Abstract
Potential alternatives, including linezolid, adjunctive rifampin, and moxifloxacin, were evaluated against vancomycin-tolerant (P9802-020) and vancomycin-susceptible clinical isolates of Streptococcus pneumoniae in an in vitro pharmacodynamic model. Vancomycin exhibited maximal killing of 2-log10 CFU/ml against P9802-020. Linezolid, moxifloxacin, and linezolid plus rifampin exhibited 99.9% killing against both isolates. These alternatives should be considered for further evaluation against vancomycin-tolerant S. pneumoniae.
During a period of increasing pneumococcal resistance (4-6, 9, 11, 12, 19), the first report of Streptococcus pneumoniae exhibiting tolerance to vancomycin was published (17). This report was soon followed by the first clinical characterization of a meningitis infection due to S. pneumoniae exhibiting tolerance to vancomycin based on confirmatory in vitro analyses (15). The potential for the identification of additional infections due to vancomycin-tolerant S. pneumoniae substantiates the search for treatment alternatives. Oxazolidinones, extended-spectrum quinolones, and adjunctive rifampin therapy may represent potential alternatives. We used an in vitro pharmacodynamic model to compare linezolid and vancomycin, alone and in combination with rifampin, with moxifloxacin against multidrug-resistant and vancomycin-tolerant S. pneumoniae clinical isolates.
Two S. pneumoniae clinical isolates were evaluated in this study: 79, a vancomycin-susceptible isolate from Detroit Receiving Hospital, Detroit, Mich., and P9802-020, a vancomycin-tolerant isolate contributed by E. Tuomanen, St. Jude Children's Research Hospital, Memphis, Tenn. Isolate 79 is resistant to penicillin and erythromycin, and isolate P9802-020 is intermediate to penicillin and resistant to cefaclor, tetracycline, trimethoprim-sulfamethoxazole, and all macrolides.
Microdilution MICs and minimal bactericidal concentrations (MBCs) were determined 10 times with Mueller-Hinton broth supplemented with calcium, magnesium, and 5% lysed horse blood and Todd-Hewitt broth supplemented with 0.5% yeast extract according to NCCLS guidelines (16). MICs were confirmed by E tests. Eligibility for vancomycin tolerance was an MBC/MIC ratio of ≥32 and/or a reduction in the inoculum of <2.0 ± 0.6-log CFU/ml by 6 h in time-kill analyses (15, 17). Resistance was assessed by plating samples on tryptic soy agar-5% lysed horse blood containing two and four times the preexperiment MICs. Additionally, postexperiment MICs were determined.
A previously described in vitro pharmacodynamic model was used (3, 7). All experiments were performed in duplicate for each strain. A peristaltic pump was used to simulate the half-lives of the antibiotics. Regimen simulations of total drug concentrations were as follows (peak concentration in milligrams per liter and half-life in hours, respectively): moxifloxacin (Bayer Corporation, West Haven, Conn.), 400 mg every 24 h (4.5 and 12); linezolid (Pharmacia-Upjohn Laboratories, Kalamazoo, Mich.), 600 mg every 12 h (18 and 6); vancomycin (Sigma-Aldrich Corporation, St. Louis, Mo.), 1,000 mg every 12 h (30 and 6); and rifampin (Marion-Merrell Dow Pharmaceuticals Inc., Kansas City, Mo.), 600 mg every 24 h (7 and 3). The growth control was simulated at the shortest half-life of the tested antimicrobial agents. For combinations, an additional supplemental model was used to compensate for different half-lives (2).
Samples were collected at 0, 1, 2, 4, 6, 8, 24, 28, 32, and 48 h for concentration assays and bacterial quantification. Concentrations of linezolid were determined by a previously described validated high-pressure liquid chromatography method (1). The standard curve for linezolid ranged from 0.5 to 30 mg/ml. The interday coefficient of variation (CV) was <4.39% for all standards. Vancomycin concentrations were determined by a fluorescence polarization immunoassay (TDx; Abbott Laboratories, Irving, Tex.) with a lower limit of detection of 2.0 μg/ml and an interday CV of 10% for all standards. Moxifloxacin and rifampin concentrations were determined by a microbioassay (7, 18). The moxifloxacin microbioassay is linear over the concentration range of 5 to 0.3125 μg/ml, with an interday CV of 5.4%. The rifampin microbioassay is linear over the range of 0.06 to 1.0 μg/ml, with an interday CV of 7%.
