Abstract
The in vitro leishmanicidal effects of a linalool-rich essential oil from the leaves of Croton cajucara against Leishmania amazonensis were investigated. Morphological changes in L. amazonensis promastigotes treated with 15 ng of essential oil per ml were observed by transmission electron microscopy; leishmanial nuclear and kinetoplast chromatin destruction, followed by cell lysis, was observed within 1 h. Pretreatment of mouse peritoneal macrophages with 15 ng of essential oil per ml reduced by 50% the interaction between these macrophages and L. amazonensis, with a concomitant increase by 220% in the level of nitric oxide production by the infected macrophages. Treatment of preinfected macrophages with 15 ng of essential oil per ml reduced by 50% the interaction between these cells and the parasites, which led to a 60% increase in the amount of nitric oxide produced by the preinfected macrophages. These results provide new perspectives on the development of drugs with activities against Leishmania, as linalool-rich essential oil is a strikingly potent leishmanicidal plant extract (50% lethal doses, 8.3 ng/ml for promastigotes and 8.7 ng/ml for amastigotes) which inhibited the growth of L. amazonensis promastigotes at very low concentrations (MIC, 85.0 pg/ml) and which presented no cytotoxic effects against mammalian cells.
Parasites of the genus Leishmania are transmitted by the bite of sand flies and infect cells of the mononuclear phagocyte lineage of their vertebrate hosts (2, 21). Depending both on the virulence factors of the parasite itself and on the immune response established by the host, a spectrum of diseases known as leishmaniasis can appear, and these can be cutaneous and/or visceral (30). Approximately 350 million people live in areas of active transmission of Leishmania, with 12 million people throughout Africa, Asia, Europe, and the Americas directly affected by leishmaniasis. More than 90% of the cutaneous cases appear in Afghanistan, Saudi Arabia, Algeria, Brazil, Iran, Iraq, Syria, and Sudan (13). Cutaneous leishmaniasis either can resolve spontaneously after a few months or, depending on the causative Leishmania species, can evolve into diffuse cutaneous, relapsing cutaneous, or mucocutaneous leishmaniasis, while untreated visceral leishmaniasis leads to death in the majority of patients (2). Leishmania amazonensis is one of the principal agents of diffuse cutaneous leishmaniasis, which is usually unresponsive to all treatments known to date (31). Also, visceralization of Leishmania strains that are classically restricted to cutaneous leishmaniasis has often been observed in patients with Leishmania-human immunodeficiency virus coinfection (3).
The control of leishmaniasis remains a problem because no vaccines exist and the available chemotherapy still relies on the potentially toxic pentavalent antimonials, which cause serious side effects and require long-term treatment (38). The rise in the rates of in vitro antimonial resistance due to intermittent drug exposure (15, 16), the isolation of antimonial-resistant Leishmania strains from patients with unresponsive cutaneous leishmaniasis (2, 9), and recently, the numerous cases of visceral leishmaniasis among patients infected with the human immunodeficiency virus (3) make the search for new agents for the treatment of leishmaniasis urgent. Extensive studies of new drugs with antileishmanial activities, including both natural products and synthetic compounds, have been undertaken worldwide (9), although problems with the side effects of the chemotherapies used at present have not yet been solved.
In recent years, there has been growing interest in alternative therapies and the use of natural products, especially those derived from plants (39). The bark of Croton cajucara is used in Brazilian folk medicine as an infusion to treat gastrointestinal disorders (22). Experimental studies with laboratory animals have identified potential applications for some purified substances from C. cajucara bark extracts, which present anti-inflammatory, antiulcerogenic, antitumorigenic, antimutagenic, antiestrogenic, hypoglycemic, and triglyceride-lowering effects (1, 6-8, 14, 20, 22-24, 33, 34, 40).
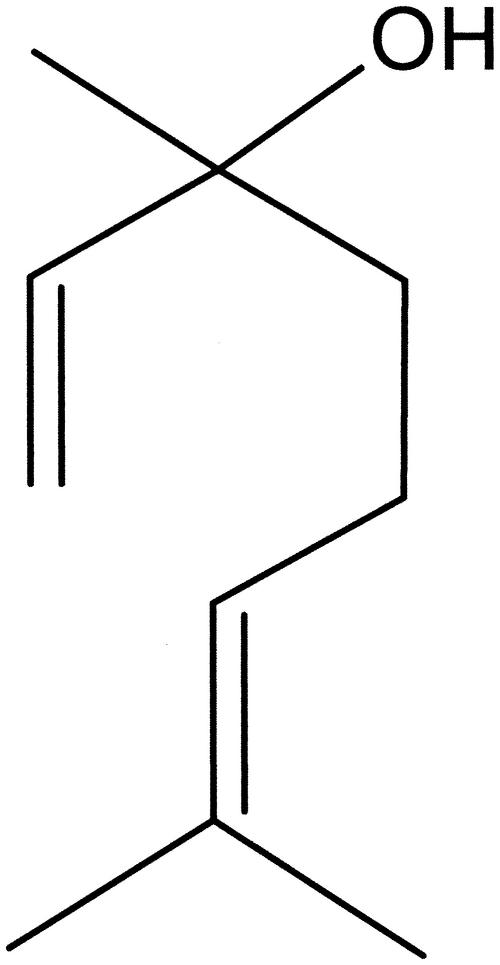
Linalool, a terpenic alcohol (Fig. 1), is the principal component of rosewood (Aniba rosaeodora var. amazonica Ducke syn Aniba duckei Kostermans) and Ho tree (Cinamomon camphora) oils (37). It is also obtained as a by-product in the industrial synthesis of vitamin E (37). The antimicrobial and anesthetic activities of linalool-containing essential oil have been reported (4, 17, 35). Recently, the linalool-rich essential oil from the leaves of C. cajucara has been purified, and its contents have been analyzed by gas chromatography-mass spectrometry (GC-MS) (32). Based upon the facts that the essential oil extracted from the bark of C. cajucara presents anti-inflammatory activity in rodents (22, 23) and that linalool-rich essential oil extracted from other plants presents antimicrobial properties (35), in this work we investigated the effects of essential oil extracted from the leaves of C. cajucara on L. amazonensis parasites, on the interaction of these flagellates with mouse peritoneal macrophages, and on nitric oxide production by the infected macrophages.
