Abstract
1. A study has been made of the effects of tetrodotoxin on the impulse activity, resting membrane potential, input resistance, and the generator potential and its after-hyperpolarization of the slowly adapting stretch receptor neurone of the lobster.
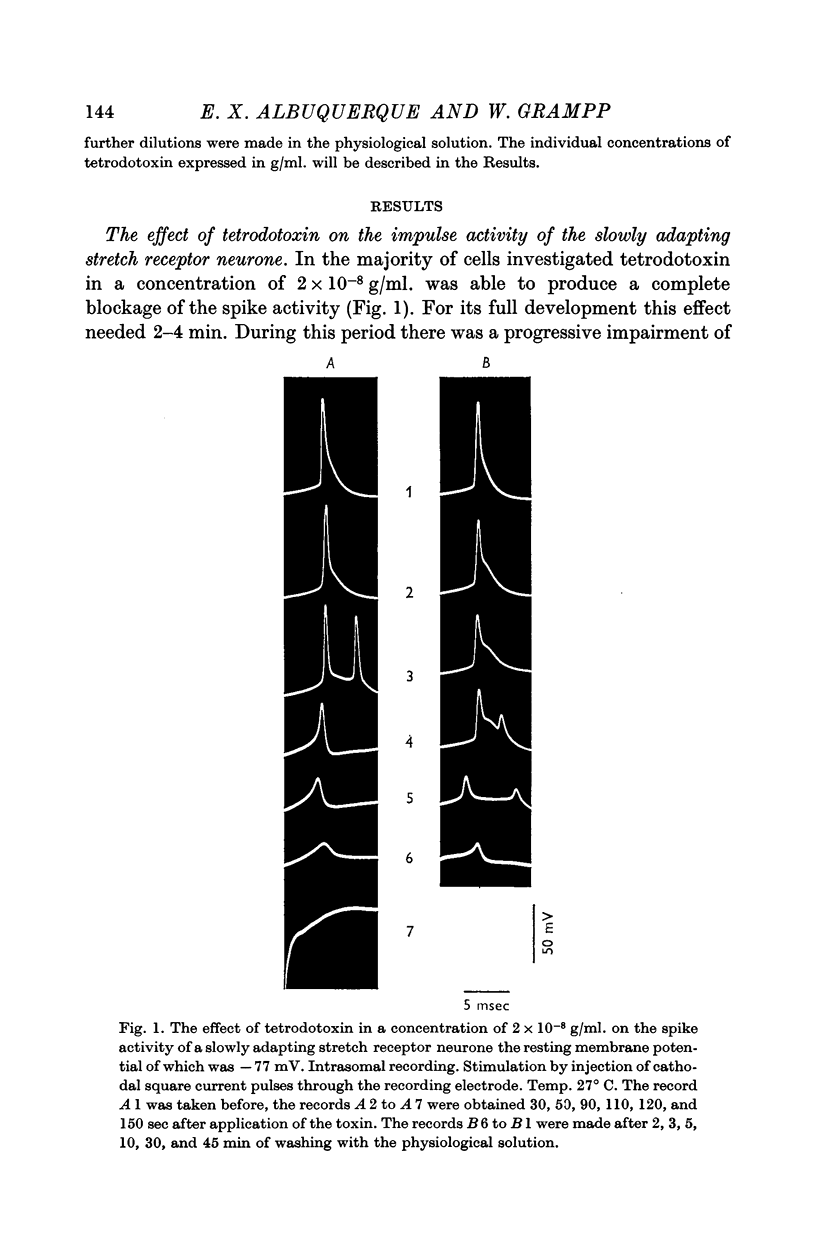
2. Tetrodotoxin was able to abolish completely within about 2 min the impulse activity in most cells, when given in a dose of 2 × 10-8 g/ml., but in all cells, when administered in a dose of 4 × 10-8 g/ml. After blockage by the toxin in concentrations as high as 4 × 10-6 g/ml. for periods of up to 30 min the action potential was restored by washing the preparation in physiological solution for 1 hr.
