Abstract
Background
Burkholderia pseudomallei is a saprophyte in tropical environments and an opportunistic human pathogen. This versatility requires a sensing mechanism that allows the bacterium to respond rapidly to altered environmental conditions. We characterized a two-component signal transduction locus from B. pseudomallei 204, mrgR and mrgS, encoding products with extensive homology with response regulators and histidine protein kinases of Escherichia coli, Bordetella pertussis, and Vibrio cholerae.
Results
The locus was present and expressed in a variety of B. pseudomallei human and environmental isolates but was absent from other Burkholderia species, B. cepacia, B. cocovenenans, B. plantarii, B. thailandensis, B. vandii, and B. vietnamiensis. A 2128 bp sequence, including the full response regulator mrgR, but not the sensor kinase mrgS, was present in the B. mallei genome. Restriction fragment length polymorphism downstream from mrgRS showed two distinct groups were present among B. pseudomallei isolates. Our analysis of the open reading frames in this region of the genome revealed that transposase and bacteriophage activity may help explain this variation. MrgR and MrgS proteins were expressed in B. pseudomallei 204 cultured at different pH, salinity and temperatures and the expression was substantially reduced at 25°C compared with 37°C or 42°C but was mostly unaffected by pH or salinity, although at 25°C and 0.15% NaCl a small increase in MrgR expression was observed at pH 5. MrgR was recognized by antibodies in convalescent sera pooled from melioidosis patients.
Conclusion
The results suggest that mrgRS regulates an adaptive response to temperature that may be essential for pathogenesis, particularly during the initial phases of infection. B. pseudomallei and B. mallei are very closely related species that differ in their capacity to adapt to changing environmental conditions. Modifications in this region of the genome may assist our understanding of the reasons for this difference.
Background
The saprophyte Burkholderia pseudomallei is an opportunistic pathogen that is capable of intracellular survival [1,2] and causes melioidosis, a frequently fatal disease of humans and animals, which can be difficult to diagnose [3]. Although the pathogen is mainly distributed in the soil and water of tropical regions, especially south-east Asia and northern Australia, it is highly adaptable, nutritionally versatile and able to survive and grow in a wide range of environments [4-8]. The disease encompasses a broad spectrum of clinical symptoms and outcomes, including long periods of latency up to 62 years [9], and affects the lives of many millions of people [10].
The pathogenesis of B. pseudomallei infections involves the expression of cell-associated components such as lipopolysaccharide, pili, extracellular polysaccharide, and flagella [3] as well as secreted factors including toxins [11], protease [12], siderophore [13] and phospholipase [14]. Although capsular polysaccharide has been shown to enhance the intracellular survival and virulence of the pathogen, the role of this and other factors in pathogenicity and host resistance has not been conclusively resolved [15-17]. It is possible that pathogenesis involves the expression of many genes that are regulated in response to complex environmental signals.
Many bacterial pathogens possess signal transduction systems that are able to elicit adaptive responses to environmental variations and consequently have an important role in regulating the expression of genes that are crucial for survival and infection [18]. The prevalence of two-component transduction systems in a wide variety of bacterial species has stimulated interest and some limited success in designing new classes of broad-spectrum antimicrobials that can block these signalling pathways [19,20]. The most attractive reason for targeting these systems, such as PhoP/PhoQ from Salmonella typhimurium and BvgA/BvgS from Bordetella pertussis, is that they control the expression of genes required for infectivity and virulence in pathogenic bacteria [21]. Bacterial two-component systems are typically composed of a transmembrane histidine protein kinase that serves as an environmental sensor and a cytoplasmic response regulator that uses reversible phosphorylation to regulate gene expression in response to changing environmental conditions [22,23].
Most, if not all, bacteria can respond and adapt to changes in environmental conditions, such as temperature, osmotic pressure and pH, by regulating gene expression [24,25]. Understanding how these conditions affect the expression of genes which can regulate an adaptive response is important for improving our understanding of opportunistic pathogens, such as B. pseudomallei, that are able to survive a wide range of environmental variations. In this study we report the identification of a two-component signal transduction system from B. pseudomallei encoding proteins most similar in structure to RcsB and RcsC that regulate capsule synthesis in Escherichia coli. We cloned and characterized the genes and examined their expression under conditions that B. pseudomallei may encounter during its life cycle. In order to more fully characterize this region of the B. pseudomallei genome we examined genetic variation downstream from the mrgRS locus in a variety of B. pseudomallei isolates and show that 2 distinct groups of isolates can be distinguished.
Results and discussion
Identification of genes encoding a two-component signal transduction system
A DNA sequence from the coding region of a two-component regulatory gene was amplified by PCR from B. pseudomallei 204 chromosomal DNA, subcloned into pUC18, sequenced on both strands to confirm the identity, labelled and used to probe B. pseudomallei λZAP and λGEM-11 genomic DNA libraries. A λGT-11 genomic library constructed from B. pseudomallei strain 204 by Dr C. Davies, University of Plymouth was also screened. Approximately 4000, 5000 and 8000 recombinant phage plaques were screened from which 5, 3, and 2 positive plaques were isolated from the λGEM-11, λZAP Express, and λGT-11 libraries, respectively. Each was found to contain identical sequence information in the regions of overlap (Figure 1). This work was initiated and completed well before the B. pseudomallei genome sequence was published, and was not based on any genome sequence information.
Figure 1.
Map of the mrgRS locus of the B. pseudomallei genome (GenBank accession no. DQ418486). Open reading frames and direction of transcription are marked (arrows). See text for details of mrgR and mrgS, ORFs1-4, and oligonucleotide probes FR1-3. E = EcoRI site, B = BclI site. The positions of the additional EcoRI site that is described in the text and which is present in many B. pseudomallei isolates, and the 450 bp bacteriophage-like insertion and 650 bp transposase-like sequence that accompany this extra restriction site, are indicated by dotted lines.
Of the two open reading frames (ORFs) identified, one (666 bp) designated mrgR for melioidosis agent regulatory gene regulator, possesses a promoter but lacks an obvious transcriptional termination sequence after the stop codon. The translated product (23,860 Da) shares extensive similarity with a large family of response regulators, particularly the RcsB proteins of Erwinia amylovora, E. coli, and Salmonella typhi, and BvgA of Bordetella pertussis (28–31% identity). A helix-turn-helix DNA binding domain is located in the C-terminal portion (residues 158–215) which is a common feature of response regulators of the LuxR/FixJ family of cytoplasmic transcriptional regulators. In addition, the protein possesses conserved residues that characterize this family, including Asp-61 which is believed to be phosphorylated, and Asp-15, Asp-16, Thr-191, and Lys-202, which are believed to contribute to the acidic pocket for the phosphorylation site [18,26]. These features strongly suggest that MrgR should be assigned to the LuxR/FixJ family of transcriptional regulators that are located in the cytoplasm and are thought to bind to specific promoter sequences upstream of regulated genes.
The second ORF (3237 bp) was designated mrgS for melioidosis agent regulatory gene sensor and is transcribed in the same direction as mrgR. The initiation codon of mrgS overlaps by a single nucleotide with the termination codon of mrgR i.e. TGATG. This feature has been described for genes encoding two-component regulator systems from different bacterial species such as ompR/envZ of E. coli [27] and irlRS operon of B. pseudomallei [28]. Eight nucleotides upstream from the ATG start codon and in the distal portion of mrgR, there is a potential S-D sequence (AAGGA) and 17 bp downstream from the stop codon lies a 36 bp GC-rich inverted repeat (ΔG = -26 kcal) which could act as a strong transcriptional terminator for mrgS. The absence of secondary structure around the stop codon of mrgR suggests that transcription does not terminate at that point and the lack of an obvious promoter region for mrgS suggests that transcription may be controlled from the mrgR promoter and that both ORFs are transcribed as a polycistronic unit.
The translated product of mrgS (118,267 Da) shares homology with a family of two-component histidine kinases that function by sensing environmental stimuli, particularly RcsC of E. coli and also sensor kinases of S. typhi and Vibrio cholerae (18–22% identity). RcsC is the sensor regulator of capsule synthesis in E. coli and many other species, including E. amylovora,P. mirabilis and S. typhi [29]. MrgS possesses many of the features common to this family of sensor proteins including two strongly hydrophobic transmembrane sequences located in the N-terminal portion of the protein between residues 29–49 and 322–342, and five blocks of conserved functional subdomains located in the C-terminal portion of the protein including His-503 that is the proposed site of phosphorylation, and the N, G1 (DTGVG), F, and G2 (GTGLG) boxes at positions 620, 647–651, 661 and 677–681, respectively [30]. These motifs are presumed to form a nucleotide-binding surface within the active site of the molecule. In addition, several conserved Asp residues are present in MrgS, at positions 860, 898, 903, 910 and 949, and may contribute to the additional response regulator domain that is a characteristic of hybrid histidine kinases [31]. Like BvgS of B. pertussis, MrgS is a member of a family of complex sensor proteins, the hybrid kinases, which contain multiple cytoplasmic domains that are believed to participate in a phosphorylation cascade in signal transduction. The presence of two hydrophobic transmembrane domains separated by a large loop of 273 amino acids which may act as a periplasmic environmental monitor is appropriate for a sensor protein that lends itself to signal transduction [18].
