Abstract
Adult AIDS Clinical Trials Group (AACTG) Protocol 886 examined the dispositions of indinavir, efavirenz, and abacavir in human immunodeficiency virus-infected subjects who received indinavir at 1,000 mg every 8 h (q8h) and efavirenz at 600 mg q24h or indinavir at 1,200 mg and efavirenz at 300 mg q12h with or without abacavir 300 at mg q12h. Thirty-six subjects participated. The median minimum concentration in plasma (Cmin) for indinavir administered at 1,200 mg q12h was 88.1 nM (interquartile range [IR], 61.7 to 116.5 nM), whereas the median Cmin for indinavir administered at 1,000 mg q8h was 139.3 nM (IR, 68.8 to 308.7 nM) (P = 0.19). Compared to the minimum Cmin range for wild-type virus (80 to 120 ng/ml) estimated by the AACTG Adult Pharmacology Committee, the Cmin for indinavir administered at 1,200 mg q12h (54 ng/ml) is inadequate. The apparent oral clearance (CL/F) (P = 0.28), apparent volume of distribution at steady state (Vss/F) (P = 0.25), and half-life (t1/2) (P = 0.80) of indinavir did not differ between regimens. The levels of efavirenz exposure were similar between regimens. For efavirenz administered at 600 mg q24h and 300 mg q12h, the median maximum concentrations in plasma (Cmaxs) were 8,968 nM (IR, 5,784 to 11,768 nM) and 8,317 nM (6,587 to 10,239 nM), respectively (P = 0.66), and the Cmins were 4,289 nM (IR, 2,462 to 5,904 nM) and 4,757 nM (IR, 3,088 to 6,644 nM), respectively (P = 0.29). Efavirenz pharmacokinetic parameters such as CL/F (P = 0.62), Vss/F (P = 0.33), and t1/2 (P = 0.37) were similar regardless of the dosing regimen. The median Cmax, Cmin, CL/F, Vss/F, and t1/2 for abacavir were 6,852 nM (IR, 5,702 to 7,532), 21.0 nM (IR, 21.0 to 87.5), 43.7 liters/h (IR, 37.9 to 55.2), 153.9 liters (IR, 79.6 to 164.4), and 2.0 h (IR, 1.8 to 2.8), respectively. In summary, when indinavir was given with efavirenz, the trough concentration of indinavir after administration of 1,200 mg q12h was inadequate. Abacavir did not influence the pharmacokinetics or exposure parameters of either indinavir or efavirenz. The levels of efavirenz exposure were similar in subjects receiving efavirenz q12h or q24h.
Combination therapy with antiretroviral agents is recommended as routine clinical care for human immunodeficiency virus (HIV) type 1 (HIV-1)-infected individuals with access to nucleoside analog reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors on the basis of clinical presentation, the plasma HIV RNA concentration, and CD4+-cell count. However, the optimal use of these different drug classes during chronic combination therapy is dependent on an awareness of potential drug-drug interactions that may alter the pharmacokinetic disposition of one or more components of the regimen.
At the time that Adult AIDS Clinical Trials Group (AACTG) Protocol 368 was designed, there were additional areas of pharmacologic interest because (i) pharmacokinetic data describing the metabolic interaction between efavirenz and indinavir were limited, (ii) the optimal dosing schedule for indinavir (every 8 h [q8h] versus q12h) was controversial, and (iii) the relationship of indinavir to efavirenz pharmacokinetics during dosing q24h or q12h was unknown. In addition, abacavir was newly approved and its pharmacokinetic characteristics when it was combined with indinavir and efavirenz were not well studied.
Therefore, the primary objectives of AACTG Protocol 886, a pharmacokinetic substudy of AACTG Protocol 368, included examination of (i) the disposition of indinavir administered either q8h or q12h, (ii) the disposition of efavirenz administered q12h or q24h, and (iii) the disposition of abacavir in patients receiving indinavir and efavirenz. Secondary objectives included examination of the relationship between antiretroviral exposure parameters such as the minimum drug concentration achieved in plasma during a dosing interval (Cmin), the area under the concentration-time curve (AUC) over 24 h (AUC24), and the antiviral response as measured by the change in the plasma HIV RNA concentration.