FIG. 1.
Chemical structure of linalool.
MATERIALS AND METHODS
Plant material.
Plant material from C. cajucara Benth was obtained from Embrapa Experimental Farm, Amazonas, Brazil. A voucher specimen was deposited at the Embrapa Occidental Amazon Herbarium (registry no. IAN 165013).
Essential oil extraction.
Leaves of C. cajucara were dried at room temperature and coarsely powdered. The oil was obtained by hydrodistillation (5 h) of C. cajucara leaves with a modified Clevenger apparatus (18); the yield was 0.40% (on a dry weight basis).
Linalool purification.
Linalool was purified and identified by GC and GC coupled with MS (GC-MS). GC was performed with a Hewlett-Packard (HP) 6890 gas chromatograph fitted with a BP-5 fused-silica capillary column (25 m by 0.33 mm; film thickness, 0.5 μm), with helium used as the carrier gas (1 ml/min). Retention indices were obtained by injection of a series of n-alkanes and were compared with those in the literature. The GC-MS system used was an HP 5973 MSD system coupled with an HP 6890 gas chromatograph; helium was used as the carrier gas, and the same column and conditions described above were used (32). Results were compared with the Wiley library of spectra.
Parasite culture.
Promastigote forms of L. amazonensis (Raimundo strain, MHOM/BR/76/Ma-5) were maintained by weekly transfers in brain heart infusion medium supplemented with 10% fetal bovine serum (FBS) at 26°C. The infectivities of the parasites were maintained by periodic inoculation into hamster footpads.
MIC evaluation.
L. amazonensis promastigotes (106 parasites/ml) were incubated at 26°C for 120 h in fresh medium (brain heart infusion medium) supplemented with 10% FBS in the absence or presence of several concentrations (1 pg/ml to 1 mg/ml) of essential oil or purified linalool (cell growth was determined daily by assessment of visible turbidity) in order to evaluate the MIC, as described previously (36). The MIC was considered the lowest concentration of each substance used that prevented the growth of L. amazonensis in vitro.
Mouse peritoneal macrophages.
Thioglycolate-elicited peritoneal macrophages from female Swiss mice (age, 6 to 8 weeks) were collected in 0.85% saline and allowed to adhere onto coverslips placed in 24-well culture plates for 30 min at 37°C in a 4% CO2 atmosphere. Nonadherent cells were then removed, and the adhered macrophages were washed twice with 0.9% saline and cultured for 24 h in culture medium (RPMI; Gibco-BRL, Gaithersburg, Md.) supplemented with 10% FBS.
Purification of amastigotes.
Intact living L. amazonensis promastigotes in the stationary growth phase were added to plate wells containing cultured peritoneal macrophages. Free promastigotes were removed after 2 h of incubation, and the infected macrophages were kept for 48 h under the conditions described above. Free amastigotes were then collected from the supernatants of the culture plate wells. The amastigotes were washed twice in 0.1 M phosphate buffer (pH 7.2) and then resuspended in RPMI culture medium for the antileishmanial activity assays.
Antileishmanial activity.
L. amazonensis promastigotes or amastigotes (106 parasites/ml) were incubated in RPMI culture medium in the absence or presence of several concentrations (1 pg/ml to 1 mg/ml) of essential oil or purified linalool at 37°C in order to evaluate parasite survival and cell morphology by optical microscopy at 10-min intervals. Parasite viability was assessed before and after the incubations by evaluation of the motility and by the trypan blue method (5) with a hemocytometer. Cell viability was determined by using the following formula: [100 − (L2/L1)] × 100, where L1 is the percentage of viable control cells and L2 is the percentage of viable treated cells, as described previously (11). The 50% lethal dose (LD50) was determined by logarithmic regression analysis of the data obtained by the formula described above, as described previously (36). Cell morphology evaluation was performed with fresh as well as Giemsa-stained preparations, as described previously (31). The essential oil and the purified linalool were diluted in dimethyl sulfoxide (DMSO; Sigma Chemical Co., St. Louis, Mo.) at 100 mg/ml and then in RPMI. In all tests, 1% DMSO (the same concentration present in the highest dose of the compounds) and medium alone were used as controls.