Because the deduced amino acid sequences of mrgR and mrgS are most similar to the sequences of genes encoding known response regulators and sensors, it has been inferred that these two genes constitute a two-component signal transduction system. Since the completion of our work the B. pseudomallei genome sequence has been published [32] and mrgR and mrgS are now known to correspond to locus tags BPSL1633 and BPSL1634, respectively, in the genome sequence of B. pseudomallei K96243.
The mrgRS locus is present in B. pseudomallei but not other Burkholderia species
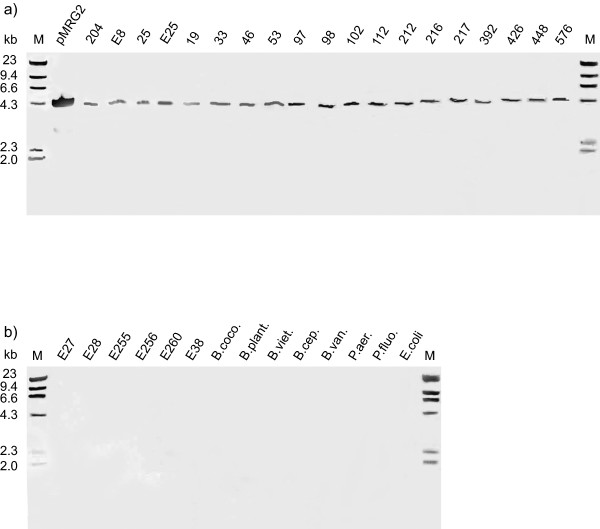
Southern hybridization analysis of genomic DNA extracted from 19 isolates of B. pseudomallei derived from environmental and clinical samples from different areas of the world (Table 1), showed that all of the isolates possessed a single EcoRI fragment of ~4.3 kb in size (Figure 2a) corresponding to the EcoRI fragment spanning most of the mrgRS locus. The locus was not detected in genomic DNA from E. coli, Pseudomonas aeruginosa, Pseudomonas fluorescens, or other Burkholderia species including the closely related Burkholderia thailandensis (Figure 2b). We compared the mrgRS locus from B. pseudomallei 204 with the completed genome sequences for B. pseudomallei isolates K96243 and 1710b and with the unfinished sequences of a further 8 strains that are available on GenBank and found that there is some variation amongst the different strains within this region of the genome. Although the mrgR reading frame is preserved there are modifications within the 5' region upstream from the mrgR start codon that may have implications for the expression of the gene. In some strains there are also modifications within the coding region of mrgS, including a single nucleotide addition (nt 3051) that changes the reading frame and adds 4 additional amino acids to the last 61 residues of the C-terminus of MrgS and which may have functional consequences. A search of the genome sequences of 8 strains of the obligate pathogen Burkholderia mallei which are available on GenBank, revealed that 2128 bp encompassing the entire mrgR gene sequence and including 1319 bp upstream of the mrgR start codon, is highly conserved in 4 of the 8 strains of this organism (98% identity over 2128 bp). The full mrgR gene sequence shares 100% identity with B. mallei. Apart from the initial 144 bp downstream from the start codon, the mrgS sequence is absent from B. mallei and this feature may contribute to its inability to adapt to conditions outside a suitable host, in contrast with B. pseudomallei [33,34].
Table 1.
Bacterial strains used in this study
| Strain | Strain origin | Sample | Geographical origin | Date | Source |
| Burkholderia pseudomallei | |||||
| E8 | Environment | Soil | NE Thailand | 1990 | 1 |
| 19 | Environment | Soil | Singapore | 1991 | 1 |
| 22 | Environment | Soil | Burkina Faso | 1973 | 2 |
| 25 | Environment | Soil | Madagascar | 1977 | 1 |
| E25 | Environment | Soil | Thailand | - | 1 |
| 33 | Environment | Manure | France | 1976 | 1 |
| 46 | Human | Blood | NE Thailand | 1988 | 1 |
| 53 | Human | Urine | NE Thailand | 1987 | 1 |
| 97 | Environment | Soil | Australia | - | 1 |
| 98 | Environment | Soil | Australia | - | 1 |
| 102 | Environment | Soil | Australia | - | 1 |
| 112 | Human | Multiple | NE Thailand | 1992 | 1 |
| 204 | Human | Blood | Thailand | - | 1 |
| 212 | Environment | Soil | NE Thailand | 1990 | 1 |
| 216 | Environment | Soil | NE Thailand | 1990 | 1 |
| 217 | Environment | Soil (wet) | NE Thailand | 1990 | 1 |
| 392 | Human | Pus | NE Thailand | 1989 | 1 |
| 426 | Environment | Soil | Vietnam | - | 1 |
| 448 | Environment | Soil | Vietnam | - | 1 |
| 576 | Human | Blood | Thailand | - | 1 |
| Hainan 1 (H1) | Human | Abscess | China | 1996 | 2 |
| Hainan 2D (H2D) | Human | Abscess | China | 1996 | 2 |
| Hainan 55 (H55) | Human | Abscess | China | 1996 | 2 |
| Hainan 706 (H706) | Human | Abscess | China | 1996 | 2 |
| Burkholderia thailandensis | |||||
| E27 | Environment | Soil | Thailand | - | 2 |
| E38 | Environment | Soil | Thailand | - | 2 |
| E82 | Environment | Soil | NE Thailand | 1990 | 2 |
| E255 | Environment | Soil | NE Thailand | - | 2 |
| E256 | Environment | Soil | Thailand | - | 2 |
| E260 | Environment | Soil | Central Thailand | 1993 | 2 |
| Other Burkholderia species | |||||
| B. cocovenenans LMG11626 | 2 | ||||
| B. plantarii LMG10908 | 2 | ||||
| B. vietnamiensis LMG6998 | 2 | ||||
| B. cepacia IIIa non-epidemic | 2 | ||||
| B. vandii LMG10620 | 2 | ||||
| Other species | |||||
| Pseudomonas aeruginosa | 3 | ||||
| Pseudomonas fluorescens | 3 | ||||
| Escherichia coli DH5α | 3 | ||||
1: David Dance HPA South West, Derriford, Plymouth; 2: Ty Pitt, Centre for Infections, HPA, Colindale, London; 3: University of Plymouth culture collection
Figure 2.
Southern blot hybridisation of EcoRI-digested genomic DNA from different bacterial species using a digoxigenin-labeled oligonucleotide probe for mrgRS: a) B. pseudomallei isolates, pMRG2 contains a 4.3 kb EcoRI fragment of B. pseudomallei genomic DNA, spanning most of the mrgRS locus, inserted in pUC18 and digested with EcoRI b) B. thailandensis, other Burkholderia species, including B. cocovenenans (B. coco.), B. plantarii (B. plant.), B. vietnamiensis (B. viet.), B. cepacia (B. cep.) and B. vandii (B. van.), Pseudomonas aeruginosa (P. aer.), Pseudomonas fluorescens (P. fluo.) and E. coli. Lane M, λ-HindIII markers with molecular sizes (in kilobases) indicated on the left. See Table 1 for isolate details. All of the samples in the figure were separated on the same single agarose gel, transferred to the same single nylon membrane, and hybridized in the same single hybridization tube using the same labeled probe. The image of the resulting single Southern blot is reproduced in the figure as 2 panels, a) and b), for convenience and clarity and therefore pMRG2 represents a positive control for all samples.