MATERIALS AND METHODS
AACTG Protocol 368 was a randomized, phase II, placebo-controlled trial of abacavir in combination with open-labeled indinavir and efavirenz in HIV-1-infected subjects who had received zidovudine (AZT) or stavudine (d4T) plus lamivudine (3TC). Subjects in AACTG Protocol 368 received efavirenz at 600 mg q24h and indinavir at 1,000 mg q8h with placebo (arm I) or abacavir at 300 mg q12h (arm II) or efavirenz at 300 mg q12h and indinavir at 1,200 mg q12h with placebo (arm III) or abacavir at 300 mg q12h (arm IV). The subjects were eligible for enrollment if they were either continuing from AACTG Protocol 320 or non-AACTG Protocol 320 subjects who were NNRTI treatment naïve and who had been actively taking AZT (or d4T) plus 3TC for at least 3 months prior to enrollment and had a CD4 count ≤200/mm3 at the time of initiation of AZT (or d4T) plus 3TC. Participation in the substudy, AACTG Protocol 886, was limited to subjects enrolled in AACTG Protocol 368 who were NNRTI treatment naïve. The substudy was performed at four designated AIDS Clinical Trials Units with experience with conducting pharmacokinetic study protocols.
Subjects were instructed to initiate efavirenz dosing in the evening and to switch to morning administration on day 8. The blood sampling protocol for AACTG Protocol 886 consisted of collection of venous blood samples on day 14 immediately predosing and at 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after dosing at 8 a.m. Subjects arrived in a fasted state since 11 p.m. the night before, and all study medications were taken simultaneously on the morning of the study day. A light breakfast was allowed 60 min after the administration of study medication, and the subjects were instructed to take all other doses of medication as scheduled over the 24-h study period. Adherence was determined at week 2 by means of a pill count, and the importance of adherence was reinforced. Subjects were considered compliant if all of the prescribed study drug was taken. For calculation of pharmacokinetic parameters, adherence was determined by recording the time of administration of the last three doses before blood sample collection began.
Each blood sample was centrifuged at 800 × g for 10 min. Plasma was separated into three aliquots and stored at −70°C until it was shipped to the pharmacology laboratory. Samples containing indinavir and efavirenz were analyzed at the University at Buffalo AACTG Pharmacology Support Laboratory, which is also a participant in the quality assurance and proficiency testing program of the AACTG Pharmacology Committee. Abacavir samples were analyzed (abacavir [Ziagen] product information, 2002; GlaxoSmithKline, Research Triangle Park, N.C.) by GlaxoSmithKline.
Indinavir and efavirenz were quantified by a validated high-performance liquid chromatography (HPLC) assay with UV detection. For indinavir, the interassay coefficients of variation (CVs) were 3.2% at 75 ng/ml and 2.8% at 3,500 ng/ml, and the intra-assay CVs were 1.7 to 8.5% at 75 ng/ml and 0.3 to 3.4% at 3,500 ng/ml. The lower limit of quantification for the indinavir assay was 12.5 ng/ml. For efavirenz, the interassay CVs were 3.6% at 100 ng/ml and 0.16% at 10,000 ng/ml, and the intra-assay CVs were 12.1 to 12.3% at 160 ng/ml and 3.94 to 4.31% at 8,000 ng/ml. The lower limit of quantification for the efavirenz assay was 100 ng/ml. Abacavir-containing samples were analyzed by a validated reverse-phase HPLC assay with UV detection. The interday CVs were 6.2, 4.3, and 5.0% for abacavir at 0.070, 0.700, and 8.02 μg/ml, respectively, while the interday variability (biases) were −6.0, −1.9, and 0.5%, respectively. The lower limit of detection for abacavir was 25 ng/ml.