Electron microscopy.
L. amazonensis promastigotes were incubated in the absence or presence of 1 ng of linalool-rich essential oil per ml for 5, 10, 15, 20, 25, or 30 min. The parasites were washed twice in Ringer's solution (0.9% NaCl, 5.0% KCl, 5.0% CaCl2) and fixed in a solution containing 2.5% glutaraldehyde, 4% formaldehyde, and 3.7% sucrose in 0.1 M phosphate buffer (pH 7.2) for 1 h at room temperature. The parasites were then washed in 0.1 M cacodylate buffer (pH 7.2), and the parasites were gently scraped off with a rubber policeman and postfixed in a solution containing 1% OsO4, 0.8% potassium ferricyanide, and 5 mM calcium chloride in 0.1 M cacodylate buffer (pH 7.2) for 1 h at room temperature in the dark. The parasites were then rinsed in cacodylate buffer, dehydrated in acetone, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and were examined in a transmission electron microscope (900; Carl Zeiss, Oberkochen, Germany) operated at 80 kV.
Infection of macrophages and nitric oxide production.
Mouse peritoneal macrophages were obtained as described above. The parasites and/or the macrophages were either not treated or treated with 15, 1.5, or 0.2 ng of essential oil per ml 20 min prior to the macrophage-parasite interactions. The adherent cultured macrophages and the free parasites were washed once and resuspended in fresh culture medium. Dead parasites were removed from the medium by centrifugation (1,000 × g, 5 min), and intact living L. amazonensis promastigotes in the stationary growth phase were then added to the macrophage culture plate wells. The parasite-macrophage interaction studies were performed at 37°C for 90 min by using parasites and/or macrophages pretreated with the essential oil or macrophages that had already been infected with the parasites for 24 h and then treated with the essential oil. In the last system, all promastigotes had already differentiated into amastigotes before the treatment with the essential oil. A ratio of 10 promastigotes to 1 macrophage was used for both infection assays. After the interaction assays were done, the coverslips were fixed and Giemsa stained, and the percentage of infected macrophages was determined by counting 600 cells in triplicate coverslips. The association indices were determined by multiplying the percentage of infected macrophages by the mean number of parasites per infected cell. Association indices were the number of parasites that actually infected the macrophages. The supernatants from control and L. amazonensis-infected macrophages were analyzed for their nitrite contents by the Griess reaction, as described previously (19). The absorbance at 550 nm was measured, and the concentration of nitrite was calculated by using a linear regression of a standard curve, as described previously (25). The mice were first killed to obtain mouse peritoneal macrophages for both infection with Leishmania and nitric oxide measurements, and all federal guidelines and institutional policies for the treatment of mice were adhered to.
Cytotoxicity assay.
The cytotoxicity of the drug for mammalian cells was measured by the neutral red uptake assay (26) with mouse peritoneal macrophages and a transformed cell line (Vero cells) (29). The cells were cultivated in 96-well microtiter plates (150 μl containing 105 cells/ml in Eagle minimal essential medium [Eagle MEM]/well) at 37°C in a humidified 5% CO2 atmosphere. The medium was completed by the addition of l-glutamine (0.10 g/liter), HEPES (2.38 g/liter), penicillin G (105 IU/liter), streptomycin sulfate (0.10 g/liter), and 4% FBS. After a 24-h incubation, 50 μl of ethanolic or aqueous crude extracts, essential oil, or linalool was added to the cell cultures at the respective MICs and LD50s for L. amazonensis. A total of 50 μl of Eagle MEM was added to the control cells. After further incubation for 48 h, control and treated cells were washed three times with phosphate-buffered saline (PBS) solution (pH 7.2). A total of 100 μl of neutral red solution (0.3% in Eagle MEM) was added to each well. After a 3-h incubation at 37°C in this solution, the cells were then washed three times with PBS. A total of 100 μl of a solution containing acetic acid (1%; vol/vol) and ethanol (50%; vol/vol) was added to the wells, and the optical densities of the supernatants were measured at 540 nm.
Statistical analysis.
All experiments were performed in triplicate. The mean and standard deviation of at least three experiments were determined. Statistical analysis of the differences between mean values obtained for the experimental groups was done by Student's t test. P values of 0.05 or less were considered significant.
RESULTS
Inhibition of parasite growth.
The MICs of C. cajucara essential oil and purified linalool for the growth of L. amazonensis promastigotes were 85.0 and 22.0 pg/ml, respectively.
Antileishmanial activity.