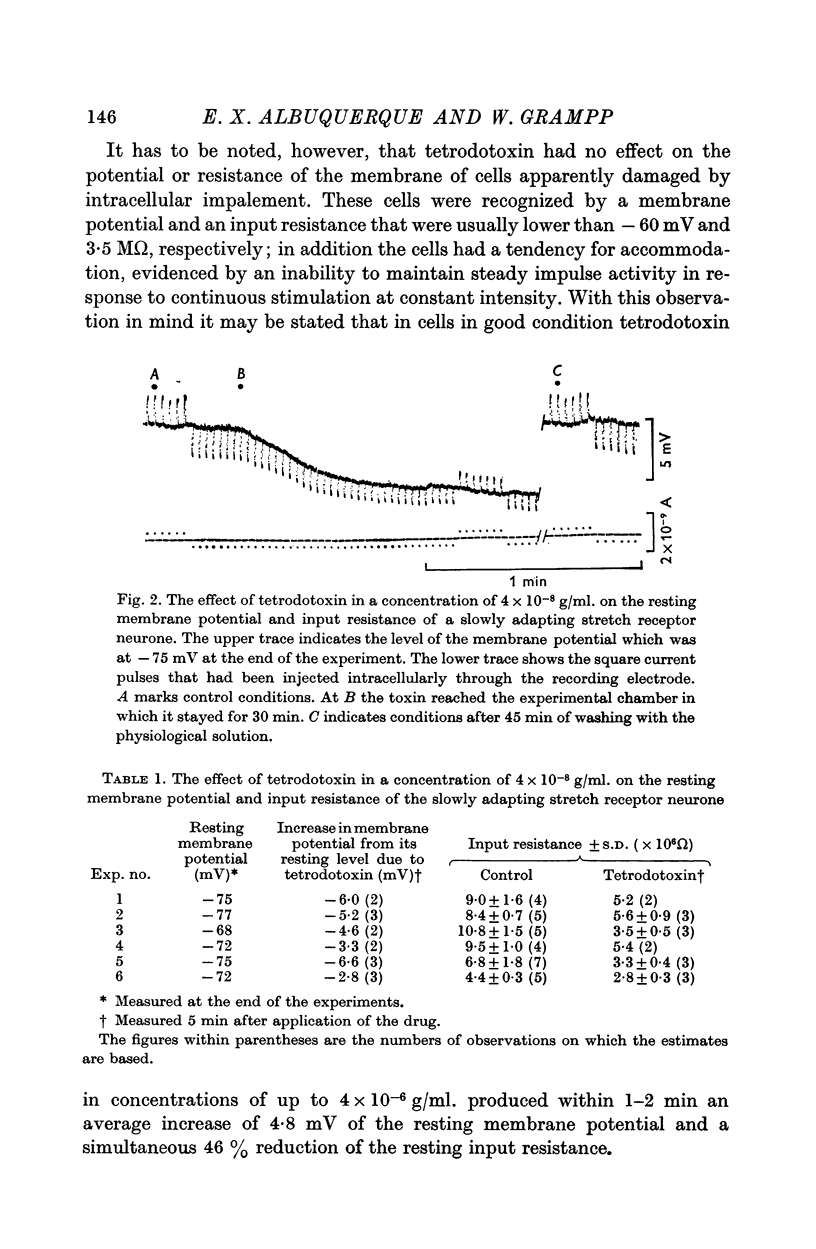
3. In a concentration of 4 × 10-8 g/ml. tetrodotoxin produced within 1-2 min an average increase of 4·8 mV of the resting membrane potential and a simultaneous 47% reduction of the resting input resistance. These effects were reversed by washing the preparation in physiological solution for 1 hr.
