are shown below the alignment.
Discussion
In this paper, we demonstrate M68 mRNA and protein overexpression in human GI cancers, characterize the relationship of this M68 overexpression to gene amplification, and report the existence of other genes in the amplified region that may contribute to tumorigenesis. Although genomic amplification of M68 in about half of colon and lung tumors has been demonstrated previously (8), this is, to our knowledge, the first evidence that M68 protein is overexpressed in tumors. We also report the first association of M68 with stomach, esophagus, and rectum cancers, demonstrating that tumors of all levels of the human GI tract are characterized by M68 overexpression. Significantly, we find that overexpression of M68/DcR3 protein may occur without genomic amplification, suggesting that M68 overexpression may precede gene amplification in tumors. Finally, we elucidated the genomic structure of the M68 region and identified three other immediately adjacent or overlapping genes, raising the possibility that they may be associated with tumorigenesis.
The identification of tumors with high-level M68 protein overexpression with no significant gene amplification raises the possibility that M68 protein overexpression may be an early event in oncogenesis, possibly driving selection for gene amplification. The mechanism of nongenomic M68 overexpression remains unclear; possibilities include direct transcriptional induction, methylation changes at the promoter, alternative splicing, and change in mRNA stability, and will be the focus of future work. The discrepancy between our data and that of Pitti et al. (8) may be caused by the use of tumor specimens from different stages and sources, preserved in different ways. Testing of larger numbers of tumors will be needed to determine the overall frequency of M68 gene amplification, mRNA overexpression, and protein overexpression; at this point, it is clear only that the three are not invariably correlated. The mechanism by which M68 transcripts is up-regulated deserves further study, given the known progressive mutation of transcriptional suppressors such as p53 in some tumors.
The extensive experience with HER-2/neu testing of breast and ovarian cancer specimens provides a useful precedent to which the M68/DcR3 data may be compared. Overexpression and/or amplification of HER-2/neu has been demonstrated in 25–30% of breast cancers (33) and predicts prognosis and response to therapy (34). By comparison, M68/DcR3 has been hypothesized to confer growth advantage by inhibiting Fas-mediated destruction of tumor cells (8). M68/DcR3 gene amplification has so far been demonstrated in approximately 50% of lung and colon tumors (8) and M68/DcR3 overexpression, in 44% of GI tract tumors (this work). In this report, we demonstrate overexpression of M68/DcR3 in the absence of amplification; although this discordance was quite common in our sample, we studied only a small number of tumors. At the same time, protein overexpression is likely to be the eventual diagnostic method of choice for M68/DcR3, given that IHC reflects the functional protein level and is more easily performed than FISH in most clinical centers. IHC is currently the preferred diagnostic method in advance of anti-HER-2/neu immunotherapy with trastuzumab (Herceptin) (35–37).
Gene amplification is a well established mechanism by which oncogene activation may occur during tumorigenesis, and it may encompass several hundreds to thousands of kilobases (36). For example, the HER-2/ErbB2 amplicon is known to contain multiple genes, most of which are overexpressed in tumors (38, 39), although the significance of their coamplification/overexpression is not known. The identified gene, NHL, is most interesting, given the association of DNA helicases with multiple inherited human neoplastic disorders, including xeroderma pigmentosum, Cockayne's syndrome, Bloom's syndrome, and Werner's syndrome, and the role of the RAD3/ERCC2 subfamily in genome stability. It will be important to determine whether there are mutations in NHL in inherited human disorders. SCLIP belongs to a small family of microtubule-destabilizing phosphoproteins that includes stathmin/oncoprotein 18, RB3, and SCG10. Stathmin is overexpressed in 30% of breast tumors (40). Though SCLIP appears to be specifically expressed in brain, more detailed study of SCLIP expression in tumors is warranted. Of note, both NHL and SCLIP are duplicated (Fig. 5A), so future studies must be done carefully to distinguish between the duplicated counterparts. In contrast to NHL and SCLIP, it is unlikely that ARP is etiologic in oncogenesis, given that ARP mRNA levels were unchanged in tumors with highly elevated M68 mRNA levels (data not shown).