Antibiotic peak and trough concentrations and elimination rates were estimated by using PKANALYST (version 1.10; MicroMath Scientific Software, Salt Lake City, Utah). Bacteria were quantified by plating of serial saline dilutions on tryptic soy agar with 5% sheep blood (Difco Laboratories, Detroit, Mich.) followed by overnight incubation in candle jars. Bactericidal activity was defined as a reduction in colony counts of ≥3-log10 CFU/ml from the starting inoculum, and the time to achieve 99.9% killing was determined. Enhancement of antimicrobial activity was defined as an increase in killing of ≥2-log10 CFU/ml by a combination of antimicrobial agents in comparison to the most active single agent of that combination. Improvement was defined as an increase in killing of <2-log10 CFU/ml in comparison to the most active single agent, while combinations that resulted in bacterial growth of ≥1-log10 CFU/ml in comparison to the least active single agent represented antagonism. All samples underwent multiple serial dilutions, and binding resin was added to moxifloxacin samples to circumvent antibiotic carryover (20).
Preexposure MICs and MBCs are reported in Table 1. No significant (>1 dilution change) differences were observed between susceptibility results in either medium. MICs were highly reproducible, while MBCs ranged from 1 to 32 (mode, 8) for isolate P9802-020 and from 0.5 to 2 (mode, 1) for isolate 79. MIC results obtained from E tests were consistent with microtiter results. No resistant colonies were seen, and no significant differences were observed between pre- and postexposure MICs.
TABLE 1.
MICs and MBCs for study isolates
| Antibiotic | MIC/MBC (mg/ml) for isolate:
|
|
|---|---|---|
| 79 | P9802-020 | |
| Linezolid | 1.0/2.0 | 0.5/2.0 |
| Moxifloxacin | 0.25/0.25 | 0.125/0.125 |
| Rifampin | 0.016/0.032 | 0.016/0.032 |
| Vancomycin | 0.5/1.0 | 0.5/8.0 |
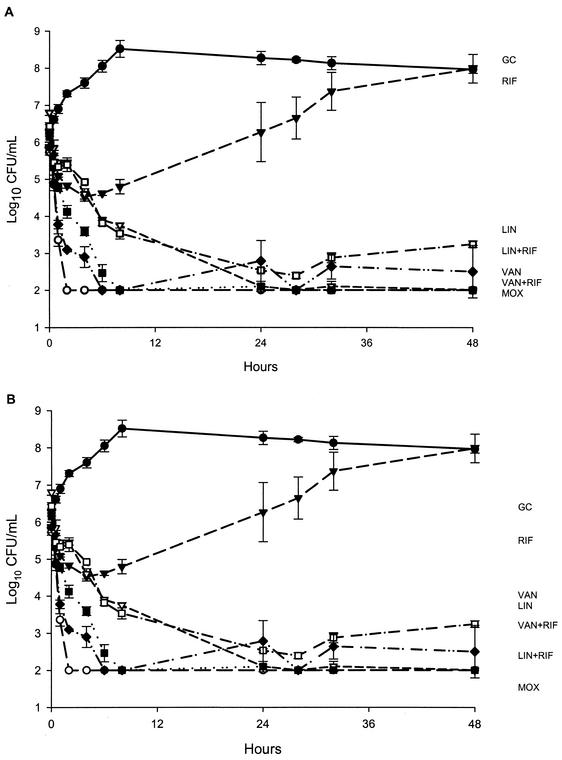
The results of simulations with isolates 79 and P9802-020 are presented in Fig. 1. All study regimens, except for rifampin alone, exhibited bactericidal activity by 8 h, and there was no regrowth of isolate 79. Bactericidal activity and limits of detection were achieved and sustained against isolate 79 by moxifloxacin and vancomycin plus rifampin from 2 to 48 h and 8 to 48 h, respectively.
FIG. 1.