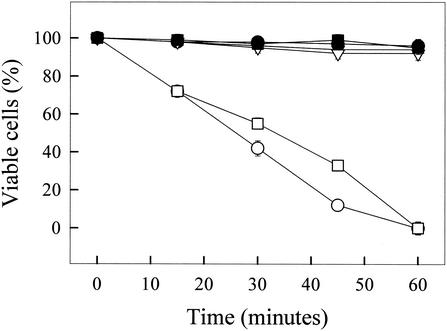
The effects of C. cajucara essential oil and purified linalool on the viability of L. amazonensis were tested. LD50s for promastigotes were 8.3 ng/ml for essential oil and 4.3 ng/ml for purified linalool, and the LD50s for amastigotes were 22.0 ng/ml for essential oil and 15.5 ng/ml for purified linalool. Figure 2 shows the time course of the viabilities of L. amazonensis promastigotes, amastigotes, and mouse peritoneal macrophages in the absence or presence of linalool-rich essential oil. Essential oil at 15.0 ng/ml was able to kill 100% of the parasites in 60 min. On the other hand, mouse macrophages were unaffected by essential oil at 15.0 ng/ml (Fig. 2).
FIG. 2.
Time courses of the viabilities of L. amazonensis promastigotes and mouse peritoneal macrophages in the absence or presence of linalool-rich essential oil (15.0 ng/ml) extracted from C. cajucara. The viabilities of both parasites and macrophages were calculated as described in the text. •, L. amazonensis promastigotes (control); ○, L. amazonensis promastigotes and essential oil; ▪, L. amazonensis amastigotes (control); □, L. amazonensis amastigotes and essential oil; ▾, mouse peritoneal macrophages (control); ▿, mouse peritoneal macrophages and esssential oil
Transmission electron microscopy.
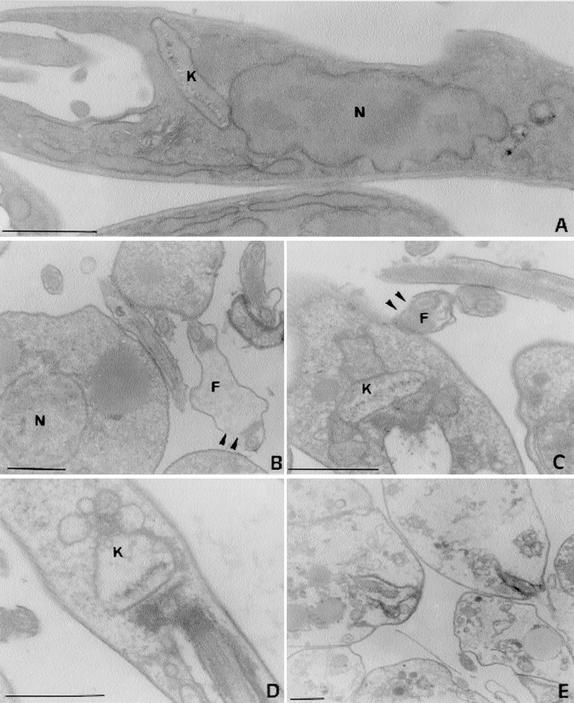
Untreated and treated (15.0 ng of essential oil per ml) promastigotes were observed by transmission electron microscopy, and photomicrographs of the promastigotes are shown in Fig. 3A to E, which show promastigotes with different degrees of damage. Disruption of flagellar membranes, mitochondrial swelling, and gross alterations in the organization of the nuclear and kinetoplast chromatins were detected. After 30 min in the presence of essential oil the parasites were completely destroyed.
FIG. 3.
Effects of linalool-rich essential oil (15.0 ng/ml) extracted from C. cajucara on promastigote forms of L. amazonensis observed by transmission electron microscopy. (A) Control parasites; (B to E) parasites treated for 5 (B), 10 (C), 15 (D), and 30 (E) min, showing promastigotes with different degrees of damage. Note the disruption of flagellar membranes (arrowheads in panels B and C), the mitochondrial swelling (C and D), and the gross alterations in the organization of the nuclear and kinetoplast chromatins (C and D). After 30 min in the presence of essential oil the parasites were completely destroyed (E). N, nucleus; K, kinetoplast, F, flagellum. Bars, 1 μm.
Cytotoxicity for mammalian cells.
The effects of the essential oil and linalool (at the MICs and LD50s for L. amazonensis) on mouse peritoneal macrophages and a transformed cell line (Vero cells) were tested as described previously (12). No cytotoxic effects on mammalian cells were observed at the concentrations used (data not shown).
Infection of macrophages.
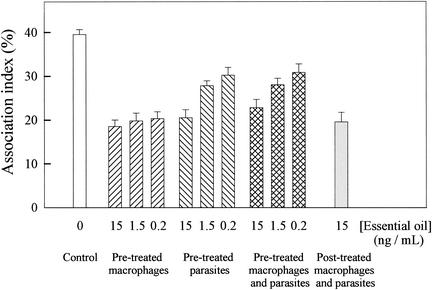
Figure 4 shows the effects of the essential oil on the L. amazonensis-macrophage interaction. The parasites and/or the mouse peritoneal macrophages were either not treated or treated with different concentrations (15.0, 1.5, and 0.2 ng/ml) of essential oil 20 min prior to the macrophage-parasite interactions. When macrophages were pretreated with essential oil, the association indices were 50% lower than those in the control system (in which control macrophages and control parasites were used), regardless of the concentration of essential oil used in those assays. When parasites were pretreated with 15, 1.5, and 0.2 ng of essential oil per ml, the association indices were 50, 30, and 25% lower, respectively, than those in the control system; these results were approximately the same as those obtained for pretreated macrophages and parasites. When the macrophages were preinfected with L. amazonensis for 24 h and then treated with 15 ng of essential oil per ml, the association index was 50% lower than that for the control system.
