Abstract
Nicotinic acetylcholine receptors (nAChRs) play an essential role in neurotransmission. Recent studies have indicated that nAChRs may be involved in the regulation of some bacterial infections through immunological mechanisms in macrophages. However, the regulation of infection with Chlamydia pneumoniae, which is a ubiquitous pneumonia-causing bacterium, by an nAChR-mediated mechanism is still unclear. In the present study, it was found that stimulation of nAChRs with ligands such as nicotine and acetylcholine altered the growth of C. pneumoniae in epithelial HEp-2 cells. Thus, the results revealed a possible pathophysiological role of nAChRs in the regulation of intracellular bacterial infection.
It is well documented that nicotinic acetylcholine (ACh) receptors (nAChRs), which are found mainly in the central and peripheral nervous systems and on many other tissue cells throughout the body, are also present on immune cells (4, 8). Although the function of peripheral nAChRs has not been well investigated, such localization suggests that peripheral nAChRs have a nonsynaptic role. Nicotine, which is the primary constituent that regulates the maintenance of tobacco use and is also one of the key constitutes of tobacco products causing adverse health consequences (21), and ACh, a neurotransmitter in cholinergic neurons, are major ligands for nAChRs. It has been recently demonstrated that activation of nAChRs by a ligand such as nicotine resulted in alteration of immune functions, besides facilitation of cation flow (11). In addition, modulation of growth of Legionella pneumophila, an intracellular pathogen that causes bacterial pneumonia in immunocompromised hosts, has also been shown following stimulation of nAChRs with nicotine (13). Thus, the nonsynaptic role of nAChRs seems important in nonneuronal cells.
Chlamydia (Chlamydophila) pneumoniae is an obligate intracellular bacterium that causes a variety of respiratory illnesses, including community-acquired pneumonia, bronchitis, pharyngitis, and sinusitis (1, 2, 17). Current studies indicate the possible involvement of C. pneumoniae in chronic inflammatory diseases, such as atherosclerosis, besides respiratory illness (10). It is known that tobacco smoking accelerates pneumonia caused by bacteria, including C. pneumoniae (12, 14). In addition, the prevalence of C. pneumoniae in clinical specimens obtained from tobacco smokers is significantly higher than that from nonsmokers (18). Although these clinical findings indicate a possible linkage between tobacco components and acceleration of C. pneumoniae infection, the mechanisms of infection modulation by tobacco smoking are unclear. Nicotine appears to be one of the major immunomodulatory components of tobacco smoke because it has been shown that treatment of human peripheral blood mononuclear cells with nicotine significantly inhibited the production of a variety of cytokines in response to anti-CD3 stimulation (16). Therefore, in the present study, a possible alteration of C. pneumoniae infection mediated by nAChRs with nicotine, as well as endogenous ACh, was assessed in order to determine a possible pathophysiological role of nAChRs in the regulation of bacterial infection.
Human epithelial HEp-2 cells (American Type Culture Collection, Manassas, Va.) cultured in Dulbecco's modified Eagle's medium (Sigma Chemical, St. Louis, Mo.) supplemented with 10% heat-inactivated fetal bovine serum and antibiotics (gentamicin at 10 μg/ml, vancomycin at 10 μg/ml, and amphotericin B at 1 μg/ml) were used in this study. The nAChR agonist nicotine hydrogen bitartrate, 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP), and ACh and the antagonist d-tubocurarine (d-TC), were purchased from Sigma. All reagents were dissolved in pyrogen-free water, sterilized by filtration with a membrane, and diluted to working concentrations with medium. C. pneumoniae TW183, confirmed Mycoplasma free by PCR (15), was propagated in HEp-2 cells (7). The numbers of inclusion-forming units (IFU) in chlamydia preparations were determined by counting chlamydial inclusions in HEp-2 cells (19). Infection of HEp-2 cells for the study of