MrgR and MrgS are present in B. pseudomallei cell lysates but not culture supernatant
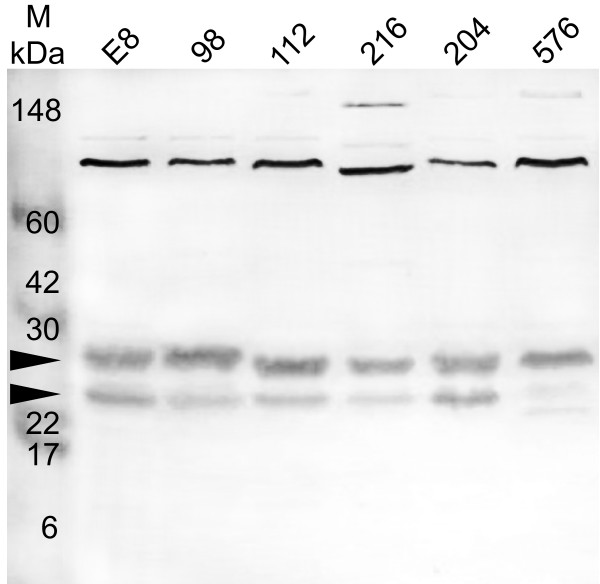
Strains of B. pseudomallei representing isolates from clinical (112, 204, 576) and environmental (E8, 98, 216) samples were cultured at 37°C and whole cell lysates and concentrated culture supernatants were prepared, proteins were separated by SDS-PAGE, transferred to nitrocellulose and probed with antibodies recognising MrgR and MrgS. Anti-MrgR and anti-MrgS each recognized more than one band on Western blots of whole cell lysates of all strains. In all strains, anti-MrgR recognized 3 major bands close to the expected size of MrgR, 24–28 kDa, as well as a strong band of ~80 kDa (Figure 3). Members of the response regulator protein family are known to be phosphorylated at specific residues [26] and because MrgR possesses the same features as these proteins we propose that the 24–28 kDa bands may represent different phosphorylation states of the MrgR protein. In some strains, bands of ~90 and/or ~150 kDa were faintly visible. Anti-MrgS strongly stained a band of ~115 kDa, close to the predicted size of MrgS, and another band of ~30 kDa (Figure 4). In some strains a band of ~90–100 kDa was also visible. Preincubating anti-MrgR and anti-MrgS with their cognate immunising peptide, as described in the antibody specificity data supplement (see Additional file 1), abolished all staining. Hence additional bands possess the same epitope and may represent either breakdown products of the 118 kDa MrgS protein or, in the case of MrgR, an additional antigenically-related 80 kDa protein. MrgR and MrgS were not detected in concentrated broth culture supernatant (result not shown) supporting the suggestion that MrgR and MrgS are not secreted proteins.
Figure 3.
Western blot probed with an affinity purified antibody, anti-MrgR, showing the detection of MrgR in whole cell lysates from 6 isolates of B. pseudomallei grown at 37°C. The isolate number is indicated above each lane. Lane M: molecular weight markers indicated in kilodaltons (kDa). See Table 1 for isolate details. Arrows indicate the expected position of the 24 kDa MrgR protein and slightly larger phosphorylated forms of MrgR. See text for details.
Figure 4.
Western blot probed with an affinity purified antibody, anti-MrgS, showing MrgS in cell lysates from 6 isolates of B. pseudomallei grown at 37°C. The isolate number is indicated above each lane. Lane M: molecular weight markers indicated in kilodaltons (kDa). See Table 1 for isolate details. Arrow indicates the expected position of the 118 kDa MrgS protein. Smaller bands may represent processed forms of MrgS. See text for details.
Convalescent sera from melioidosis patients reacts with MrgR
Western blots of whole cell lysates from 6 isolates of B. pseudomallei that were probed with convalescent sera pooled from 6 melioidosis patients (supplied by Vanaporn Wuthiekanun, Wellcome Trust-Oxford University-Mahidol University Tropical Medicine Research Programme, Bangkok, Thailand) demonstrated strong antibody recognition of multiple components in all isolates (see Additional file 4). Furthermore, Western blots of cell lysates of E. coli expressing MBP-MrgR that were probed with the same sera stained a band of ~60 kDa that was present in soluble, insoluble, and amylose resin purified extracts from bacteria that had been induced with IPTG (Figure 5). A low level of recognition was evident in uninduced cell extracts, similar to that observed when Western blots of the same cell extracts were probed with anti-MrgR. The convalescent sera did not recognize purified MBP, MBP-MrgS or any other E. coli components and no recognition of MBP-MrgR was seen when Western blots of the same extracts were probed with serum from an individual not previously exposed to B. pseudomallei (data not shown). This raises the possibility that MrgR may be expressed by B. pseudomallei during melioidosis infection although it is unclear how a regulatory protein that is not apparently secreted in vitro would be exposed to the host immune system and the presence of cross reactive epitopes on related proteins can not be excluded.
Figure 5.
Western blot showing the recognition of MBP-MrgR fusion protein by antibodies in convalescent sera pooled from 6 melioidosis patients. Lane 1: Uninduced cells, lane 2: cells induced for 3 h with IPTG, lane 3: amylose resin purified cell extract, lane 4: crude cell extract, and lane 5: insoluble matter, lane 6: purified maltose binding protein. Lane M: molecular weight markers indicated in kilodaltons (kDa).
Expression of MrgR and MrgS proteins under different growth conditions
B. pseudomallei 204, from which the mrgRS locus was cloned and characterized, was cultured under a variety of different conditions. A range of temperatures, pH and salinities were chosen to reflect a variety of natural and host environments to which B. pseudomallei might be exposed within the tropics [6]. Although B. pseudomallei can grow over a wide range of variation in these parameters, we obtained the highest yields of protein at 42°C, pH 6.8 and 0.5% NaCl. Substantial yields of protein were also obtained at either 37 or 42°C, pH 5 and 0.15% NaCl, and the lowest yields were recorded at pH 8. These observations support in vitro and in vivo studies showing that B. pseudomallei readily adapts to acidic environments [7,35,36] and has an optimum growth temperature in vitro of 37–42°C [6]. Birds are considered to be relatively resistant to B. pseudomallei infection [37] but the strong growth at 42°C of all of the isolates of B. pseudomallei raises the possibility that birds might act as a reservoir of B. pseudomallei and pose a risk for the transmission of active melioidosis to other species [38].
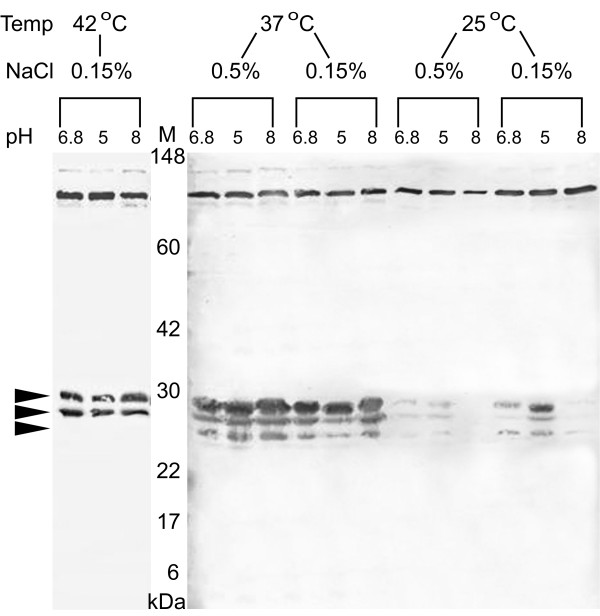
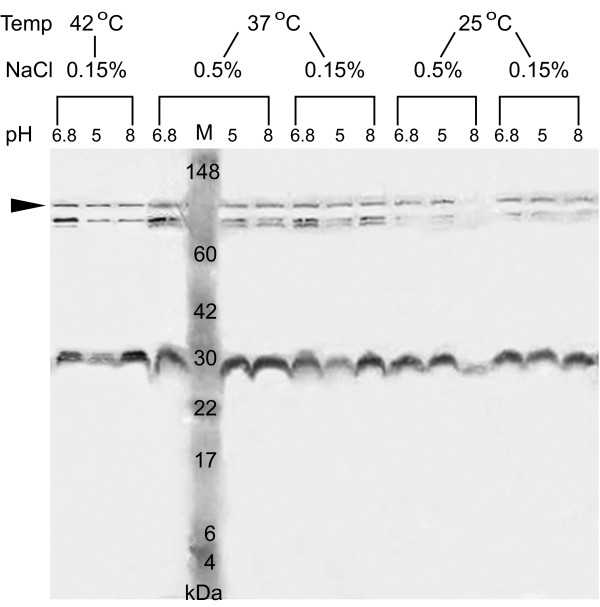
The expression of the 24–28 kDa MrgR bands was substantially reduced when B. pseudomallei 204 was cultured at 25°C compared with 37 or 42°C but was mostly unaffected by different pH or NaCl concentration, although at 25°C and 0.15% NaCl a small increase in expression was observed at pH 5 (Figure 6). This was particularly evident at 42°C, where the lower molecular weight band that we believe represents the dephosphorylated form of MrgR is absent, demonstrating that at higher temperatures the gene is co-ordinately up-regulated. It is possible that at least some of the observed effects could be explained by differences in gene expression at different growth stages, but because equivalent quantities of protein from each sample were used in these studies and the expression of the 80 kDa immunoreactive band was mostly unaffected by pH, salt concentration or temperature then this possibility seems unlikely. A similar response was observed for MrgS expression (Figure 7).
Figure 6.
Expression of MrgR in B. pseudomallei 204 cultured under different combinations of temperature, pH and NaCl concentration. Western blots of whole cell lysates were probed with anti-MrgR. The identity of each lane is indicated at the top. Lane M: molecular weight markers indicated in kilodaltons (kDa). Arrows indicate the expected position of the 24 kDa MrgR protein and slightly larger phosphorylated forms of MrgR.