Plasma indinavir concentrations were first modeled in the Adapt II program by using maximum likelihood (5, 6). For all modeling methods, observed data were weighted by the inverse of the fitted variance. The variance model assumed a linear relationship between the standard deviation and the fitted concentration. Model discrimination was performed by using Akaike's information criterion and the rule of parsimony. Once the structural model was developed, final pharmacokinetic parameter estimates were calculated by a MAP Bayesian approach by iterative two-stage analysis, and both the maximum concentration of drug in plasma (Cmax) and Cmin were determined by visual inspection. For one subject, the indinavir concentrations were missing for the last two time points; therefore, the Cmin was determined by extrapolating the concentration at the last observed time point to Cmin by using the estimated terminal half-life. Efavirenz and abacavir pharmacokinetic parameters were determined by noncompartmental analysis with the WinNonlin program (version 2.1; Pharsight, Palo Alto, Calif.). Statistical analyses were performed by using either the variable or the log transform of the variable when appropriate. All statistical comparisons between groups were made by the Kruskal-Wallis test. Fisher's exact test was used to determine associations between categorical variables when the assumptions of the chi-square test were not met. The relationships between the fractional change in the plasma HIV RNA concentration [(concentration at week 0 − concentration at week 2 or 4)/concentration at week 0] and exposure parameters (indinavir AUC24, efavirenz AUC24, abacavir AUC24, indinavir Cmax and Cmin, efavirenz Cmax and Cmin, and abacavir Cmax and Cmin), the viral load at week 0, and study arm were explored via multiple linear regression. Multiple linear regression was also used to determine if the efavirenz AUC24, abacavir AUC24, efavirenz and abacavir Cmax and Cmin, and viral load at weeks 0, 2, and 4 were significant predictors of indinavir clearance. All statistical analyses were conducted by using the SAS system (version 8; SAS Institute, Cary, N.C.).
RESULTS
Demographics.
Thirty-six subjects participated in the study, and data for all except one of the subjects were included in the indinavir pharmacokinetic analysis. The one exception received another dose during the sampling period; therefore, the results are for the remaining 35 subjects. The average age of the subjects was 40.0 years (standard deviation, 8.6 years), with 83% of the subjects being male and 46, 31, 17, 3, and 3% of the subjects being white, non-Hispanic; black, non-Hispanic; Hispanic, Latino; Asian, Pacific Islander; and American Indian or Alaska Native, respectively. Eight-six percent of the patients reported no prior intravenous drug use. The median plasma HIV RNA concentration at week 0 was 15,848 copies/ml (interquartile range [IR], 5,483 to 42,586 copies/ml). Seventy-one percent of the 34 patients were determined to be 100% adherent by means of pill count. Adherence was not associated with study arm (P = 0.25), abacavir use (P = 0.23), or indinavir dosing q8h versus q12h (P = 0.25).
Indinavir and efavirenz pharmacokinetic parameters with abacavir use.
Of the 36 subjects from whom plasma samples were collected, data for 35, 25, and 13 subjects were included in the indinavir, efavirenz, and abacavir pharmacokinetic analyses, respectively. Protocol irregularities concerning scheduled dosing times resulted in the exclusion of the data for one subject receiving indinavir and the data for four subjects receiving efavirenz from the pharmacokinetic analysis. The data for an additional seven subjects from arms I and II were excluded from the efavirenz analysis because the efavirenz dose was not switched to every morning after day 8, resulting in erroneous blood sampling times. Knowing that once the full effect of enzyme induction by efavirenz is achieved, the total level of exposure over time (versus the variation in the concentration in plasma at a single time point) is thought to determine the influence of efavirenz on the induction of indinavir metabolism, and since estimation of indinavir pharmacokinetic parameters was not influenced by the inclusion or exclusion of data for these subjects, the plasma indinavir concentrations for these seven subjects were included in the indinavir analysis.