nAChR involvement in the control of bacterial growth was performed as follows. Cells (2 × 105/well, 24-well plates) were infected by incubation with C. pneumoniae at a multiplicity of infection of 10 for 2 h and then washed to remove noninfected bacteria. The infected cells were treated with or without nicotine (0.001 to 10 μg/ml [0.00064 to 6.4 μM]), DMPP (1 μM), or ACh (1 μM) for 72 h at 37°C in 5% CO2. In some experiments, infected cells were pretreated with d-TC (10 μM) for 15 min before nAChR agonist treatment. The concentrations of nAChR agonists and antagonist used were previously confirmed to be appropriate concentrations showing stimulation and blocking of nAChRs (13). At the concentrations used, these agonists and antagonist did not show any cytotoxicity for HEp-2 cells during incubation for up to 72 h, as determined by trypan blue dye exclusion assay. Assessment of C. pneumoniae growth in cells was performed by detection of infective progeny bacteria (IFU) as described previously (20). The transcripts of nAChR subunit genes (α4, α7, β2, and β4) in HEp-2 cells were determined by reverse transcription (RT)-PCR as described previously (13). The primers for these genes were designed from GenBank cDNA sequences by using the website program Primer 3 (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3.cgi). The primer sequences for nAChR α4 (fragment size, 172 bp) were 5′-CAC GTT TGC CAA ATT TTC CT-3′ (sense) and 5′-CCG AGT CCT GCA GGT AGA AG-3′ (antisense). The primer sequences for nAChR α7 (fragment size, 180 bp) were 5′-CCC AAG TGG ACC AGA GTC AT-3′ (sense) and 5′-GAT GTA CAG CAG GTT CCC GT-3′ (antisense). The primer sequences for nAChR β2 (fragment size, 201 bp) were 5′-GGC ATG TAC GAG GTG TCC TT-3′ (sense) and 5′-CAC CTC ACT CTT CAG CAC CA-3′ (antisense). The primer sequences for nAChR β4 (fragment size, 173 bp) were 5′-GTT CAT GTT TGT GTG CGT CC-3′ (sense) and 5′-AAC CCA GAA AGA AGC AGC AA-3′ (antisense). The primers for β actin were synthesized on the basis of sequences described previously (6). The specificity of the PCR was confirmed by oligonucleotide sequencing of PCR products (Qiagen, Valencia, Calif.).
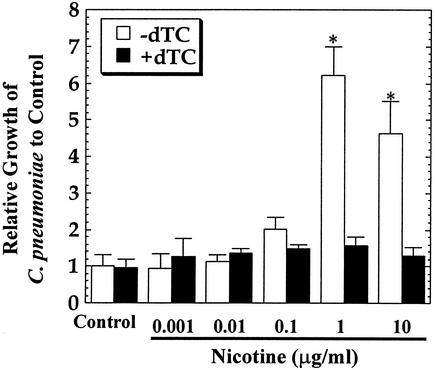
As shown in Fig. 1, treatment of HEp-2 cells with nicotine after infection with bacteria resulted in a significant increase in C. pneumoniae inclusion numbers in cells at 72 h after cultivation. The size and morphology of chlamydia inclusions were not significantly affected by the treatment. The concentration required for significant enhancement of bacterial growth was more than 1 μg/ml, which is higher than the level in the plasma of heavy smokers (33 ± 15 ng/ml) (5). It has been reported that the mean nicotine yield of tobacco smoking is ∼0.91 mg/cigarette (9). Therefore, the concentration of nicotine in the respiratory tract after tobacco smoking may be higher than the level in plasma. Nevertheless, the findings revealed that nicotine alters the growth of C. pneumoniae in cells. Such an effect of nicotine on bacterial growth was almost completely blocked by the addition of d-TC, a nonselective nAChR antagonist, at all of the nicotine concentrations tested. This result indicates that the effect of nicotine on bacterial growth may be mediated by nAChRs. Since the small molecular size and lipophilic characteristics of nicotine may allow a small amount to directly cross cell membranes, blocking of the effect of nicotine by a receptor antagonist indicates the possibility that nAChRs are involved in bacterial growth regulation.
FIG. 1.
Effect of nicotine on C. pneumoniae growth in HEp-2 cells. Cells infected with bacteria were treated with or without the indicated concentrations of nicotine in the presence or absence of d-TC (10 μM) and then incubated for 72 h. The number of IFU in cells was assessed and expressed as bacterial growth relative to that of the control group. The data shown are the mean plus the standard deviation for triplicate cultures and are representative of three experiments. The number of IFU per culture of the control group 72 h after infection was 2.8 × 104 ± 0.9 × 104. ∗, P < 0.05 (significantly different from the control group).