Figure 7.
Expression of MrgS in B. pseudomallei 204 cultured under different combinations of temperature, pH and NaCl concentration. Western blots of whole cell lysates were probed with anti-MrgS. The identity of each lane is indicated at the top. Lane M: molecular weight markers indicated in kilodaltons (kDa). Arrow indicates the expected position of the 118 kDa MrgS protein.
Cloning and sequence analysis of mrgRS downstream flanking region
Four ORFs, referred to here as ORFs1-4, were identified downstream from the mrgRS locus (Figure 1) and the conceptual translations of each ORF were analysed. The sequence and arrangement of the ORFs is available on GenBank (accession no. DQ418486). We cloned, sequenced and analyzed the genes well before the completion of the B. pseudomallei genome sequence, which is now known to correspond to the region of the B. pseudomallei K96243 genome sequence containing locus tags BPSL1635-1637. These locus tags do not exactly match ORF2 and ORF3 that we have identified. Indeed, there are differences in the nucleotide sequences and also in the gene and protein annotations for this region of the two completed genome sequences, B. pseudomallei K96243 and 1710b that are listed on GenBank. The strong transcriptional termination sequence following the mrgS gene suggests that the downstream sequences are transcribed separately. ORF1 (BPSL1635) is immediately downstream of mrgS and transcribed in the same direction and the predicted protein (31,527 kDa) is similar to sensor transduction proteins containing EAL (GluAlaLeu) domains, such as the oxygen sensing protein of E. coli O157:H7 (31% identity) and BvgR of B. pertussis (25% identity). EAL domains are found in a wide variety of bacterial signalling proteins where they can act to stimulate degradation of the second messenger, cyclic di-GMP, and may function as a diguanylate phosphodiesterase [39]. Enzymatically active and inactive forms have been described [39] and the variation in EAL domain sequences may reflect the convergent evolution of successful structure-function motifs rather than the usual primary sequence similarity. This structure is conserved in the conceptual translation of ORF1. Although the precise functions of many of these proteins are unknown, their open reading frames are within or are tightly linked to operons with well-defined functions. For example, bvgR is required for regulation of all known bvg-repressed genes in B. pertussis, and is located on a separate regulatory unit immediately downstream from the bvgAS locus encoding a two-component regulatory system that upregulates virulence gene expression in B. pertussis [40]. The similarities between the genomic organisation in bvgAS-bvgR and mrgRS-ORF1 together with the fact that both BvgAS and MrgRS are members of the two-component signal transduction family allows for the possibility that ORF1 may also act to repress the mrgRS locus.
Further downstream and in the reverse direction of the complementary strand three open reading frames were identified, ORF2, ORF3, and ORF4. For ORF2, the predicted protein (28,251 kDa) is similar to the arginine/serine rich regions of functionally diverse proteins that are important for binding RNA and DNA and for protein-protein interactions [41], such as LigA of Burkholderia cenocepacia (33% identity). The conceptual translation of ORF3 (16,856 kDa) is similar to a variety of transmembrane-transport proteins, such as the ABC thiamine transporter of Roseovarius nubinhibens (31% identity) and capsular polysaccharide export protein of Rhodobacter sphaeroides (28% identity), and possesses 2 transmembrane helices (TopPRED2 scores 0.71 and 0.88). For ORF4 (BPSL1637), the predicted protein (39,493 kDa) has a lipase (class 3) domain spanning residues 103–252 and including the lipase serine active site [42], GHSLG, located at positions 187–191. This protein lacks the features usually associated with a signal peptide, although this is not an essential component for secretion [43], and is strongly predicted to be secreted by non-classical mechanisms, SecP score = 0.933881 (B. pseudomallei isolate 204) and 0.930609 (B. pseudomallei isolate 112) [44]. B. pseudomallei has been shown to utilize a wide variety of lipid substrates [45,46] and to confirm the presence of an extracellular lipase concentrated culture supernatants were prepared from all isolates of B. pseudomallei (Table 1). Lipase activity was determined to be present in all samples using the fluorogenic substrate 4-methylumbelliferyl oleate and so it is possible that this gene could be expressed, secreted and contribute to this lipase activity, either alone or in concert with other molecules.
The full response regulator gene mrgR is present in the genome of many B. mallei isolates but mrgS and the downstream flanking region are absent. Instead, ORFs immediately downstream of the mrgR homolog in B. mallei have strong homology to penicillin amidase, carbamoyl transferase and transposase and their presence provides circumstantial evidence that transposition may partly explain the absence of this region in B. mallei.
Restriction fragment length analysis of the mrgRS downstream flanking region
Three oligonucleotide probes, FR1, FR2 and FR3, covering almost 5 kb of the B. pseudomallei genome downstream from mrgRS (Figure 1) were used to investigate EcoRI digests of genomic DNA from different B. pseudomallei strains as well as B. thailandensis (Table 1). No signal was obtained with any of the probes in any of the B. thailandensis isolates. Furthermore, this region is not present in the recently completed B. thailandensis E264 genome sequence [33] that is available on GenBank.
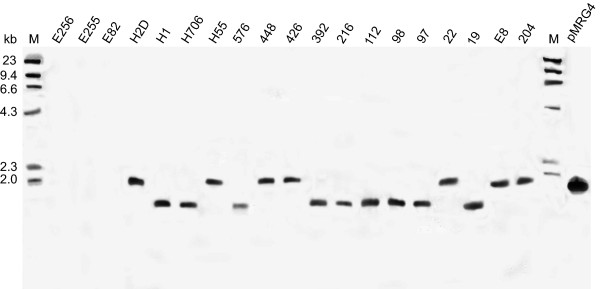
The FR1 probe spans the distal end of mrgS and 285 nucleotides further downstream from the mrgS stop codon including an EcoRI site, and was expected to hybridise two EcoRI genomic DNA fragments of 684 and 592 bp. These two bands were present in most isolates of B. pseudomallei except 392 and H55 which possessed the 592 bp fragment only (data not shown). In isolate 392 this band is very strongly stained and may represent a doublet. In addition, H55 possessed an additional unique band of approximately 4.5 kb. There were no obvious variations in fragment size between isolates in the adjoining region that was probed with FR2 (data not shown). However, the region probed with FR3, which lacked an internal EcoRI site, revealed two distinct patterns between B. pseudomallei isolates. One pattern indicated the presence of a single band of ~1781 bp in seven isolates, while the other pattern demonstrated a band of ~1421 bp in nine isolates (Figure 8). To determine the reasons for the difference, we sequenced this part of the genome of isolates possessing each size of EcoRI fragment.
Figure 8.
Southern blot hybridisation of EcoRI-digested genomic DNA from isolates of B. pseudomallei and B. thailandensis using oligonucleotide probe FR3. Lane M, λ-HindIII markers with molecular sizes (in kilobases) indicated on the left. Lane pMRG4 contains an 8.4 kb BclI fragment of the B. pseudomallei 204 genome spanning most of the mrgRS locus and 5 kb downstream, inserted in the phagemid pBK-CMV which was excised from λZAP Express and digested with EcoRI. See Table 1 for isolate details.
Alignment of 1472 nucleotides covering the same region of the genomes of isolates 112 and 204 revealed 19 base substitutions including one substitution of "A" for "G" at position 1119 which introduces an EcoRI site within ORF4 as described below (GenBank accession no. DQ418487). An examination of the genome sequences of B. pseudomallei that are available on GenBank showed that 8 of the 10 strains possess the same modification at this position as isolate 112. Among all of the B. pseudomallei isolates, including 112 and 204, there are between 4 and 19 nucleotide substitutions within this 1472 bp region. Furthermore, isolates that possess the "A" substitution at position 1119 have substantial modifications to sequences flanking this region of the genome (Figure 1), including the presence of a 450 bp insertion (between nucleotides 9565 and 9566 of GenBank accession no. DQ418486) encoding a product with homology to a bacteriophage phiE125 gp30 protein (GenBank accession no. AF447491), and ~650 bp sequence homologous to mutator type transposases from a wide variety of bacterial species, including Ralstonia solanacearum, Pseudomonas syringae, and B. thailandensis. The transposase is probably inactive because of the organization of the sequence, which includes at least 4 stop codons and incomplete reading frames.
Among the isolates of B. pseudomallei that were examined up to 16 nucleotide substitutions fall within ORF4 and although most of these, including the "A" for "G" substitution which introduces the EcoRI site described above, do not alter the predicted primary translation there are from 2–4 conservative amino acid substitutions. Non-conservative substitutions which are present, G180E (6/12 isolates) and V229A (10/12 isolates), flank the serine active site and may have functional consequences (Figure 9). Lipases hydrolyze long-chain acyl-triglycerides, a process that usually involves activation of the enzyme at the lipid/water interface, and are known to facilitate the colonisation of plants by fungi and bacteria [47]. The active site is hidden until activation occurs when the "lid" covering the active site opens, exposes the active site catalytic triad, S-D-H, and allows access to the substrate. Mutating specific residues flanking the active site can affect this process, for example by altering the enantiomeric selectivity of B. cepacia lipase [48]. B. pseudomallei is often associated with disease among workers in rice fields in Thailand and it has been suggested that it may interact closely with plants by using a plant pathogen-like TTS gene cluster [49-51]. In this case, the delivery of lipases could play an essential role in the colonization of plants by B. pseudomallei.