The pharmacokinetic parameters for indinavir and efavirenz and the concentrations of indinavir and efavirenz in plasma (Cmax and Cmin) are listed in Table 1 by study arm. The final indinavir pharmacokinetic model included central and tissue distribution volumes, first-order absorption following a lag time, and first-order elimination from the central compartment. As predicted from previous knowledge of the disposition of abacavir, abacavir did not influence the pharmacokinetics of indinavir. The apparent oral clearance (CL/F; P = 0.66), apparent volume of distribution at steady state (Vss/F; P = 0.38), and half-life (t1/2; P = 0.72) were not influenced by the administration of abacavir. Abacavir also did not influence the level of exposure to indinavir. When the indinavir q8h regimens were compared, Cmin (P = 0.95) and Cmax (P = 0.32) were similar regardless of abacavir administration. The indinavir Cmin (P = 0.10) and Cmax (P = 0.14) were also similar when the indinavir q12h regimens were compared.
TABLE 1.
Pharmacokinetic parameters for indinavir and efavirenz by study arm
| Regimena | Cmax (nM) | Cmin (nM) | CL/F (liters/h) | Vss/F (liters) | t1/2 (h) |
|---|---|---|---|---|---|
| Indinavir | |||||
| 1,000 mg q8h (arm I) | 13,040 (6,184-22,260)b | 168.2 (81.9-257.0) | 98.4 (38.7-207.0) | 103.4 (28.4-135.1) | 2.3 (1.7-2.6) |
| 1,000 mg q8h and abacavir (arm II) | 9,253 (4,082-11,220) | 110.40 (68.8-308.7) | 103.0 (62.0-231.1) | 108.8 (71.0-179.0) | 1.9 (1.4-3.4) |
| 1,200 mg q12h (arm III) | 20,000 (8,655-22,390) | 55.0 (10.2-93.5) | 73.9 (51.8-104.4) | 29.1 (25.6-136.4) | 1.9 (1.4-2.4) |
| 1,200 mg q12h and abacavir (arm IV) | 13,695 (8,305-17,060) | 97.7 (73.6-144.5) | 71.3 (60.7-96.9) | 48.3 (35.0-109.2) | 2.3 (1.9-2.9) |
| Efavirenz | |||||
| 600 mg q24h (arm I) | 10,723.6 (7,513.1-12,439.4) | 5,630.4 (3,408.4-6,181.6) | 10.8 (8.6-19.9) | 363.7 (272.2-864.4) | 23.6 (20.2-29.4) |
| 600 mg q24h and abacavir (arm II) | 7,826.25 (5,783.5-10,791.2) | 3,016.4 (2,462.3-5,623.4) | 16.8 (11.2-20.0) | 554.5 (407.6-688.7) | 21.8 (19.4-31.6) |
| 300 mg q12h (arm III) | 8,381.72 (4,994.48-11,126.32) | 3,922.95 (2,976.7-6,643.6) | 13.3 (9.9-19.5) | 397.0 (283.5-549.1) | 20.9 (10.1-37.6) |
| 300 mg q12h and abacavir (arm IV) | 8,316.98 (6,587.03-10,239.15) | 4,909.7 (3,389.9-7,258.7) | 12.4 (9.2-15.8) | 478.1 (227.9-602.9) | 18.3 (12.6-39.8) |
The numbers of subjects included in indinavir arms I, II, III and IV were 5, 9, 7, and 14, respectively, and the numbers of subjects included in efavirenz arms I, II, III, and IV were 4, 8, 6, and 7, respectively.
The values are medians (IRs).