Simulations of activities of study agents against S. pneumoniae clinical isolates 79 (A) and P9802-020 (B). GC, growth control; RIF, rifampin;LIN, linezolid; VAN, vancomycin; MOX, moxifloxacin. Plotted data represent the means and standard deviations for duplicate simulations.
Despite initial activity exhibited by rifampin alone, significant regrowth was noted at 24 h for both isolates. Contrary to the pronounced reduction in the level of isolate 79, vancomycin alone exhibited maximal killing of isolate P9802-020 of 2-log10 CFU/ml for the study duration. Against P9802-020, 99.9% killing was achieved by linezolid alone, linezolid plus rifampin, and moxifloxacin by 24, 24, and 8 h, respectively. Adjunctive rifampin resulted in enhancement only when it was added to linezolid. No antagonistic activity was noted. Limits of detection were achieved against P9802-020 by moxifloxacin as early as 8 h and were sustained for the remaining 48 h.
Observed antimicrobial drug exposures and pharmacokinetic parameters (means and standard deviations) for simulations were as follows (peak in milligrams per liter and t1/2 in hours, respectively): moxifloxacin, 4.9 ± 0.4 and 11.1 ± 1.1; linezolid, 18.6 ± 1.9 and 5.6 ± 0.6; vancomycin, 34 ± 5.7 and 6.5 ± 0.7; and rifampin, 6.6 ± 0.9 and 3.4 ± 0.5.
Multidrug-resistant S. pneumoniae infections have become a worldwide dilemma, leaving clinicians with limited treatment options for relatively common infections. Probable consequences of the selective pressure are the identification and characterization of the first S. pneumoniae isolate that demonstrated tolerance to vancomycin (17). Lack of lysis was evident in time-kill analyses that demonstrated persistent viability (a decrease of <2.0 ± 0.6-log CFU/ml) of the vancomycin-tolerant S. pneumoniae isolate.
In general, the significance of tolerance has been difficult to assess due to difficulties in reproducibility. The reproducibility of MBC determinations has been hampered by manual technical errors and such phenomena as the Eagle effect, adhesion of bacteria to wells, the possible existence of microenvironments where there is limited antimicrobial distribution, growth phase effects, antibiotic carryover, and many others. Laborious time-kill analyses have been advocated as a reliable method for the determination of tolerance (10, 14). The results from the present study with a single tolerant isolate suggested that tolerance was more accurately described by time-kill analyses rather than MBC/MIC ratios, which were high (1 to 64). In addition to the aforementioned reasons, some of the variations in MBC determinations in the present study could be attributable to the different media used to evaluate antimicrobial agents against this unique isolate, and this possibility should be further evaluated. The characteristic contrast in vancomycin activities against tolerant and susceptible strains observed in previously published standard time-kill experiments paralleled our observations in pharmacodynamic simulations and was sustained for the entire study.
Consistent with the results of other in vitro studies of streptococci, moxifloxacin exhibited substantial and rapid bactericidal activity against both tested isolates (7, 8, 13). Linezolid exhibited significant activity (99.9% killing) against both isolates, with a more pronounced effect against the vancomycin-susceptible isolate. Adjunctive rifampin did not exhibit antagonistic activity for any simulation and displayed enhancement only in combination with linezolid. The reduced potential for resistance with adjunctive rifampin was indiscernible, since no resistance or alterations in susceptibility were noted for single-drug regimens.
Some observations made in this study that require additional evaluation include the impact of growth phase and viability on the activities of different mechanistic agents and different killing rates obtained with various drug exposures (e.g., maximum concentration in serum/MIC and area under the concentration-time curve/MIC). The relationships between exposures and pharmacodynamic activities against these isolates should be further explored. Evaluation of additional combination regimens including rifampin and repeated dosing in studies with longer durations would be beneficial for determining potential enhanced efficacies. Larger studies with meningitis-related animal models that incorporate protein and penetration might provide additional information on the pharmacodynamics of clinically relevant drug exposures for these isolates. The results of this pharmacodynamic study emphasize the importance of further evaluation of potential treatment options, including linezolid, moxifloxacin, and adjunctive rifampin, for vancomycin-tolerant S. pneumoniae infections.