Figure 9.
Alignment of lipases of B. pseudomallei isolates 112 and 204. Boxes enclose the "lid" motif (residues 112–127) and serine active site (residues 187–191). Asterisks mark the catalytic triad of serine, aspartic acid and histidine. +: conservative amino acid substitutions. Non-conservative amino acid substitutions are underlined. The 6 substitutions that are marked were also present in many of the B. pseudomallei genome sequences of 10 isolates that are available in GenBank: S47N: 10 isolates; I157V: 10 isolates; G180E: 5 isolates; V229A: 9 isolates; N340D: 4 isolates; E346Q: 3 isolates. Other substitutions were also found occasionally: S252F: 1 isolate; D341A: 1 isolate; V249L: 1 isolate.
Studies investigating the molecular diversity of different isolates of B. pseudomallei using a wide variety of methods have demonstrated that although distinct groupings can be defined substantial diversity exists and many isolates do not fall into readily definable groupings or clinical outcomes [3]. Recently, whole genome DNA and RNA spotted arrays have been used to examine large scale genomic variation among different Burkholderia species [33,34]. This work has demonstrated the close relationship that exists between the non-pathogenic environmental saprophyte B. thailandensis, the obligate equine parasite B. mallei and the opportunistic pathogen B. pseudomallei and particularly between B. mallei and B. pseudomallei, supporting work by Godoy et al. [52] who considered B. mallei to be a clone of B. pseudomallei rather than a separate species. Kim et al. [33] showed that the expression of a substantial number of genes is highly conserved between all 3 species and suggested that the evolution of small sets of genes rather than large scale acquisitions and losses define the different adaptations of each species. On the other hand, Ong et al. [34] proposed that the same 3 species may have diverged through the loss of large chromosomal segments and defined 3 distinct molecular subtypes of B. pseudomallei on the basis of regions of difference, constituting areas of DNA recombination that are often characterized by open reading frames (ORFs) encoding products related to phage proteins, transposase or integrase proteins. However, apart from rRNA genes [53] few studies have assessed the extent of variation at specific loci in B. pseudomallei.
The diversity of organisms in tropical countries in which melioidosis is endemic and the frequent interactions that may be possible in that environment and within the human host could create opportunities for genetic change [54]. However, a mutation that confers a selective advantage in one environment is unlikely to be a benefit in all environments [55]. Winstanley et al. [56] assessed the extent of variation in the flagellin genes of B. pseudomallei by comparing the sequences that were obtained from environmental and clinical isolates of the pathogen. The sequences showed either 100% identity or differed by a single nucleotide demonstrating a remarkably conservative nature in these genes. Here, we found a consistent variation in a small region of the B. pseudomallei genome downstream from the mrgRS locus involving up to 19 nucleotide substitutions including one which changes GAGTTC to GAATTC, the recognition sequence for the restriction enzyme EcoRI. The consequent restriction fragment length polymorphism (RFLP) was shown to separate B. pseudomallei isolates from clinical and environmental sources and from spatially and temporally separate origins, into two distinct groups of almost equal proportions. There was no apparent association between the source of the isolates, the date and place of isolation, and the RFLP pattern. Nevertheless, the substitutions represent a stable genetic modification since they have been retained over time. Furthermore, a search of the genome sequences for B. pseudomallei isolates that are available on GenBank revealed the presence of the same modification in many of the isolates. While it is possible that these sequence differences may have occurred at random, the almost equal distribution of the polymorphism among the isolates, the presence of a 450 bp insertion and the close proximity of an inactive transposase suggest that it is much more likely that some process of selection has occurred. For example, differences in primary or secondary host factors may act to select isolated subpopulations according to transmission route or host immune genotypes and play a role in the selection for a mutation or recombination event arising within a bacterial population [57,58]. How this may have occurred in the isolates we examined here is not known. Ong et al. [34] described B. pseudomallei strain subtypes isolated from different primary hosts, pigs (G1 strains) and melioidosis patients (G2 strains), and so we propose that a selective process involving primary hosts or other vectors, including amoebae, fungi or plants [59-61], may have played a role.
Conclusion
This work was initiated and completed well before the B. pseudomallei genome sequence was published. Although the completion of the B. pseudomallei genome sequence has provided a huge body of annotated nucleotide sequence data and an examination of this data revealed at least 33 potential two-component systems in the sequence annotations, very few of the putative genes have been specifically confirmed to be expressed at the protein level in different B. pseudomallei strains. In fact, there are substantial discrepancies in the gene and protein annotations for the completed genome sequences of B. pseudomallei isolates K96243 and 1710b that are available on the GenBank database. Our work demonstrates that mrgR and mrgS are present and expressed as protein in a variety of B. pseudomallei isolates and that the expression of the locus is temperature-regulated which is an essential step that must be completed before pursuing more complex studies aimed at understanding the biological function and pathogenic significance of the mrgRS locus. A similar approach will be necessary to prove the specific protein expression of each of the putative genes that have been identified in the B. pseudomallei genome rather than simply assuming that all open reading frames that are annotated in the nucleotide sequence of the genome are expressed as the conceptually translated protein.
Because of its' nutritional and environmental adaptability, B. pseudomallei has the potential to spread to areas of the world outside southeast Asia and northern Australia. Two-component transduction systems are a major mechanism by which bacteria regulate adaptive responses to environmental stimuli [18]. Although there have been extensive studies of the biology of B. pseudomallei, very few of these have investigated the influence of environmental factors on gene expression and the adaptive responses of the bacterium. We have identified and characterized a two-component transduction system from B. pseudomallei that is present and expressed in a variety of strains of the bacterium, encoding proteins that are most similar to the regulators of capsular synthesis in other bacteria. In B. pseudomallei, the expression of these proteins is regulated in response to temperature rather than pH or salinity and they are more strongly expressed at temperatures found in mammalian and avian hosts. The recognition of MrgR by antibodies in convalescent sera from melioidosis patients suggests that the mrgRS locus may have a role in regulating adaptive responses during the course of infection. Because the expression of the genes is increased at higher temperatures then the adaptive responses they control may be particularly important during the initial phases of infection.
It has been proposed that B. mallei, B. pseudomallei, and B. thailandensis represent 3 states of ecological niche adaptation, namely, obligate pathogen, opportunistic pathogen, and saprophyte, respectively [33]. The 3 species are very closely related and the mechanisms of evolutionary divergence are subject to debate [33,34,52]. The absence of the mrgRS locus and downstream flanking region from the genome of B. thailandensis shows that it is not essential for a non-pathogenic existence. Because of the similarities in gene organization and structure with other signal transduction systems involved in phase change and the regulation of virulence gene expression, it is possible that mrgS and the downstream ORFs may have a similar role in adaptive responses of B. pseudomallei, which in the case of mrgS is regulated by changes in temperature. The absence of this region in B. mallei suggests that this function is no longer required but mrgR may still have a regulatory role.
Nucleotide sequence accession numbers
Methods
Bacterial strains and growth conditions
Bacterial strains used in this study are listed in Table 1. All strains of Burkholderia species were grown at 37°C, except B. plantarii and B. vandii which were grown at 25°C, on Luria-Bertani (LB) agar or broth, statically. Pseudomonas aeruginosa and Pseudomonas fluorescens were grown on nutrient agar at 37°C. The identity of B. pseudomallei was confirmed by the API 20NE biochemical test [62]. For expression studies, 10 ml LB broths containing NaCl (0.15, 0.5 or 2.2% w/v) were adjusted to pH 5, 6.8 or 8 and a standardized inoculum of 107 cells was added and incubated for 48 h at 25, 37 or 42°C. E. coli strains were cultured on LB agar or broth [63] and when required, culture media were supplemented with IPTG (100 μM), X-gal (100 μg ml-1), ampicillin (100 μg ml-1), chloramphenicol (25 μg ml-1) or kanamycin (25 μg ml-1).
DNA manipulations
Restriction enzyme digestions, DNA blunting and kinasing, DNA ligation, bacterial transformation and DNA hybridisation were performed according to standard methods [63]. Plasmid DNA was isolated using the Miniprep plasmid DNA extraction kit (BioRad), lambda DNA was purified using the Wizard λ prep DNA purification kit (Promega) and DNA fragments, PCR products and ligation mixtures were recovered using the Prep-a-gene DNA purification kit (BioRad). Genomic DNA was extracted using the Puregene D-6000 DNA isolation kit (Gentra Systems) and quantified by comparison with lambda DNA standards (Sigma Aldrich) using digital images of 1% agarose gels stained with ethidium bromide.