Future studies will need to elucidate the mechanism by which M68/DcR3 overexpression contributes to oncogenesis. While this manuscript was in preparation, a report by Yu et al. (41) showed that M68/TR6/DcR3 binds to and inhibits LIGHT-mediated apoptosis. LIGHT is a TNF family member that is involved in the cytotoxic killing of HEMV- and LT-R-expressing cells. Finally, the prognostic significance of M68 overexpression in human cancers will need to be determined, as will the therapeutic value of down-regulating M68 overexpression by genetic or immunotherapeutic means.
Acknowledgments
We are grateful to M. Oshima and T. Ishikawa for discussions, to G. Xie and Y. Liu for expertise on bioinformatics, and to G. Wollenberg for pathology.
Abbreviations
- TNFR
tumor necrosis factor receptor
- IHC
immunohistochemistry
- GI
gastrointestinal tract
- FISH
fluorescence in situ hybridization
- EST
expressed sequence tag
- BAC
bacterial artificial chromosome
Footnotes
References
- 1.Siegel R M, Fleisher T A. J Allergy Clin Immunol. 1999;103(5):729–738. doi: 10.1016/s0091-6749(99)70412-4. [DOI] [PubMed] [Google Scholar]
- 2.Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 3.Baker S J, Reddy E P. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- 4.Keystone E C. J Rheumatol. 1999;26 Suppl 57:22–28. [PubMed] [Google Scholar]
- 5.Mueller H. Cell Mol Life Sci. 1998;54:1291–1298. doi: 10.1007/s000180050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter M E. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Dixit V M. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 8.Pitti R M, Marsters S A, Lawrence D A, Roy M, Kischkel F C, Dowd P, Huang A, Donahue C J, Sherwood S W, Baldwin D T, et al. Nature (London) 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 9.Green D R. Nature (London) 1998;396:629–630. doi: 10.1038/25248. [DOI] [PubMed] [Google Scholar]
- 10.Muleris M, Almeida A, Gerbault-Seureau M, Malfoy B, Dutrillaux B. Genes Chromosomes Cancer. 1995;14:155–163. doi: 10.1002/gcc.2870140302. [DOI] [PubMed] [Google Scholar]
- 11.Shinomiya T, Mori T, Ariyama Y, Sakabe T, Fukuda Y, Murakami Y, Nakamura Y, Inazawa J. Genes Chromosomes Cancer. 1999;24:337–344. [PubMed] [Google Scholar]
- 12.Parkin D M, Pisani P, Ferlay J. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Wingo P A, Ries L A G, Giovino G A, Miller D S, Rosenberg H M, Shopland D R, Thun M J, Edwards B K. J Natl Cancer Inst. 1999;91:675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 14.Petrukhin K, Koisti M J, Bakall B, Li W, Xie G, Marknell T, Sandgren O, Forsman K, Holmgren G, Andreasson S, et al. Nat Genet. 1998;19:241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- 15.Cox D R, Burmeister M, Price E R, Kim S, Myers R M. Science. 1990;250:245–250. doi: 10.1126/science.2218528. [DOI] [PubMed] [Google Scholar]
- 16.Wisecarver J L. Am J Clin Pathol. 1999;111:299–301. doi: 10.1093/ajcp/111.3.299. [DOI] [PubMed] [Google Scholar]