Acknowledgments
We acknowledge Charles Peloquin, Division of Infectious Diseases, National Jewish Medical and Research Center, Denver, Colo., for analysis of linezolid samples.
REFERENCES
- 1.Allen, G. P., R. Cha, and M. J. Rybak. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 3.Cappelletty, D. M., and M. J. Rybak. 1996. Bactericidal activities of cefprozil, penicillin, cefaclor, cefixime, and loracarbef against penicillin-susceptible and -resistant Streptococcus pneumoniae in an in vitro pharmacodynamic infection model. Antimicrob. Agents Chemother. 40:1148-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1999. Defining the public health impact of drug-resistant Streptococcus pneumoniae: report of a working group. Morb. Mortal. Wkly. Rep. 45:RR-1. [PubMed]
- 5.Centers for Disease Control and Prevention. 1999. Geographic variation in penicillin resistance in Streptococcus pneumoniae—selected sites, United States, 1997. Morb. Mortal. Wkly. Rep. 48:656-661. [PubMed] [Google Scholar]
- 6.Chen, D. K., A. McGeer, J. C. De Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 7.Coyle, E. A., G. W. Kaatz, and M. J. Rybak. 2001. Activities of newer fluoroquinolones against ciprofloxacin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:1654-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalhoff, A., C. Krasemann, S. Wegener, and G. Tillotson. 2001. Penicillin-resistant Streptococcus pneumoniae: review of moxifloxacin activity. Clin. Infect. Dis. 32(Suppl. 1):S22-S29. [DOI] [PubMed]
- 9.Diekema, D. J., A. B. Brueggemann, and G. V. Doern. 2000. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg. Infect. Dis. 6:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handwerger, S., and A. Tomasz. 1985. Antibiotic tolerance among clinical isolates of bacteria. Rev. Infect. Dis. 7:368-386. [DOI] [PubMed] [Google Scholar]
- 11.Ho, P. L., R. W. H. Yung, D. N. C. Tsang, T. L. Que, M. Ho, W. H. Seto, T. K. Ng, W. C. Yam, and W. W. Ng. 2001. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J. Antimicrob. Chemother. 48:659-665. [DOI] [PubMed] [Google Scholar]
- 12.Linares, J., A. G. Campa, and R. Pallares. 1999. Fluoroquinolone resistance in Streptococcus pneumoniae. N. Engl. J. Med. 20:1546-1548. [DOI] [PubMed] [Google Scholar]
- 13.Lister, P. D., and C. C. Sanders. 2001. Pharmacodynamics of moxifloxacin, levofloxacin and sparfloxacin against Streptococcus pneumoniae. J. Antimicrob. Chemother. 47:811-818. [DOI] [PubMed] [Google Scholar]
- 14.May, J., K. Shannon, A. King, and G. French. 1998. Glycopeptide tolerance in Staphylococcus aureus. J. Antimicrob. Chemother. 42:189-197. [DOI] [PubMed] [Google Scholar]
- 15.McCullers, J. A., B. K. English, and R. Novak. 2000. Isolation and characterization of vancomycin-tolerant Streptococcus pneumoniae from the cerebrospinal fluid of a patient who developed recrudescent meningitis. J. Infect. Dis. 181:369-373. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2000. Approved standard. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Novak, R., B. Henriques, E. Charpentier, S. Normark, and E. Tuomanen. 1999. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 399:590-593. [DOI] [PubMed] [Google Scholar]
- 18.Palmer, S. M., and M. J. Rybak. 1996. Pharmacodynamics of once- or twice-daily levofloxacin versus vancomycin, with or without rifampin, against Staphylococcus aureus in an in vitro model with infected platelet-fibrin clots. Antimicrob. Agents Chemother. 40:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., R. N. Jones, G. V. Doern, and K. Kugler. 1998. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997). Antimicrob. Agents Chemother. 42:1762-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zabinski, R. A., A. J. Larsson, K. J. Walker, S. S. Gilliland, and J. C. Rotschafer. 1993. Elimination of quinolone antibiotic carryover through use of antibiotic-removal beads. Antimicrob. Agents Chemother. 37:1377-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]