Preparation of oligonucleotide probes
PCR primers (Sigma Genosys), 5'-GATTTCACGATGCATCAGGCGAAC-3' and 5'-TTCTGGATCGCCGCGATGTCCGTG-3', were derived from the sequences of response regulator genes from other bacterial species to amplify a 377 bp sequence from B. pseudomallei 204 genomic DNA. The PCR reaction contained 1 unit Taq DNA polymerase in reaction buffer (Roche), 1.5 mM MgCl2, 10 pmol of each primer, 0.2 mM of each dNTP and 10 ng genomic DNA. Reactions were placed at 96°C for 2 min, followed by 35 cycles consisting of 96°C for 30 s, annealing at 45°C (1st cycle), 50°C (2nd and 3rd cycles) and 60°C (remaining 33 cycles) for 30 s, 72°C for 90 s and a final extension at 72°C for 5 min. Amplicons were analysed in 1% agarose gels, purified and subcloned into pUC18 using E. coli DH5α. Cloned DNA was fully sequenced in both directions (MWG Biotech). Following confirmation, the insert was excised from pUC18 by EcoRI and PstI digestion, purified by agarose gel electrophoresis and labelled with digoxigenin-11-dUTP (Roche).
For RFLP analysis of 4994 bp of the genome downstream from the mrgRS locus, three pairs of PCR primers (Table 2) were designed on the basis of nucleotide sequence data (GenBank accession no. DQ418486) to amplify oligonucleotide probes to sequentially examine 5 contiguous EcoRI fragments of the genome. The probes were designated FR1, a 665 bp probe targeting a 1276 bp segment with an internal EcoRI site and including the mrgS stop codon, FR2, a 1062 bp probe targeting a 1937 bp segment with an internal EcoRI site, and FR3, a 1136 bp probe targeting a 1781 bp segment. The PCR reaction contained 1 unit Taq polymerase in reaction buffer (Roche), 1.5 mM MgCl2, 24 pmol of each primer, 0.2 mM of each dNTP and 10 ng genomic DNA. Reactions were placed at 96°C for 2 min, followed by 35 cycles of 96°C for 40 s, 65°C for 40 s, 72°C for 90 s and a final extension at 72°C for 5 min. Amplicons were analysed in 1% agarose gels, purified and subcloned into pUC18 using E. coli DH5α. Cloned DNA was fully sequenced in both directions (MWG Biotech) and was labelled with digoxigenin-dUTP (Roche).
Table 2.
PCR primers for preparing oligonucleotide probes FR1, FR2, and FR3
| Primer | Sequence 5'-3' |
| FR1 forward | ATGATGGACGGTTTCCAGTTGCTC |
| FR1 reverse | AACGTTAAATCAAGTCGCGGGTGG |
| FR2 forward | AGAGCGCTGTCGCAACTGAATCTG |
| FR2 reverse | TCGCTTCGCTTGCTGAGAAA |
| FR3 forward | GGTCCGGGCCAAATATTACGATCC |
| FR3 reverse | AGCGGAACCAATCCGAACTCACAG |
Construction and screening of genomic DNA libraries
B. pseudomallei 204 chromosomal DNA libraries were constructed using λGEM-11 and λZAP Express vectors. For λGEM-11, genomic DNA was partially digested with Sau3A to yield fragments between 9 and 23 kb which were inserted into the BamHI site and packaged recombinants were plated on E. coli LE392 using NZCYM-agarose containing 0.2% maltose (Promega). For λZAP Express, genomic DNA was completely digested with BclI and ligated into the BamHI site, packaged, and propagated on E. coli XL1-Blue. Plasmid DNA, in the form of the kanamycin-resistant phagemid pBK-CMV, containing cloned DNA inserts was excised from λZAP Express using helper phage and E. coli XLOLR (Stratagene).
For screening B. pseudomallei genomic libraries, phage plaques were overlaid with gridded nitrocellulose filters (Schleicher and Schuell) for 30 min at 4°C. Filters were processed for hybridization with a labelled oligonucleotide probe at high stringency overnight at 68°C according to standard methods [63]. Positive phage plaques were detected with anti-digoxigenin antibody (Roche).
DNA sequencing and sequence analysis of cloned products
Both strands of cloned DNA were sequenced (MWG Biotech) using either M13 universal and reverse primers or custom synthesized primers (Sigma Genosys). Gene sequences have been deposited in the GenBank database (GenBank accession nos. DQ418486, DQ418487). Gene promoter predictions were made using software provided by Berkeley Drosophila Genome Project [64]. Homology and conserved domain searches were conducted using BLAST software provided by NCBI [65]. Software provided by the Swiss Institute of Bioinformatics [66] and Institut Pasteur [67] was used to analyse secondary structure (PHDtopology), hydrophobicity (Pepwindow), helix-turn-helix motifs (Helixturnhelix), and transmembrane regions (TopPRED2, DAS). Multiple amino acid sequence alignments were performed using MultAlin software [68]. SecretomeP 2.0 [44] was used to predict non-classical protein secretion [69].
Southern blotting
Genomic DNA (3 μg) was completely digested with EcoRI and the fragments separated in 1% agarose gels in TBE buffer, depurinated, denatured and neutralized using standard methods [63]. DNA was capillary blotted onto positively charged nylon membranes (Amersham Biosciences) and fixed to the membrane using UV irradiation. The blots were prehybridized, hybridized with the labelled oligonucleotide probe and developed, according to standard methods [63].
Synthetic peptides and production of affinity purified antibodies
Synthetic peptides consisting of 9 amino acid residues, DTNVDLINC and RKFYSLESN, corresponding to amino acids 206 to 214 located toward the C-terminus of MrgR and amino acids 14 to 22 located in the N-terminus of MrgS, respectively, were synthesized, conjugated to keyhole limpet hemocyanin (KLH) and used to immunize rabbits (Bethyl Laboratories). To facilitate coupling to maleimide-activated KLH a glycine residue was attached to the C-terminus of the MrgR oligopeptide and a cysteine residue was attached to the N-terminus of the MrgS oligopeptide. Antibodies were purified from the hyperimmune serum of immunized rabbits by affinity chromatography using a peptide-Sepharose matrix, filter sterilized and stored at 4°C. The affinity purified antibodies, anti-MrgR and anti-MrgS, were tested for antigen recognition and specificity as described in Additional file 1, Additional file 2 and Additional file 3.
Preparation of B. pseudomallei protein samples and Western blotting
Cells from 1 ml aliquots of B. pseudomallei cultures that had been prepared as described above were centrifuged (4,000 g, 10 min, 4°C), washed in PBS and resuspended in lysis buffer (0.75 M Tris-HCl pH 8.8 containing 0.2% SDS). B. pseudomallei broth culture supernatants containing extracellular products (ECPs) were centrifuged (15,000 g, 10 min, 4°C) and the supernatant was concentrated 250-fold using Amicon Minicon Miniplus units, 10 K nominal mw cutoff (Millipore). The total protein concentration of cell lysates and ECPs was determined using a protein assay kit (Bio-Rad). Lipase activity in the concentrated supernatants was determined by comparison with uninoculated broth using the fluorogenic substrate 4-methylumbelliferyl oleate (Sigma). For each sample, equivalent amounts of B. pseudomallei whole cell lysate (5 μg) or ECPs (2 μg), were diluted in Laemmli buffer and separated on 12% polyacrylamide-SDS gels. Proteins were electrotransferred to nitrocellulose membranes under standard conditions [70]. Membranes were blocked for 2 h in PBS containing 1% casein, incubated overnight with the primary antibody diluted in the blocking solution, washed 3 × 10 min, and then incubated for 2 h at room temperature in secondary antibody conjugated to horseradish peroxidase (Dako A/S). A list of the primary and secondary antibodies and dilutions that were used is provided in Table 3.
Table 3.