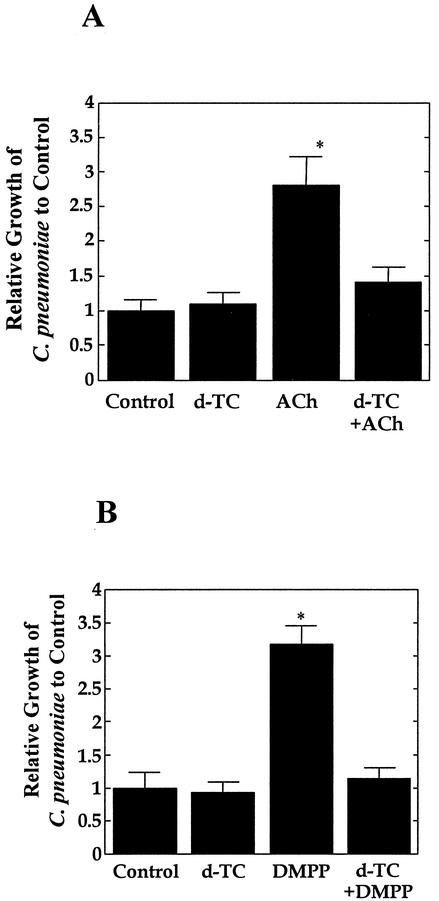
In order to define the possible involvement of nAChRs in the regulation of C. pneumoniae growth in cells, bacterium-infected HEp-2 cells were treated with other nAChR agonists, such as ACh and DMPP, and growth of the bacteria was assessed. Figure 2 shows the results of such experiments. Stimulation of nAChRs by these agonists significantly enhanced the growth of C. pneumoniae in cells, similar to the effect of nicotine. Furthermore, these bacterial-growth-enhancing effects of agonists were completely abolished by treatment with the antagonist d-TC, similar to the case of nicotine and d-TC treatment experiments. These results clearly show the involvement of nAChRs in the regulation of C. pneumoniae growth in cells.
FIG. 2.
Effects of the nAChR agonists ACh (A) and DMPP (B) on C. pneumoniae growth in HEp-2 cells. Cells were infected with bacteria and then treated with or without ACh (1 μM) or DMPP (1 μM) in the presence or absence of d-TC (10 μM). The number of IFU was assessed at 72 h after infection and expressed as bacterial growth relative to that of the control. The data shown are the mean plus the standard deviation for triplicate cultures and are representative of three experiments. The numbers of IFU per culture of the control group at 72 h after infection in the ACh and DMPP experiments were 2.2 × 104 ± 0.4 × 104 and 2.7 × 104 ± 0.6 × 104, respectively. ∗, P < 0.05 (significantly different from the control group).
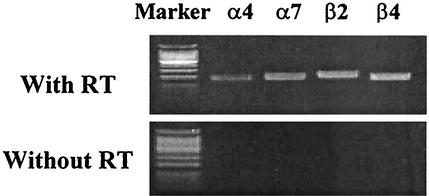
It is widely accepted that nAChRs form a family of receptors and that they are differentially expressed in many tissues (3). However, it is not known whether and which type of nAChRs are present in HEp-2 cells. Therefore, in order to define the presence of nAChRs in HEp-2 cells, transcripts of nAChR subunit genes (α4, α7, β2, and β4) were assessed by RT-PCR. The PCR products of HEp-2 cells without RT were examined as a negative control. As shown in Fig. 3, HEp-2 cells expressed all of the subunits of nAChRs tested. Even though the study demonstrated only receptor message expression, the results of the blocking study with the antagonist and the receptor message study indicate the expression of nAChRs in HEp-2 cells. Whereas the presence of nAChRs in the cells was indicated, the type of nAChRs, which are assembled from five subunits in accordance with defined combination rules (3), present in HEp-2 cells was not made clear by this study.
FIG. 3.
Transcripts of nAChR subunit genes determined by RT-PCR. Expression of nAChR mRNAs (α4, α7, β2, and β4 subunits) in HEp-2 cells was analyzed by RT-PCR.
In conclusion, nAChRs are involved in the regulation of C. pneumoniae growth in cells. The findings highlight the importance of nAChRs in not only the neurotransmissional but also the pathophysiological aspects of infection.