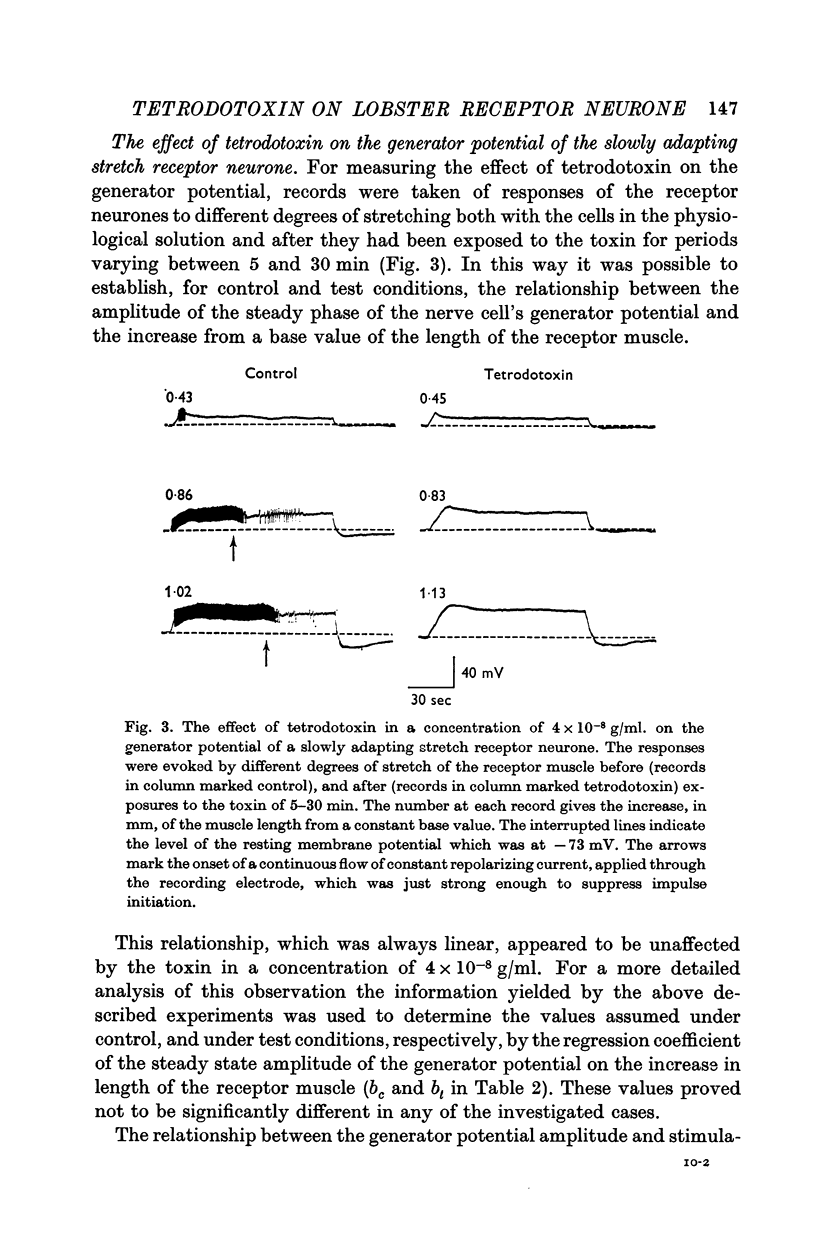
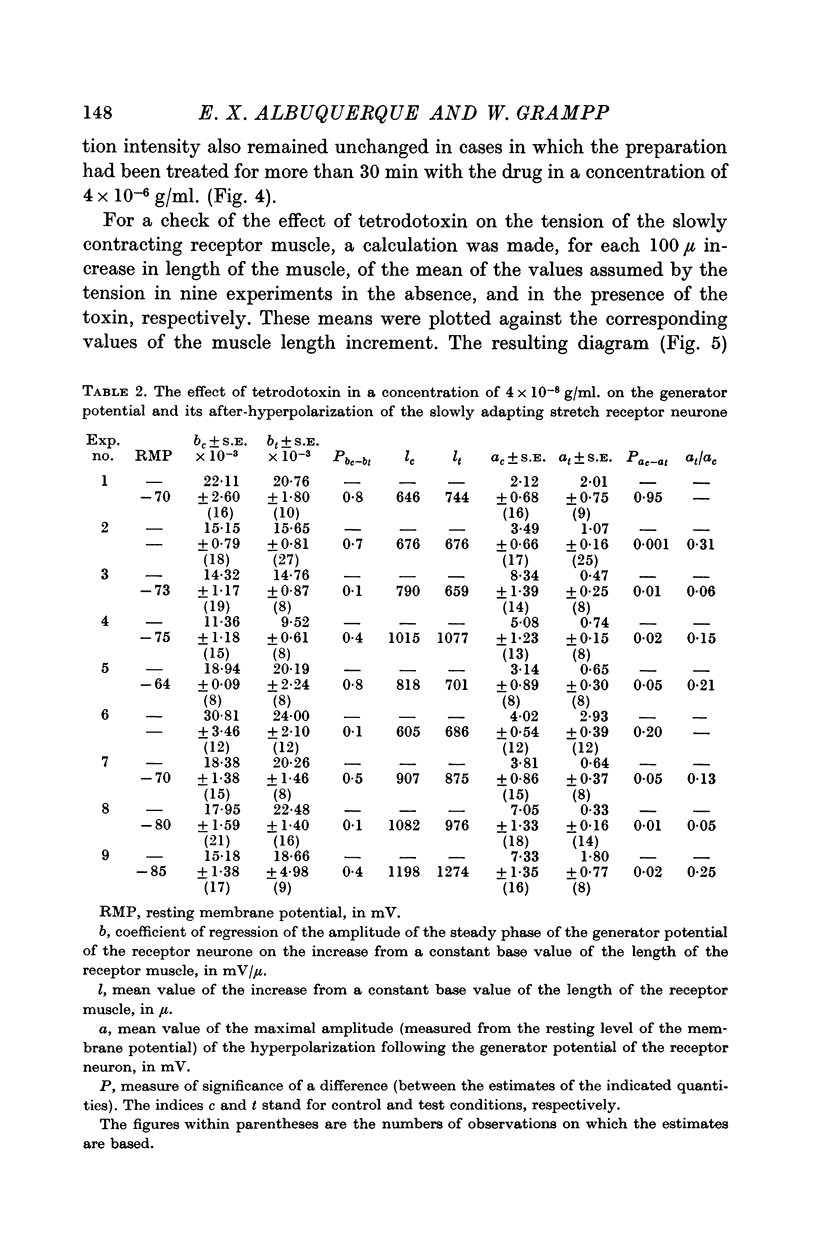
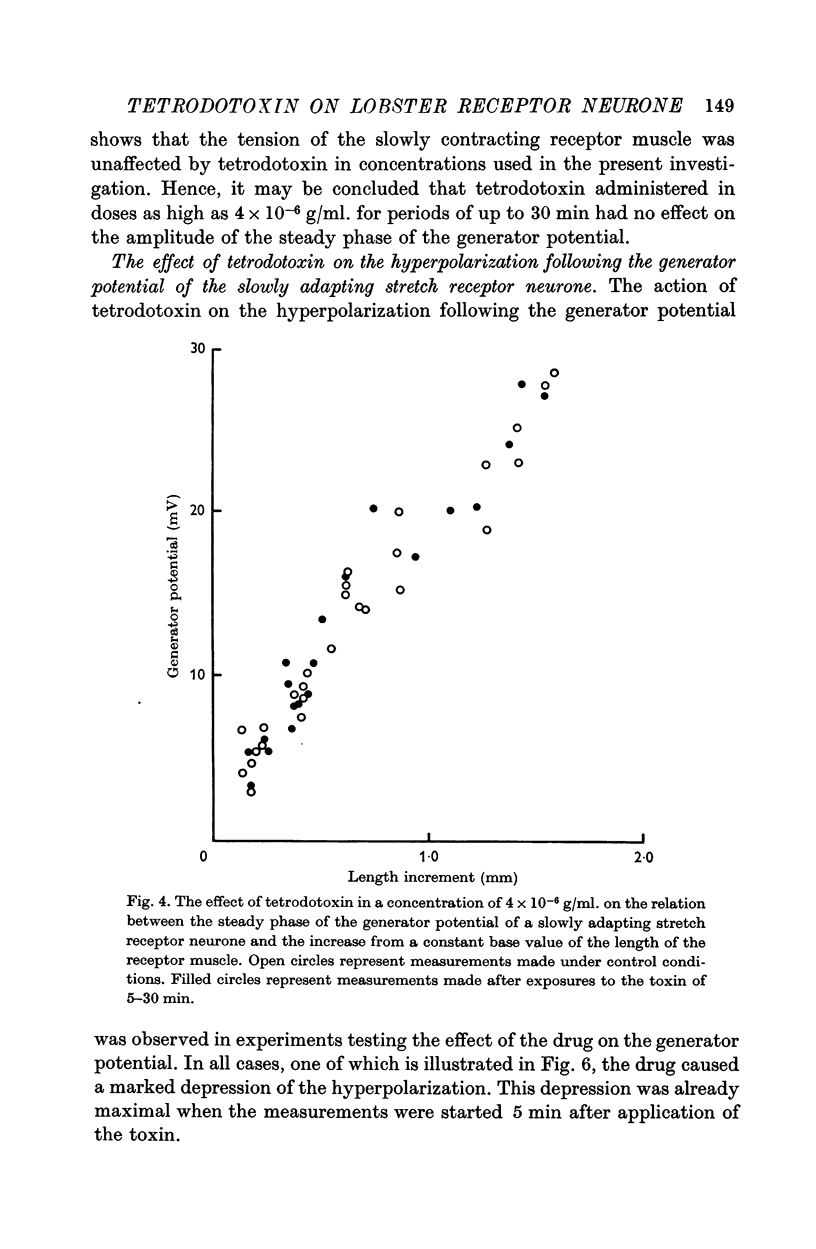