- 17.Schwendel A, Richard F, Langreck H, Kaufmann O, Lage H, Winzer K J, Petersen I, Dietel M. Br J Cancer. 1998;78:806–811. doi: 10.1038/bjc.1998.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakakura C, Mori T, Sakabe T, Ariyama Y, Shinomiya T, Date K, Hagiwara A, Yamaguchi T, Takahashi T, Nakamura Y, et al. Genes Chromosomes Cancer. 1999;24:299–305. doi: 10.1002/(sici)1098-2264(199904)24:4<299::aid-gcc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Altura R A, Maris J M, Li H, Boyett J M, Brodeur G M, Look A T. Genes Chromosomes Cancer. 1997;19:176–184. doi: 10.1002/(sici)1098-2264(199707)19:3<176::aid-gcc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Hall P A, Coates P J, Ansari B, Hopwood D. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 21.Potten C S, Wilson J W, Booth C. Stem Cells. 1997;15:82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- 22.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 23.Strand S, Hofmann W J, Grambihler A, Hug H, Volkmann M, Otto G, Wesch H, Mariani S M, Hack V, Stremmel W, et al. Nat Med. 1998;4:588–593. doi: 10.1038/nm0598-588. [DOI] [PubMed] [Google Scholar]
- 24.Press M F, Bernstein L, Thomas P A, Meisner L F, Zhou J Y, Ma Y, Hung G, Robinson R A, Harris C, El-Naggar A, et al. J Clin Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 25.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozon S, Byk T, Sobel A. J Neurochem. 1998;70:2386–2396. doi: 10.1046/j.1471-4159.1998.70062386.x. [DOI] [PubMed] [Google Scholar]
- 27.Schurmann A, Massmann S, Joost H G. J Biol Chem. 1995;270:30657–30663. doi: 10.1074/jbc.270.51.30657. [DOI] [PubMed] [Google Scholar]
- 28.Naumovski L, Chu G, Berg P, Friedberg E C. Mol Cell Biol. 1985;5:17–26. doi: 10.1128/mcb.5.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds P, Higgins D R, Prakash L, Prakash S. Nucleic Acids Res. 1985;13:2357–2372. doi: 10.1093/nar/13.7.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber C A, Salazar E P, Stewart S A, Thompson L H. EMBO J. 1990;9:1437–1447. doi: 10.1002/j.1460-2075.1990.tb08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. Nucleic Acids Res. 1989;12:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog H, Darby K, Ball H, Hort Y, Beck-Sickinger A, Shine J. Genomics. 1997;41:315–319. doi: 10.1006/geno.1997.4684. [DOI] [PubMed] [Google Scholar]
- 33.Slamon D J, Godolphin W, Jones L A, Holt J A, Wong S G, Keith D E, Levin W J, Stuart S G, Udove J, Ullrich A. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 34.Pegram M D, Pauletti G, Slamon D J. Breast Cancer Res Treat. 1998;52:65–77. doi: 10.1023/a:1006111117877. [DOI] [PubMed] [Google Scholar]
- 35.Press M F, Pike M C, Chazin V R, Hung G, Udove J A, Markowicz M, Danyluk J, Godolphin W, Sliwkowski M, Akita R. Cancer Res. 1993;53:4960–4970. [PubMed] [Google Scholar]
- 36.Ross J S, Fletcher J A. Am J Clin Pathol. 1999;112,(Suppl 1):S53–S67. [PubMed] [Google Scholar]
- 37.Physicians' Desk Reference (1999) ed. Walsh, P. (Medical Economics, Montvale, NJ) 53 (Suppl A), A9.
- 38.Tanaka S, Mori M, Akiyoshi T, Tanaka Y, Mafune K, Wands J R, Sugimachi K. Cancer Res. 1997;57:28–31. [PubMed] [Google Scholar]
- 39.Akiyama N, Sasaki H, Ishizuka T, Kishi T, Sakamoto H, Onda M, Hirai H, Yazaki Y, Sugimura T, Terada M. Cancer Res. 1997;57:3548–3553. [PubMed] [Google Scholar]
- 40.Bieche I, Lachkar S, Becette V, Cifuentes-Diaz C, Sobel A, Lidereau R, Curmi P A. Br J Cancer. 1998;78:701–709. doi: 10.1038/bjc.1998.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu K Y, Kwon B, Ni J, Zhai Y, Ebner R, Kwon B S. J Biol Chem. 1999;274:13733–13736. doi: 10.1074/jbc.274.20.13733. [DOI] [PubMed] [Google Scholar]