FIG. 4.
Effects of linalool-rich essential oil extracted from C. cajucara on the L. amazonensis-macrophage interaction. The parasites and/or the mouse peritoneal macrophages were either not treated or treated with 15, 1.5, and 0.2 ng of essential oil per ml 20 min prior to the macrophage-parasite interactions. The adherent cultured macrophages and the free parasites were washed once and resuspended in fresh culture medium. Dead parasites were removed from the medium by centrifugation (1,000 × g, 5 min), and intact living L. amazonensis promastigotes were then added to the macrophage culture plate wells. Association indices were determined by light microscopy, with 600 cells in triplicate coverslips counted after 90 min of interaction. Each bar represents the mean ± standard error of at least three independent experiments, which were performed in triplicate. The association indices from assays performed with macrophages and/or parasites pretreated with essential oil are significantly different from the association indices for control (nontreated) macrophages.
Nitric oxide production.
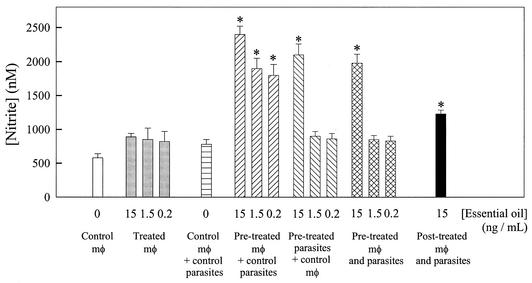
Mouse peritoneal macrophages were either noninfected or infected with L. amazonensis, and then the culture supernatants were evaluated for nitrite contents. The parasites and/or the macrophages were either not treated or treated with 15, 1.5, and 0.2 ng of essential oil per ml 20 min prior to the macrophage-parasite interactions. Figure 5 shows that noninfected macrophages that were treated with essential oil produced 40 to 50% more nitric oxide than the control (noninfected and nontreated) macrophages. Infected macrophages produced 30% more nitric oxide than noninfected macrophages. When infected macrophages were pretreated with 15, 1.5, and 0.2 ng of essential oil per ml, the levels of nitric oxide production were 220, 150, and 130% higher, respectively, than those for the control infected macrophages. When parasites were pretreated with 15 ng of essential oil per ml, the level of nitric oxide production was 170% higher than that for the control infected macrophages, although no significant difference was obtained when the parasites were pretreated with 1.5 or 0.2 ng of essential oil per ml. When both macrophages and parasites were pretreated with 15 ng of essential oil per ml, the level of nitric oxide production was 150% higher than that for the control infected macrophages, although no significant difference was obtained when the parasites were pretreated with 1.5 and 0.2 ng of essential oil per ml. When the macrophages were preinfected with L. amazonensis for 24 h and then treated with 15 ng of essential oil per ml, the level of nitric oxide production was 60% higher than that for the control infected macrophages.
FIG. 5.
Effects of linalool-rich essential oil extracted from C. cajucara on nitric oxide production by mouse peritoneal macrophages (mφ). The parasites and/or the macrophages were either not treated or treated with 15, 1.5, and 0.2 ng of essential oil per ml 20 min prior to the macrophage-parasite interactions. The adherent cultured macrophages and the free parasites were washed once and resuspended in fresh culture medium. Dead parasites were removed from the medium by centrifugation (1,000 × g, 5 min), and intact living L. amazonensis promastigotes were then added to the macrophage culture plate wells. The supernatants from control and L. amazonensis-infected macrophages were recovered, and the nitrite concentration of each system was determined by the Griess reaction, as described in the Materials and Methods section. Each bar represents the mean ± standard error of at least three independent experiments, which were performed in triplicate. The asterisks indicate that the result is significantly different from that for the infected macrophages when neither the macrophages nor the parasites were treated with essential oil.
DISCUSSION
The ability to survive and multiply within macrophages is a feature of several infectious agents including Trypanosoma cruzi and Leishmania. In order to sustain a chronic infection, parasites must subvert macrophage-accessory cell activities and ablate the development of protective immunity (2). Nevertheless, the most important mechanism for the killing of Leishmania and the control of leishmaniasis is the production of nitric oxide by macrophages of draining lymph nodes (11).