Dilutions of primary and secondary antibodies for Western blotting
| Primary Antibody | Dilution | Secondary Antibody | Dilution | Source |
| Anti-MrgR | 1:500 | Swine Anti-rabbit IgG | 1:2000 | Bethyl Labs |
| Anti-MrgS | 1:300 | Swine Anti-rabbit IgG | 1:1000 | Bethyl Labs |
| Anti-MBP | 1:1000 | Swine Anti-rabbit IgG | 1:2000 | NEB |
| Convalescent sera pooled from 6 melioidosis patients | 1:300 | Rabbit Anti-human IgG | 1:1000 | Ty Pitt, HPA, London |
Authors' contributions
MEM constructed and screened DNA libraries, prepared oligonucleotide probes, performed Southern blotting and Western blotting, participated in the analysis and interpretation of data, co-drafted and co-wrote the manuscript. THG led the conception and design of the study, co-drafted and co-wrote the manuscript, performed DNA manipulations, participated in the analysis and interpretation of data, and annotated the sequences. DABD initiated the melioidosis studies, obtained bacterial isolates, assisted in the interpretation of data, and contributed to writing the manuscript. MLG assisted with the conception and design of the study, cultured bacterial isolates, performed DNA extraction, participated in the interpretation of data, and contributed to writing the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Antibody specificity. Methods for verifying antibody specificity; Construction and expression of recombinant fusion proteins; Characterisation of antibodies recognising MrgR and MrgS; Figure legends for Additional Figures
Figure S3. Western blot showing the recognition of B. pseudomallei cellular components from 6 isolates by antibodies in convalescent sera pooled from 6 melioidosis patients.
Figure S1. Western blot showing the recognition of MBP-MrgR fusion protein by affinity purified antibodies, anti-MrgR.
Figure S2. Western blot showing the recognition of MBP-MrgS fusion protein by affinity purified antibodies, anti-MrgS.
Acknowledgments
Acknowledgements
This work was funded by the Egyptian government, through the Egyptian Education and Culture Bureau, London, UK. We thank Dr Ty Pitt, Centre for Infections, Health Protection Agency, Colindale, London for providing bacterial strains, Vanaporn Wuthiekanun, Wellcome Trust-Oxford University-Mahidol University Tropical Medicine Research Programme, Bangkok, Thailand, for providing convalescent sera from melioidosis patients, and Lynne Gay for technical assistance.
Contributor Information
Magdy E Mahfouz, Email: mmahfouz4@yahoo.co.uk.
T Hilton Grayson, Email: thomas.grayson@plymouth.ac.uk.
David AB Dance, Email: david.dance@phnt.swest.nhs.uk.
Martyn L Gilpin, Email: m.gilpin@plymouth.ac.uk.
References
- Jones AL, Beveridge TJ, Woods DE. Intracellular survival of Burkholderia pseudomallei. Infect Immun. 1996;64:782–790. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruksachartvuthi S, Aswapokee N, Thankerngpol K. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol. 1990;31:109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology and management. Clin Microb Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett ED, Nelson RA. Pulmonary melioidosis. Observations in thirty-nine cases. Am Rev Resp Dis. 1975;112:331–340. doi: 10.1164/arrd.1975.112.3.331. [DOI] [PubMed] [Google Scholar]
- So SY, Chau PY, Leung YK, Lam WK, Yu DYC. Successful treatment of melioidosis caused by a multiresistant strain in an immunocompromised host with third generation cepharosporins. Am Rev Resp Dis. 1983;127:650–654. doi: 10.1164/arrd.1983.127.5.650. [DOI] [PubMed] [Google Scholar]
- Dance DAB. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 2000;74:159–168. doi: 10.1016/S0001-706X(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Mitsuchi M, Wichaidlit P, Jeungnijnirund S. Outlines of soils of the Northeastern Plateau, Thailand: Their characteristics and constraints. Japan International Cooperation Agency Report. Thailand: Siriphan Offset Pub. Co. Ltd; 1986. [Google Scholar]
- Yabuuchi E, Wang L, Arakawa M, Yano I. Survival of Pseudomonas pseudomallei strains at 5°C. Kansenshogaku Zasshi. 1993;67:331–335. doi: 10.11150/kansenshogakuzasshi1970.67.331. [DOI] [PubMed] [Google Scholar]
- Ngauy V, Lemeshev Y, Sadkowski L, Crawford G. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J Clin Microbiol. 2005;43:970–972. doi: 10.1128/JCM.43.2.970-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dance DAB. Melioidosis. Curr Opin Infect Dis. 2002;15:127–132. doi: 10.1097/00001432-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Haase A, Janzen J, Barrett S, Currie B. Toxin production by Burkholderia pseudomallei strains and correlation with severity of melioidosis. J Med Microbiol. 1997;46:557–563. doi: 10.1099/00222615-46-7-557. [DOI] [PubMed] [Google Scholar]
- Gauthier YP, Thibault FM, Paucod JC, Vidal DR. Protease production by Burkholderia pseudomallei and virulence in mice. Acta Trop. 2000;74:215–220. doi: 10.1016/S0001-706X(99)00073-X. [DOI] [PubMed] [Google Scholar]
- Tuanyok A, Kim HS, Nierman WC, Yu Y, Dunbar J, Moore RA, Baker P, Tom M, Ling JML, Woods DE. Genome-wide expression analysis of iron regulation in Burkholderia pseudomallei and Burkholderia mallei using DNA microarrays. FEMS Microbiol Lett. 2005;252:327–335. doi: 10.1016/j.femsle.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Korbsrisate S, Suwanasai N, Leelaporn A, Ezaki T, Kawamura Y, Asrasombath S. Cloning and characterization of a nonhemolytic phospholipase C gene from Burkholderia pseudomallei. J Clin Microbiol. 1999;37:3742–3745. doi: 10.1128/jcm.37.11.3742-3745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J Med Microbiol. 2004;53:1177–1182. doi: 10.1099/jmm.0.45766-0. [DOI] [PubMed] [Google Scholar]
- Reckseidler SL, DeShazer D, Sokol PA, Woods DE. Detection of bacterial virulence genes by subtractive hybridization: Identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect Immun. 2001;69:34–44. doi: 10.1128/IAI.69.1.34-44.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckseidler-Zenteno SL, DeVinney R, Woods DE. The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect Immun. 2005;73:1106–1115. doi: 10.1128/IAI.73.2.1106-1115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Nessler S. The bacterial HPr kinase/phosphorylase: A new type of Ser/Thr kinase as antimicrobial target. Biochim Biophys Acta. 2005;1754:126–131. doi: 10.1016/j.bbapap.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Stephenson K, Hoch JA. Developing inhibitors to selectively target two-component and phosphorelay signal transduction systems of pathogenic microorganisms. Curr Med Chem. 2004;11:765–773. doi: 10.2174/0929867043455765. [DOI] [PubMed] [Google Scholar]
- Calva E, Oropeza R. Two-component signal transduction systems, environmental signals and virulence. Microb Ecol. 2000;51:166–176. doi: 10.1007/s00248-005-0087-1. [DOI] [PubMed] [Google Scholar]
- Galperin MY. Bacterial signal transduction network in a genomic perspective. Environ Microbiol. 2004;6:552–67. doi: 10.1111/j.1462-2920.2004.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolanin PM, Thomason PA, Stock JB. Histidine protein kinases: Key signal transducers outside the animal kingdom. Genome Biol. 2002;3:3013.1–3013.8. doi: 10.1186/gb-2002-3-10-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JG. Environmental sensing mechanisms in Bordetella. Adv Microb Physiol. 2001;44:141–81. doi: 10.1016/s0065-2911(01)44013-6. [DOI] [PubMed] [Google Scholar]
- Lucas RL, Lee CA. Unravelling the mysteries of virulence gene regulation in Salmonella typhimurium. Mol Microbiol. 2000;36:1024–33. doi: 10.1046/j.1365-2958.2000.01961.x. [DOI] [PubMed] [Google Scholar]
- Bourret RB, Hess JF, Simon MI. Conserved aspartate residues and phosphorylation in signal transduction by the chemotaxis protein CheY. Proc Natl Acad Sci USA. 1990;87:41–45. doi: 10.1073/pnas.87.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S, Inouye M. Environmentally regulated gene expression for membrane proteins in Escherichia coli. Annu Rev Cell Biol. 1988;4:21–42. doi: 10.1146/annurev.cb.04.110188.000321. [DOI] [PubMed] [Google Scholar]
- Jones AL, Deshazer D, Woods DE. Identification and characterization of a two-component regulatory system involved in invasion of eukaryotic cells and heavy-metal resistance in Burkholderia pseudomallei. Infect Immun. 1997;65:4972–4977. doi: 10.1128/iai.65.12.4972-4977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N, Gottesman S. The Rcs phosphorelay: A complex signal transduction system. Annu Rev Microbiol. 2005;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- Stock JB, Surette MG, Levit M, Stock AM. Two-component signal transduction system: structure-function relationships and mechanisms of catalysis. In: Hoch JA, Silhavy TJ, editor. Two-Component Signal Transduction. Washington DC: ASM Press; 1995. pp. 25–32. [Google Scholar]