Abacavir did not influence pharmacokinetic parameters for efavirenz or the level of efavirenz exposure. CL/F (P = 0.65), Vss/F (P = 0.65), and t1/2 (P = 0.81) were not influenced by the administration of abacavir. When the efavirenz q24h study arms were compared, the efavirenz Cmin (P = 0.40) and Cmax (P = 0.40) were similar. For the efavirenz q12h regimens, the efavirenz Cmin (P = 0.67) and Cmax (P = 0.89) were also similar, regardless of concurrent abacavir administration.
Table 2 includes the pharmacokinetic parameters for indinavir and efavirenz by abacavir use. The CL/F (P = 0.68), Vss/F (P = 0.20), and t1/2 (P = 0.55) of indinavir and the CL/F (P = 0.62), Vss/F (P = 0.51), and t1/2 (P = 0.87) of efavirenz were not influenced by the administration of abacavir.
TABLE 2.
Pharmacokinetic parameters for indinavir and efavirenz by abacavir use
| Antiviral agenta | CI/F (liters/h) | Vss/F (liters) | t1/2 (h) |
|---|---|---|---|
| Indinavir | 78.7 (45.2-155.7)b | 41.4 (27.0-135.7) | 2.1 (1.5-2.5) |
| Indinavir and abacavir | 74.8 (60.7-193.9) | 71.0 (35.0-179.6) | 2.3 (1.6-3.2) |
| Efavirenz | 12.6 (9.2-19.5) | 374.8 (283.5-549.1) | 23.5 (17.0-33.7) |
| Efavirenz and abacavir | 12.6 (10.5-18.9) | 522.6 (314.6-622.5) | 21.0 (18.3-31.7) |
The numbers of subjects included in the indinavir, indinavir and abacavir, efavirenz, and efavirenz and abacavir analyses were 12, 23, 10, and 15, respectively.
The values are medians (IRs).
Pharmacokinetic and exposure parameters for each regimen.
The regimen of indinavir at 1,200 mg q12h trended toward a higher Cmax and a lower Cmin compared to those for the regimen of indinavir at 1,000 mg q8h. The median Cmaxs for indinavir at 1,200 mg q12h and 1,000 mg q8h were 15,250 nM (IR, 8,655 to 20,000 nM) and 10,172 nM (IR, 4,082 to 15,960 nM), respectively (P = 0.099). The Cmin for indinavir at 1,200 mg q12h was 88.1 nM (IR, 61.7 to 116.5), whereas the Cmin for indinavir at 1,000 mg q8h was 139.3 nM (IR, 68.8 to 308.7 nM) (P = 0.19). The pharmacokinetic parameters for indinavir, CL/F (P = 0.27), Vss/F (P = 0.25), and t1/2 (P = 0.79), were similar regardless of the regimen.
The levels of efavirenz exposure were similar regardless of the dosing regimen. The median Cmaxs (P = 0.66) were 8,968 nM (IR, 5,784 to 11,768 nM) and 8,317 nM (IR, 6,587 to 10,239 nM) for efavirenz at 600 mg q24h and 300 mg q12h, respectively, and the median Cmins (P = 0.28) were 4,289 nM (IR, 2,462 to 5,904 nM) and 4,757 nM (IR, 3,088 to 6,644 nM) for efavirenz at 600 mg q24h and 300 mg q12h, respectively. The pharmacokinetic parameters for efavirenz, such as CL/F (P = 0.62), Vss/F (P = 0.33), and t1/2 (P = 0.36), were similar regardless of the dosing regimen.
When the data for arms II and IV were combined, the level of abacavir exposure was described by a median Cmax and a median Cmin of 21.0 nM (IR, 21.0 to 87.5 nM) and 6,852 nM (IR, 5,702 to 7,532 nM), respectively. Abacavir median CL/F, Vss/F, and t1/2 were 43.7 liters/h (IR, 37.9 to 55.2 liters/h), 153.9 liters (IR, 79.6 to 164.4 liters), and 2.0 h (IR, 1.8 to 2.8 h), respectively (Table 3).
TABLE 3.