New drug therapy regimens have taken advantage of the knowledge obtained from studies on Leishmania-macrophage interactions (2). Concerning mouse peritoneal macrophage infection with L. amazonensis, when the macrophages were pretreated with C. cajucara essential oil, as well as when the macrophages were preinfected with the parasites and then treated with the essential oil, the association indices were 50% lower than those for the control system (in which control macrophages and control parasites were used), regardless of the concentration of essential oil used in those assays (Fig. 4). Therefore, the finding that macrophages pretreated with C. cajucara essential oil produced twice the amount of nitric oxide as the nontreated macrophages is hardly surprising (Fig. 5). Interestingly, recent studies have demonstrated that sand fly saliva suppresses macrophage leishmanicidal activity, inhibiting nitric oxide production (10, 22). This activity has been attributed to the sand fly peptide maxadilan, which diminishes the ability of macrophages to produce nitric oxide and kill Leishmania in vitro (11). Cooperation between Leishmania species and their vectors is presumably a result of coevolution of the vector and the parasite. The epidemiological consequences of this restriction are that the spread of leishmaniases is restricted by the distribution of suitable vectors (27, 28).
Linalool-rich essential oils extracted from other plants have antimicrobial properties (35), so we decided to test the effects of C. cajucara extracts on the growth and viability of L. amazonensis. The LD50s of the essential oil and purified linalool from C. cajucara for both L. amazonensis promastigotes and amastigotes were very low. It is remarkable that 15.0 ng of essential oil per ml was able to kill 100% of both promastigotes and amastigotes in 60 min (Fig. 2). On the other hand, mouse macrophages were unaffected by 15.0 ng of essential oil per ml (Fig. 2). Also, the MICs of essential oil purified from Helichrysum italicum for fungal growth (4) are much higher than the MIC of essential oil from C. cajucara for L. amazonensis presented here (85.0 pg/ml).
Mitochondrial swelling and important alterations in the organization of the nuclear and kinetoplast chromatins were observed by electron microscopy when L. amazonensis parasites were treated for 20 to 30 min with 15.0 ng of essential oil from C. cajucara per ml (Fig. 3). Although linalool was extremely potent when used directly on L. amazonensis parasites, it had little effect when used in the assays for measurement of the association between macrophages and parasites, as well as assays for measurement of the levels of nitric oxide production by the infected macrophages (data not shown). These data could be explained by synergistic effects of the different compounds of the plant extracts used in this work, which is a phenomenon widely known for several other systems (41). On the other hand, the present work shows that the decrease in the association between macrophages and parasites, concomitant with the increase in the level of nitric oxide production by the infected macrophages when both cell types were pretreated with essential oil, was less than that when macrophages alone were pretreated. In this case one could infer that linalool and another substance of the essential oil could present distinct and yet opposite effects on the macrophages.
The results presented in this paper further support the antimicrobial activity of linalool-rich essential oil. The extreme toxicity of C. cajucara leaf extracts for L. amazonensis, with no effect upon mammalian cells, enables linalool-rich essential oil to be a source of a new lead compound for novel antileishmanial drugs.
Acknowledgments
We thank Rafael Linden and Marcio L. Rodrigues for critical comments on the manuscript and Paulo C. Miguel, Jaqueline F. de Amorim, and Barbara C. G. Da Silva for technical assistance.
This work was supported by the Brazilian agencies PRONEX (grant 0885), FAPERJ, CNPq, CNPq/UFRJ (PIBIC), FUJB, Embrapa, and FINEP.
REFERENCES
- 1.Agner, A. R., M. A. Maciel, A. C. Pinto, and I. M. Colus. 2001. Antigenotoxicity of trans-dehydrocrotonin, a cleorodane diterpene from Croton cajucara. Planta Med. 67:815-819. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, J., A. R. Satoskar, and D. G. Russel. 1999. Leishmania species: models of intracellular parasitism. J. Cell Sci. 112:2993-3002. [DOI] [PubMed] [Google Scholar]