- Mizuno T. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res. 1997;4:161–168. doi: 10.1093/dnares/4.2.161. [DOI] [PubMed] [Google Scholar]
- Holden MTG, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, Brown NF, Challis GL, Cherevach I, Chillingworth T, Cronin A, Crossett B, Davis P, DeShazer D, Feltwell T, Fraser A, Hance Z, Hauser H, Holroyd S, Jagels K, Keith KE, Maddison M, Moule S, Price C, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Simmonds M, Songsivilai S, Stevens K, Tumapa S, Vesaratchavest M, Whitehead S, Yeats C, Barrell BG, Oyston PCF, Parkhill J. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci USA. 2004;101:14240–14245. doi: 10.1073/pnas.0403302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Schell MA, Yu Y, Ulrich RL, Sarria SH, Nierman WC, DeShazer D. Bacterial genome adaptation to niches: Divergence of the potential virulence genes in three Burkholderia species of different survival strategies. BMC Genomics. 2005;6:174. doi: 10.1186/1471-2164-6-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C, Ooi CH, Wang D, Chong H, Ng KC, Rodrigues F, Lee MA, Tan P. Patterns of large-scale genomic variation in virulent and avirulent Burkholderia species. Genome Res. 2004;14:2295–2307. doi: 10.1101/gr.1608904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejsirilert S, Kondo E, Chiewsilp D, Kanai K. Growth and survival of Pseudomonas pseudomallei in acidic environments. Jpn J Med Sci Biol. 1991;44:63–74. doi: 10.7883/yoken1952.44.63. [DOI] [PubMed] [Google Scholar]
- Yabuuchi E, Miyajima N, Hotta H, Ohyama A, Tanaka N. Pseudomonas cepacia from blood of a burn patient. Med J Osaka Univ. 1970;21:1–6. [PubMed] [Google Scholar]
- Lajudie P, Brygoo ER. Contribution a l'etude du pouvoir pathogene du bacille de Whitmore. Ann Instit Pasteur. 1953;85:99–106. [PubMed] [Google Scholar]
- Vesselinova A, Najdenski H, Nikolova S, Kussovski V. Experimental melioidosis in hens. Zentralbl Veterinarmed B. 1996;43:371–378. doi: 10.1111/j.1439-0450.1996.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Schmidt AJ, Ryjenkov DA, Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: Enzymatically active and inactive EAL domains. J Bacteriol. 2005;187:4774–4781. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoo S, Foreman-Wykert AK, Cotter PA, Miller JF. Mechanisms of Bordetella pathogenesis. Front Biosci. 2001;6:E168–E186. doi: 10.2741/mattoo. [DOI] [PubMed] [Google Scholar]
- Boucher L, Ouzounis CA, Enright AJ, Blencowe BJ. A genome-wide survey of RS domain proteins. RNA. 2001;7:1693–1701. [PMC free article] [PubMed] [Google Scholar]
- Derewenda ZS, Cambillau C. Effects of gene mutations in lipoprotein and hepatic lipases as interpreted by a molecular model of the pancreatic lipase. J Biol Chem. 1991;266:23112–23119. [PubMed] [Google Scholar]
- Pallen MJ, Chaudhuri RR, Henderson IR. Genomic analysis of secretion systems. Curr Opin Microbiol. 2003;6:519–527. doi: 10.1016/j.mib.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Kiemer L, Fausbøll A, Brunak S. Non-classical protein secretion in bacteria. BMC Microbiol. 2005;5:58. doi: 10.1186/1471-2180-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol Immunol. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Wuthiekanun V, Smith MD, Dance DA, Walsh AL, Pitt TL, White NJ. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol. 1996;45:408–412. doi: 10.1099/00222615-45-6-408. [DOI] [PubMed] [Google Scholar]
- La Camera S, Geoffroy P, Samaha H, Ndiaye A, Rahim G, Legrand M, Heitz T. A pathogen-inducible patatin-like lipid acyl hydrolase facilitates fungal and bacterial host colonization in Arabidopsis. Plant J. 2005;44:810–825. doi: 10.1111/j.1365-313X.2005.02578.x. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Kariya K, Nakanishi Y, Kurono Y, Achiwa K. Inversion of enantioselectivity in hydrolysis of 1,4-dihydropyridines by point mutation of lipase PS. Tetrahedron Lett. 1995;36:1063–1066. doi: 10.1016/0040-4039(94)02454-J. [DOI] [Google Scholar]
- Winstanley C, Hales BA, Hart CA. Evidence for the presence in Burkholderia pseudomallei of a type III secretion system-associated gene cluster. J Med Microbiol. 1999;48:649–656. doi: 10.1099/00222615-48-7-649. [DOI] [PubMed] [Google Scholar]
- Attree O, Attree I. A second type III secretion system in Burkholderia pseudomallei : who is the real culprit? Microbiology. 2001;147:3197–3199. doi: 10.1099/00221287-147-12-3197. [DOI] [PubMed] [Google Scholar]
- Rainbow L, Hart CA, Winstanley C. Distribution of type III secretion gene clusters in Burkholderia pseudomallei, B. thailandensis and B. mallei. J Med Microbiol. 2002;51:374–384. doi: 10.1099/0022-1317-51-5-374. [DOI] [PubMed] [Google Scholar]
- Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Spratt BG. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–2079. doi: 10.1128/JCM.41.5.2068-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JE, Sacchi CT, Glass MB, De BK, Weyant RS, Levett PN, Whitney AM, Hoffmaster AR, Popovic T. Use of 16S rRNA gene sequencing for rapid identification and differentiation of Burkholderia pseudomallei and B. mallei. J Clin Microbiol. 2003;41:4647–4654. doi: 10.1128/JCM.41.10.4647-4654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A, Vaughan HS, Melder A, Wood Y, Janmaat A, Gilfedder J, Kemp D, Currie B. Subdivision of Burkholderia pseudomallei ribotypes into multiple types by random amplified polymorphic DNA analysis provides new insights into epidemiology. J Clin Microbiol. 1995;33:1687–1690. doi: 10.1128/jcm.33.7.1687-1690.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan FM. Genetic exchange and evolutionary divergence in prokaryotes. Trends Ecol Evol. 1994;9:175–180. doi: 10.1016/0169-5347(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Winstanley C, Hales BA, Corkill JE, Gallagher MJ, Hart CA. Flagellin gene variation between clinical and environmental isolates of Burkholderia pseudomallei contrasts with the invariance among clinical isolates. J Med Microbiol. 1998;47:689–694. doi: 10.1099/00222615-47-8-689. [DOI] [PubMed] [Google Scholar]
- Meyers LA, Levin BR, Richardson AR, Stojiljkovic I. Epidemiology, hypermutation, within-host evolution and the virulence of Neisseria meningitidis . Proc R Soc Lond B. 2003;270:1667–1677. doi: 10.1098/rspb.2003.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley KA, Wilson DJ, Kriz P, Mcvean G, Maiden MCJ. The influence of mutation, recombination, population history, and selection on patterns of genetic diversity in Neisseria meningitidis. Mol Biol Evol. 2005;22:562–569. doi: 10.1093/molbev/msi041. [DOI] [PubMed] [Google Scholar]
- Pitt TL, Trakulsomboon S, Dance DAB. Molecular phylogeny of Burkholderia pseudomallei. Acta Trop. 2000;74:181–185. doi: 10.1016/S0001-706X(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Inglis TJ, Rigby JP, Robertson TA, Dutton NS, Henderson M, Chang BJ. Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect Immun. 2000;68:1681–1686. doi: 10.1128/IAI.68.3.1681-1686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Chang BJ, Abbott LK, Kuo J, Harnett G, Inglis TJJ. Invasion of spores of the arbuscular mycorrhizal fungus Gigaspora decipiens by Burkholderia spp. Appl Environ Microbiol. 2003;69:6250–6256. doi: 10.1128/AEM.69.10.6250-6256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dance DAB, Wuthiekanun V, Naigowit P, White NJ. Identification of Pseudomonas pseudomallei in clinical practice: use of simple screening tests and API20NE. J Clin Pathol. 1989;42:645–648. doi: 10.1136/jcp.42.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2. New York: Cold Spring Habor Laboratory Press; [Google Scholar]
- Berkeley Drosophila Genome Project http://www.fruitfly.org/seq_tools/promoter.html
- National Centre for Biotechnology Information http://www.ncbi.nlm.nih.gov/
- ExPASy Proteomics Server http://www.expasy.ch/
- Institut Pasteur http://www.pasteur.fr/english.html
- MultAlin http://prodes.toulouse.inra.fr/multalin/multalin.html
- SecretomeP 2.0 server http://www.cbs.dtu.dk/services/SecretomeP/
- Towbin HM, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibody specificity. Methods for verifying antibody specificity; Construction and expression of recombinant fusion proteins; Characterisation of antibodies recognising MrgR and MrgS; Figure legends for Additional Figures
Figure S3. Western blot showing the recognition of B. pseudomallei cellular components from 6 isolates by antibodies in convalescent sera pooled from 6 melioidosis patients.
Figure S1. Western blot showing the recognition of MBP-MrgR fusion protein by affinity purified antibodies, anti-MrgR.
Figure S2. Western blot showing the recognition of MBP-MrgS fusion protein by affinity purified antibodies, anti-MrgS.