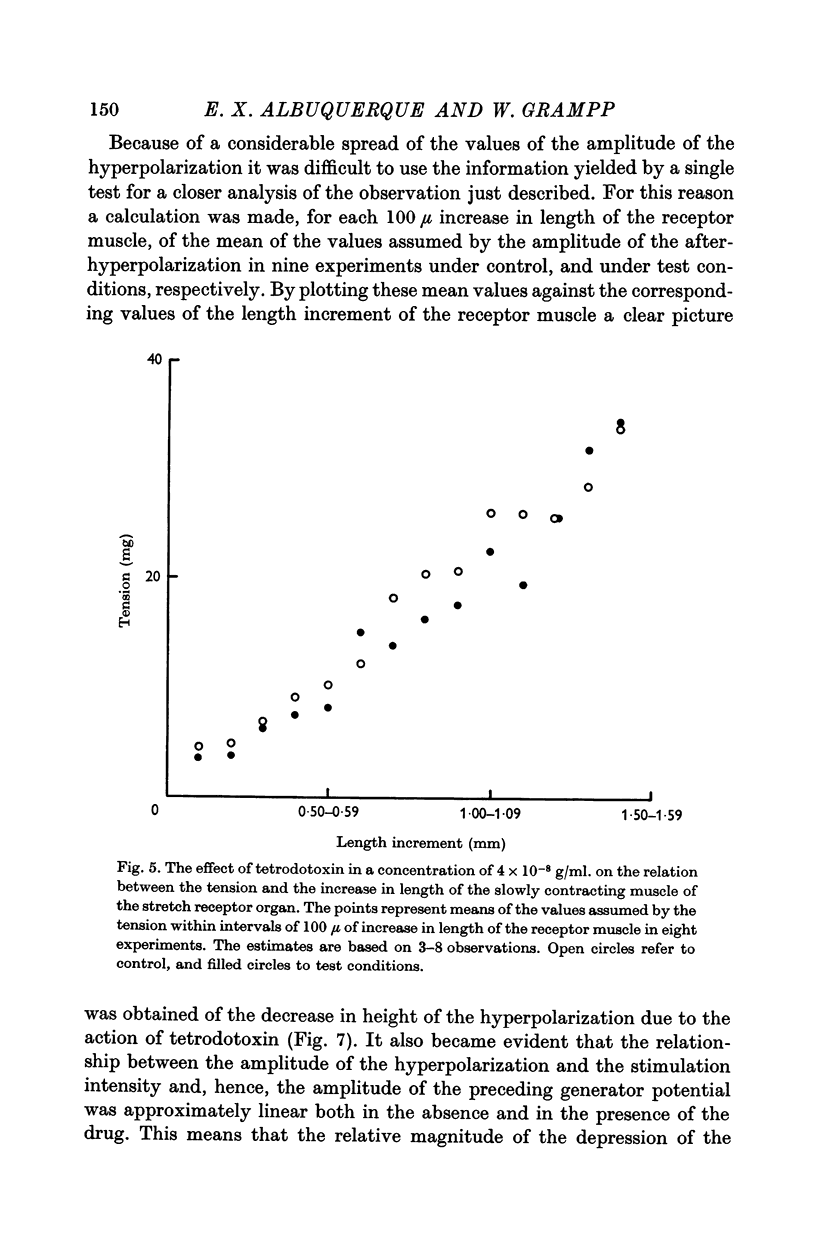
4. Tetrodotoxin administered in doses as high as 4 × 10-6 g/ml. for periods of up to 30 min had no effect on the amplitude of the steady phase of the generator potential.