- 3.Alvar, J., C. Cañavate, B. Gutiérrez-Solar, M. Jiménez, F. Laguna, R. López-Vélez, R. Molina, and J. Moreno. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angioni, A., A. Barra, M. Arlorio, J. D. Coisson, M. T. Russo, F. M. Pirisi, M. Satta, and P. Cabras. 2003. Chemical composition, plant genetic differences, and antifungal activity of the essential oil of Helichrysum italicum G. Don ssp. microphyllum (Willd.) Nym. J. Agric. Food Chem. 51:1030-1034. [DOI] [PubMed] [Google Scholar]
- 5.Barakiewicz, J., H. M. Dosch, and A. Cohen. 1988. Extracellular nucleotide catabolism in human B and T lymphocytes: the source of adenosine production. J. Biol. Chem. 263:7094-7098. [PubMed] [Google Scholar]
- 6.Bighetti, E. J., C. A. Hiruma-Lima, J. S. Gracioso, and A. R. Brito. 1999. Anti-inflammatory and antinociceptive effects in rodents of the essential oil of Croton cajucara Benth. J. Pharm. Pharmacol. 51:1447-1453. [DOI] [PubMed] [Google Scholar]
- 7.Brito, A. R., J. A. Rodriguez, C. A. Hiruma-Lima, M. Haun, and D. S. Nunes. 1998. Antiulcerogenic activity of trans-dehydrocrotonin from Croton cajucara. Planta Med. 64:126-129. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho, J. C., M. F. Silva, M. A. Maciel, A. C. Pinto, D. S. Nunes, R. M. Lima, J. K. Bastos, and S. J. Sarti. 1996. Investigation of anti-inflammatory and anticonceptive activities of trans-dehydrocrotin, a 19-nor-clerodane diterpene from Croton cajucara. Part 1. Planta Med. 62:402-404. [DOI] [PubMed] [Google Scholar]
- 9.Croft, S. L., and V. Yardley. 2002. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 8:319-342. [DOI] [PubMed] [Google Scholar]
- 10.Da Silva, S. A. G., S. S. Costa, S. C. F. Mendonça, E. M. Silva, V. L. G. Moraes, and B. Rossi-Bergmann. 1995. Therapeutic effect of oral Kalanchoe pinnata leaf extract in murine leishmaniasis. Acta Trop. 60:201-210. [DOI] [PubMed] [Google Scholar]
- 11.David, J., M. Soares, A. Satoskar, F. Brombacher, C. Shoemaker, R. Titus, and M. Bozza. 1997. Immunomodulatory properties of maxadilan, a peptide derived from sand fly saliva. Acta Parasitol. Turc. 21(Suppl.):174. [Google Scholar]
- 12.Delorenzi, J. C., M. Attias, C. R. Gattass, M. Andrade, C. Rezende, A. Cunha-Pinto, A. T. Henriques, D. C. Bou-Habib, and E. M. B. Saraiva. 2001. Antileishmanial activity of an indole alkaloid from Pesquiera australis. Antimicrob. Agents Chemother. 45:1349-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desjeux, P. 1991. Information on the epidemiology and control of the leishmaniases by country and territory. Report no. 27. World Health Organization, Geneva, Switzerland.
- 14.Farias, R. A., V. S. Rao, G. S. Viana, E. R. Silveira, M. A. Maciel, and A. C. Pinto. 1997. Hypoglycemic effect of trans-dehydrocrotonin, a nor-clerodane diterpene from Croton cajucara. Planta Med. 63:558-560. [DOI] [PubMed] [Google Scholar]
- 15.Fournet, A., R. Hocquemiller, F. Roblot, A. Cavé, P. Richomme, and J. Bruneton. 1993. Les chimanines, nouvelles quinoléines substituées en 2 isolées d′une plante bolivienne antiparasitaire: Galipea longiflora. J. Nat. Prod. 56:1547-1552. [Google Scholar]
- 16.Fournet, A., R. Hocquemiller, and J. C. Gantier. 1995. Combattre la leishmaniose. Recherche 26:424-429. [Google Scholar]
- 17.Ghelardini, C., N. Galeotti, G. Salvatore, and G. Mazzanti. 1999. Local anaesthetic activity of the essential oil of Lavandula angustifolia. Planta Med. 65:700-703. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb, O. R., and M. T. Guimarães. 1960. Modified distillation trap. Chemist-Analyst 49:114-116. [Google Scholar]
- 19.Green, S. J., M. S. Meltzer, J. B. Hibbs, and C. A. Nacy. 1990. Activated macrophages destroy intracellular Leishmania major amastigotes by l-arginine-dependent killing mechanism. J. Immunol. 144:278-283. [PubMed] [Google Scholar]
- 20.Grynberg, N. F., A. Echevarria, J. E. Lima, S. S. Pamplona, A. C. Pinto, and M. A. Maciel. 1999. Anti-tumor activity of 19-nor-clerodane diterpenes, trans-dehydrocronitin and trans-crotonin, from Croton cajucara. Planta Med. 65:687-689. [DOI] [PubMed] [Google Scholar]
- 21.Hall, L. R., and R. G. Titus. 1995. Sand fly saliva selectively modulates macrophage functions that inhibit killing of Leishmania major and nitric oxide production. J. Immunol. 155:3501-3506. [PubMed] [Google Scholar]
- 22.Hiruma-Lima, C. A., R. C. Spadari-Bratfisch, D. M. Grassi-Kassisse, and A. R. Brito. 1999. Antiulcerogenic mechanisms of dehydrocrotonin, a diterpene lactone obtained from Croton cajucara. Planta Med. 65:325-330. [DOI] [PubMed] [Google Scholar]