Editor: J. N. Weiser
REFERENCES
- 1.Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1993. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 90:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne, G. I., L. K. Lehmann, and G. J. Landry. 1986. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect. Immun. 53:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordero-Erausquin, M., L. M. Marubio, R. Klink, and J. P. Changeux. 2000. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol. Sci. 21:211-217. [DOI] [PubMed] [Google Scholar]
- 4.Drescher, D. G., K. M. Khan, G. E. Green, B. J. Morley, K. W. Beisel, H. Kaul, D. Gordon, A. K. Gupta, M. J. Drescher, and R. L. Barretto. 1995. Analysis of nicotinic acetylcholine receptor subunits in the cochlea of the mouse. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 112:267-273. [DOI] [PubMed] [Google Scholar]
- 5.Ebert, R. V., M. E. McNabb, K. T. McCusker, and S. L. Snow. 1983. Amount of nicotine and carbon monoxide inhaled by smokers of low-tar, low-nicotine cigarettes. JAMA 250:2840-2842. [PubMed] [Google Scholar]
- 6.Haranaga, S., H. Ikejima, H. Yamaguchi, H. Friedman, and Y. Yamamoto. 2002. Analysis of Chlamydia pneumoniae growth in cells by reverse transcription-PCR targeted to bacterial gene transcripts. Clin. Diagn. Lab. Immunol. 9:313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haranaga, S., H. Yamaguchi, H. Friedman, S. Izumi, and Y. Yamamoto. 2001. Chlamydia pneumoniae infects and multiplies in lymphocytes in vitro. Infect. Immun. 69:7753-7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiemke, C., M. Stolp, S. Reuss, A. Wevers, S. Reinhardt, A. Maelicke, S. Schlegel, and H. Schroder. 1996. Expression of alpha subunit genes of nicotinic acetylcholine receptors in human lymphocytes. Neurosci. Lett. 214:171-174. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis, M. J., R. Boreham, P. Primatesta, C. Feyerabend, and A. Bryant. 2001. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: evidence from a representative population survey. J. Natl. Cancer Inst. 93:134-138. [DOI] [PubMed] [Google Scholar]
- 10.Kalayoglu, M. V., P. Libby, and G. I. Byrne. 2002. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA 288:2724-2731. [DOI] [PubMed] [Google Scholar]
- 11.Klapproth, H., K. Racke, and I. Wessler. 1998. Acetylcholine and nicotine stimulate the release of granulocyte-macrophage colony stimulating factor from cultured human bronchial epithelial cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 357:472-475. [DOI] [PubMed] [Google Scholar]
- 12.Leinonen, M., and P. Saikku. 1999. Interaction of Chlamydia pneumoniae infection with other risk factors of atherosclerosis. Am. Heart J. 138:S504-S506. [DOI] [PubMed] [Google Scholar]
- 13.Matsunaga, K., T. W. Klein, H. Friedman, and Y. Yamamoto. 2001. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J. Immunol. 167:6518-6524. [DOI] [PubMed] [Google Scholar]
- 14.Nuorti, J. P., J. C. Butler, M. M. Farley, L. H. Harrison, A. McGeer, M. S. Kolczak, and R. F. Breiman. 2000. Cigarette smoking and invasive pneumococcal disease. N. Engl. J. Med. 342:681-689. [DOI] [PubMed] [Google Scholar]
- 15.Ossewaarde, J. M., A. de Vries, T. Bestebroer, and A. F. Angulo. 1996. Application of a Mycoplasma group-specific PCR for monitoring decontamination of Mycoplasma-infected Chlamydia sp. strains. Appl. Environ. Microbiol. 62:328-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang, Y., N. Virasch, P. Hao, M. T. Aubrey, N. Mukerjee, B. E. Bierer, and B. M. Freed. 2000. Suppression of human IL-1β, IL-2, IFN-γ, and TNF-α production by cigarette smoke extracts. J. Allergy Clin. Immunol. 106:280-287. [DOI] [PubMed] [Google Scholar]
- 17.Shemer, Y., and I. Sarov. 1985. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect. Immun. 48:592-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smieja, M., R. Leigh, A. Petrich, S. Chong, D. Kamada, F. E. Hargreave, C. H. Goldsmith, M. Chernesky, and J. B. Mahony. 2002. Smoking, season, and detection of Chlamydia pneumoniae DNA in clinically stable COPD patients. BMC Infect. Dis. 2:12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summersgill, J. T., N. N. Sahney, C. A. Gaydos, T. C. Quinn, and J. A. Ramirez. 1995. Inhibition of Chlamydia pneumoniae growth in HEp-2 cells pretreated with gamma interferon and tumor necrosis factor alpha. Infect. Immun. 63:2801-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi, H., S. Haranaga, H. Friedman, J. A. Moor, K. E. Muffly, and Y. Yamamoto. 2002. A Chlamydia pneumoniae infection model using established human lymphocyte cell lines. FEMS Microbiol. Lett. 216:229-234. [DOI] [PubMed] [Google Scholar]
- 21.Zia, S., A. Ndoye, V. T. Nguyen, and S. A. Grando. 1997. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res. Commun. Mol. Pathol. Pharmacol. 97:243-262. [PubMed] [Google Scholar]