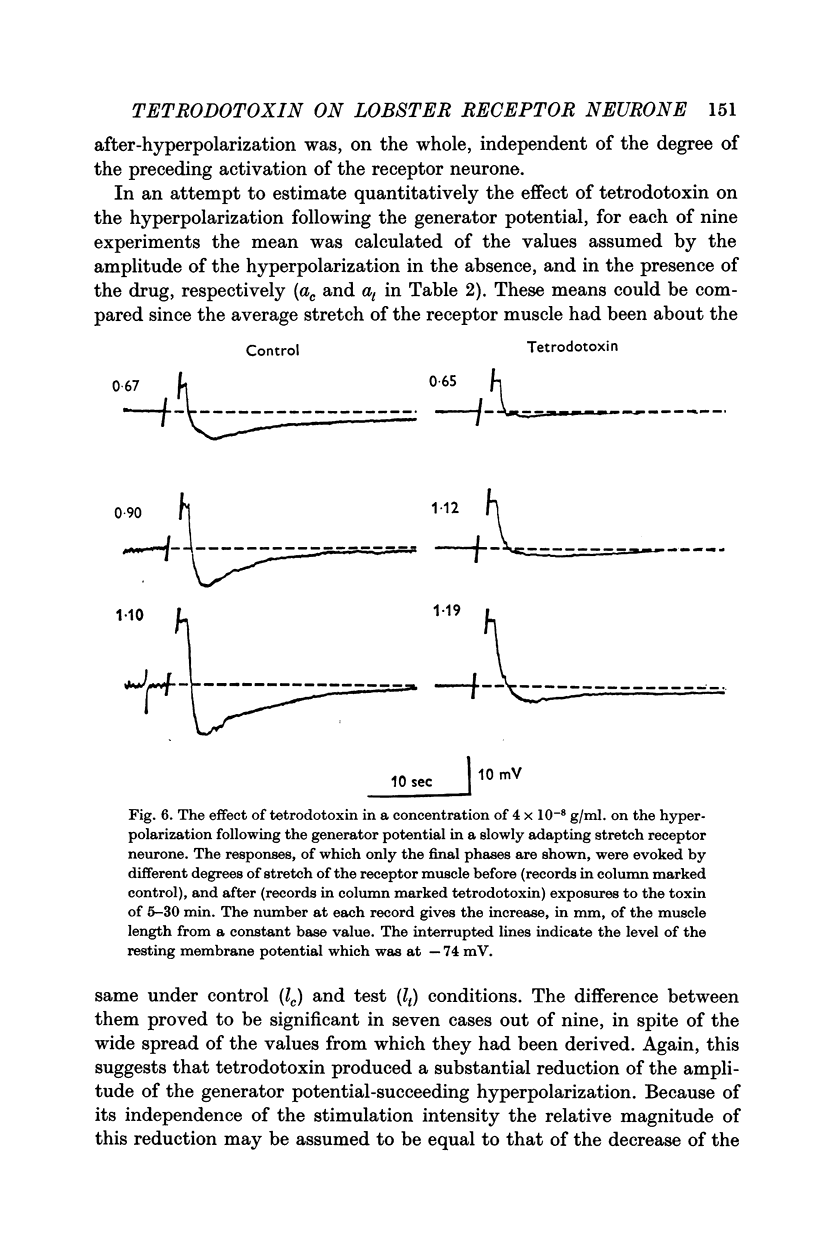
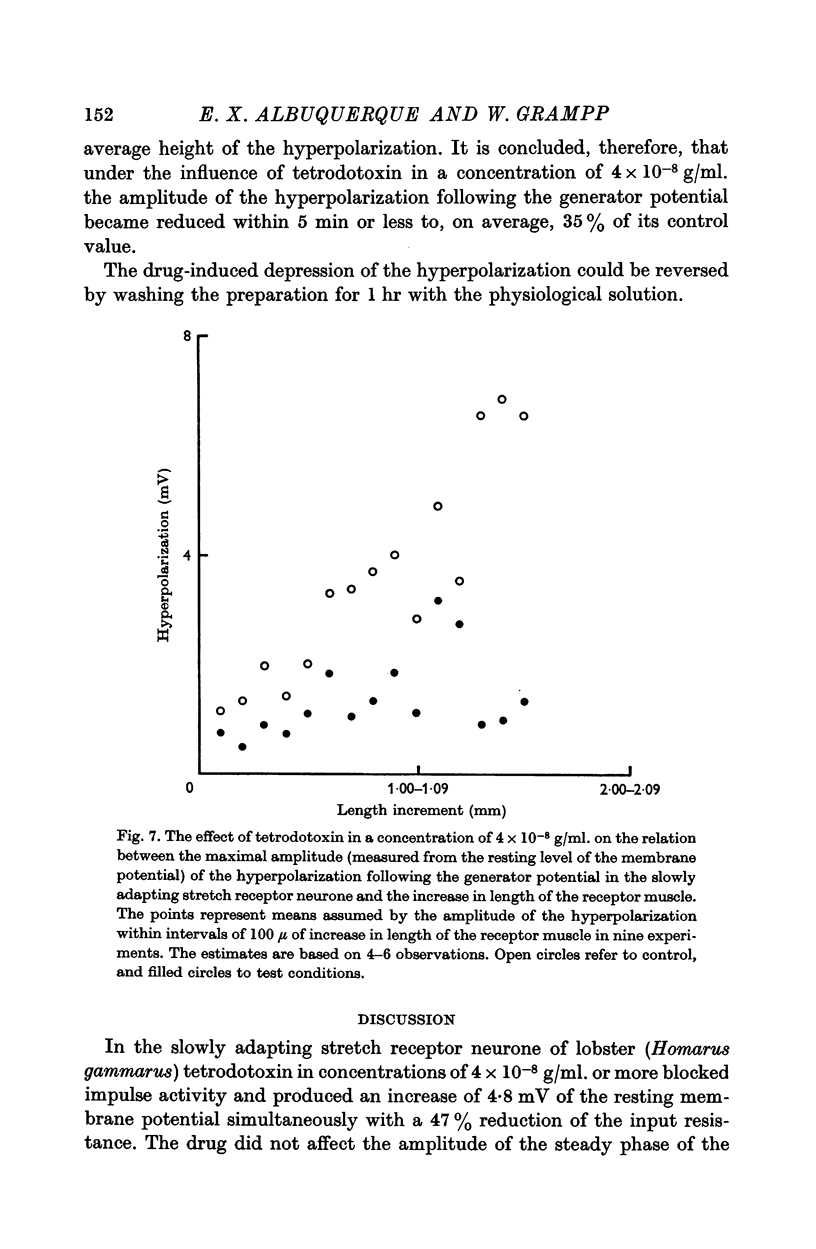
5. In a concentration of 4 × 10-8 g/ml. tetrodotoxin produced within 5 min a 65% reduction of the amplitude of the hyperpolarization following the generator potential. This effect was reversed by washing the preparation in physiological solution for 1 hr.