- 23.Hiruma-Lima, C. A., J. S. Gracioso, J. A. Rodriguez, M. Haun, D. S. Nunes, and A. R. Brito. 2000. Gastroprotective effects of essential oil from Croton cajucara Benth. (Euphorbiaceae). J. Ethnopharmacol. 69:229-234. [DOI] [PubMed] [Google Scholar]
- 24.Ichihara, Y., K. Takeya, Y. Hitotsuyanagi, H. Morita, S. Okuyama, M. Suganuma, H. Fujiki, M. Motidome, and H. Itokawa. 1992. Cajucarinolide and isocajucarinolide: anti-inflammatory diterpenes from Croton cajucara. Planta Med. 58:549-551. [DOI] [PubMed] [Google Scholar]
- 25.Ignácio, S. R. N., J. L. P. Ferreira, M. B. Almeida, and C. F. Kubelka. 2001. Nitric oxide production by murine macrophages in vitro and in vivo treated with Phyllanthus tenellus extracts. J. Ethnopharmacol. 74:181-187. [DOI] [PubMed] [Google Scholar]
- 26.Jirikowski, G. F., J. F. Ramalho-Ortigao, T. Lindl, and H. Seliger. 1989. Immunocytochemistry of 5-bromo-2′-deoxyuridine labelled oligonucleotide probes. A novel technique for in situ hybridization. Histochemistry 91:51-53. [DOI] [PubMed] [Google Scholar]
- 27.Killick-Kendrick, R. 1985. Some epidemiological consequences of the evolutionary fit between leishmaniae and their phlebotomine vectors. Bull. Soc. Pathol. Exot. Filiales 78:747-755. [PubMed] [Google Scholar]
- 28.Lanzaro, G. C., A. H. C. S. Lopes, J. M. C. Ribeiro, C. B. Shoemaker, A. Warburg, M. Soares, and R. G. Titus. 1999. Variation in the salivary peptide, maxadilan, from species in the Lutzomyia longipalpis complex. Insect Mol. Biol. 8:267-275. [DOI] [PubMed] [Google Scholar]
- 29.Leary, J. J., R. Wittrock, R. T. Sarisky, A. Weinberg, and M. J. Levin. 2002. Susceptibilities of herpes simplex viruses to penciclovir and acyclovir in eight cell lines. Antimicrob. Agents Chemother. 46:762-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liew, F. Y., and C. A. O'Donnel. 1993. Immunology of leishmaniasis. Adv. Parasitol. 32:161-259. [DOI] [PubMed] [Google Scholar]
- 31.Lopes, A. H. C. S., P. M. L. Dutra, C. O. Rodrigues, M. J. Soares, J. Angluster, and R. S. B. Cordeiro. 1997. Effect of platelet-activating factor on the process of cellular differentiation of Herpetomonas muscarum muscarum. J. Eukaryot. Microbiol. 44:321-325. [DOI] [PubMed] [Google Scholar]
- 32.Lopes, D., H. R. Bizzo, A. F. S. Sobrinho, and M. V. G. Pereira. 2000. Linalool-rich essential oil from leaves of Croton cajucara Benth. J. Essent. Oil Res. 12:705-708. [Google Scholar]
- 33.Luna-Costa, A. M., J. C. Silva, A. R. Campos, V. S. Rao, M. A. Maciel, and A. C. Pinto. 1999. Antioestrogenic effect of trans-dehydrocrotonin, a nor-clerodane diterpene from Croton cajucara Benth. Phytother. Res. 13:689-691. [DOI] [PubMed] [Google Scholar]
- 34.Maciel, M. A., A. C. Pinto., A. C. Arruda, S. G. Pamplona, F. A. Vanderline, A. J. Lapa, A. Echevarria, N. F. Grynberg, I. M. Colus, R. A. Farias, A. M. Luna-Costa, and V. S. Rao. 2000. Ethnopharmacology, phytochemistry and pharmacology: a successful combination in the study of Croton cajucara. J. Ethnopharmacol. 70:41-55. [DOI] [PubMed] [Google Scholar]
- 35.Mazzanti, G., L. Battinelli, and G. Salvatore. 1998. Antimicrobial properties of the linalool-rich essential oil of Hyssopus officinalis L. var. decumbens (Lamiaceae). Flav. Fragr. J. 13:289-294. [Google Scholar]
- 36.Newton, S. M., C. Lau, S. S. Gurcha, G. S. Besra, and C. W. Wright. 2002. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J. Ethnopharmacol. 79:57-67. [DOI] [PubMed] [Google Scholar]
- 37.Ohashi, S. T., L. S. Rosa, J. A. Santana, and C. L. Green. 1997. Brazilian rosewood oil: sustainable production and oil quality management. Perf. Flav. 22:1-5. [Google Scholar]
- 38.Olliaro, P. L., and A. D. M. Bryceson. 1993. Practical progress and new drugs for changing patterns of leishmaniasis. Parasitol. Today 9:323-328. [DOI] [PubMed] [Google Scholar]
- 39.Rates, S. M. K. 2001. Plants as source of drugs. Toxicon 39:603-613. [DOI] [PubMed] [Google Scholar]
- 40.Silva, J. C., F. A. Santos, V. S. Rao, M. A. Maciel, and A. C. Pinto. 2001. The lipid-lowering effect of trans-dehydrocrotonin, a clerodane terpene from Croton cajucara Benth. J. Pharm. Pharmacol. 53:535-539. [DOI] [PubMed] [Google Scholar]
- 41.Zee-Cheng, K. E. 1992. Shi-quan-da-bu-tang (tem significant decoction), SQT. A potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs. Methods Find. Exp. Clin. Pharmacol. 14:725-736. [PubMed] [Google Scholar]