6. The simultaneous increase in resting membrane potential and decrease in membrane resistance is suggested to be due to an elevated potassium permeability besides a reduced sodium conductance. The constancy in height of the generator potential in the presence of a decreased membrane resistance makes necessary the assumption of an augmented generator current. The decrease in amplitude of the hyperpolarization following the generator potential may be the result of an increase in potassium conductance and/or a reduction in acceleration of an electrogenic pump in consequence of a diminished sodium influx during the generator potential.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINLEY F. J., Jr SODIUM, POTASSIUM, AND CHLORIDE CONCENTRATIONS AND FLUXES IN THE ISOLATED GIANT AXON OF HOMARUS. J Neurophysiol. 1965 Jul;28:742–772. doi: 10.1152/jn.1965.28.4.742. [DOI] [PubMed] [Google Scholar]
- Chan S. L., Quastel J. H. Tetrodotoxin: effects on brain metabolism in vitro. Science. 1967 Jun 30;156(3783):1752–1753. doi: 10.1126/science.156.3783.1752. [DOI] [PubMed] [Google Scholar]
- DIAMOND J., GRAY J. A., INMAN D. R. The relation between receptor potentials and the concentration of sodium ions. J Physiol. 1958 Jul 14;142(2):382–394. doi: 10.1113/jphysiol.1958.sp006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., TERZUOLO C. A., WASHIZU H. THE EFFECT OF CHANGES OF THE IONIC ENVIRONMENT UPON AN ISOLATED CRUSTACEAN SENSORY NEURON. J Neurophysiol. 1963 Nov;26:948–957. doi: 10.1152/jn.1963.26.6.948. [DOI] [PubMed] [Google Scholar]
- EYZAGUIRRE C., KUFFLER S. W. Processes of excitation in the dendrites and in the soma of single isolated sensory nerve cells of the lobster and crayfish. J Gen Physiol. 1955 Sep 20;39(1):87–119. doi: 10.1085/jgp.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. The origin of the post-tetanic hyperpolarization of mammalian motor nerve terminals. J Physiol. 1966 May;184(2):335–352. doi: 10.1113/jphysiol.1966.sp007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundfest H. Heterogeneity of excitable membrane: electrophysiological and pharmacological evidence and some consequences. Ann N Y Acad Sci. 1966 Jul 14;137(2):901–949. doi: 10.1111/j.1749-6632.1966.tb50208.x. [DOI] [PubMed] [Google Scholar]
- HAAPANEN L., OTTOSON D. A frequency compensated input unit for recording with microelectrodes. Acta Physiol Scand. 1954 Nov;32(2-3):271–280. doi: 10.1111/j.1748-1716.1954.tb01175.x. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., TERZUOLO C. A., WASHIZU Y. SEPARATION OF TRANSDUCER AND IMPULSE-GENERATING PROCESSES IN SENSORY RECEPTORS. Science. 1963 Nov 29;142(3596):1180–1181. doi: 10.1126/science.142.3596.1180. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., DEGUCHI T., URAKAWA N., OHKUBO Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am J Physiol. 1960 May;198:934–938. doi: 10.1152/ajplegacy.1960.198.5.934. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakajima S., Grundfest H. The action of tetrodotoxin on electrogenic components of squid giant axons. J Gen Physiol. 1965 Jul;48(6):975–996. [PubMed] [Google Scholar]
- Narahashi T., Anderson N. C., Moore J. W. Comparison of tetrodotoxin and procaine in internally perfused squid giant axons. J Gen Physiol. 1967 May;50(5):1413–1428. doi: 10.1085/jgp.50.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K., Sato M. Blocking of the impulse and depression of the receptor potential by tetrodotoxin in non-myelinated nerve terminals in pacinian corpuscles. J Physiol. 1966 May;184(2):376–386. doi: 10.1113/jphysiol.1966.sp007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki M., Sato M. Changes in the membrane potential and the membrane conductance associated with a sustained compression of the non-myelinated nerve terminal in Pacinian corpuscles. J Physiol. 1965 Sep;180(1):186–208. [PMC free article] [PubMed] [Google Scholar]
- TERZUOLO C. A., WASHIZU Y. Relation between stimulus strength, generator potential and impulse frequency in stretch receptor of Crustacea. J Neurophysiol. 1962 Jan;25:56–66. doi: 10.1152/jn.1962.25.1.56. [DOI] [PubMed] [Google Scholar]
- Takata M., Moore J. W., Kao C. Y., Fuhrman F. A. Blockage of sodium conductance increase in lobster giant axon by tarichatoxin (tetrodotoxin). J Gen Physiol. 1966 May;49(5):977–988. doi: 10.1085/jgp.49.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]