Pharmacokinetic parameters of indinavir and efavirenz by regimen
| Regimena | Cmax (nM) | Cmin (nM) | CL/F (liters/h) | Vss/F (liters) | t1/2 (h) |
|---|---|---|---|---|---|
| Indinavir | |||||
| 1,000 mg q8h | 10,172 (4,082-15,960)b | 139 (68.8-308.7) | 100.8 (43.6-231.1) | 106.1 (31.5-178.1) | 2.1 (1.4-3.4) |
| 1,200 mg q12h | 15,250 (8,655-20,000) | 88 (62-117) | 72.4 (54.6-96.9) | 47.7 (29.1-109.2) | 2.2 (1.6-2.7) |
| Efavirenz | |||||
| 600 mg q24h | 8,968 (5,784-11,768) | 4,286 (2,462-5,904) | 14.2 (10.2-19.9) | 511.6 (323.9-688.7) | 22.1 (19.4-31.6) |
| 300 mg q12h | 8,317 (6,587-10,239) | 4,757 (3,088-6,644) | 12.6 (9.92-15.8) | 438.7 (283.5-549.1) | 18.3 (12.6-37.6) |
| 300 mg q12h with abacavir | 6,852 (5,702-7,532) | 21.0 (21.0-87.5) | 43.7 (37.9-55.2) | 153.9 (79.6-164.4) | 2.0 (1.8-2.8) |
The numbers of subjects included in the indinavir 1,000 mg q8h and 1,200 mg q12h analyses were 14 and 21, respectively. The number of subjects included in the efavirenz 600 mg q24h, efavirenz 300 mg q12h, and abacavir 300 mg q12h analyses were 12, 13, and 13, respectively.
The values are medians (IRs)
Influence of efavirenz exposure levels on indinavir pharmacokinetics.
There was no correlation between the efavirenz AUC24 and the indinavir CL/F (P = 0.30). A multivariate analysis found no predictive value of efavirenz exposure parameters such as efavirenz AUC24, Cmax, or Cmin on indinavir CL/F.
Predictors of fractional change in plasma HIV RNA load.
There was a positive correlation between the numbers of HIV RNA copies per milliliter of plasma at week 0 and week 4 (r2 = 0.34; P = 0.0002) and a trend toward a positive correlation between viral loads at weeks 0 and 2 (r2= 0.11; P = 0.059). The viral load at week 2 correlated positively with that at week 4 (r2 = 0.12; P = 0.048).
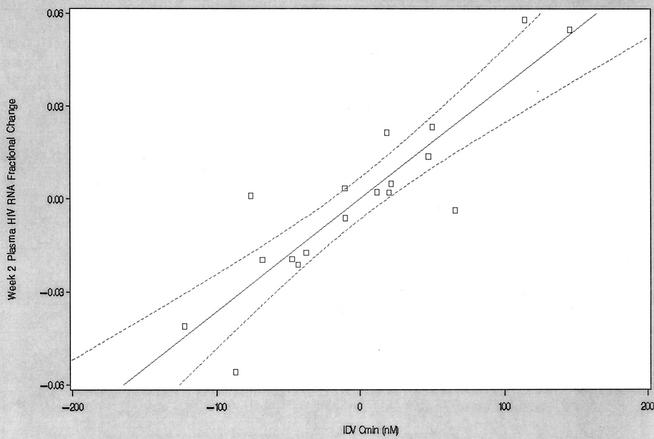
The results of multiple linear regression of the fractional change in the plasma HIV RNA load at week two are shown in Table 4. The data for three subjects were excluded from the final model due to sampling at week 6 instead of week 2, poor adherence, and a baseline viral load (181 copies/ml) considered outside the group distribution (IR, 5,483 to 42,586 copies/ml). Of the indinavir, efavirenz, and abacavir exposure parameters that were tested, only the indinavir AUC24 (P < 0.0001) and the indinavir Cmin (P < 0.0001) remained in the final model (r2 = 0.82). Figure 1 displays the partial regression plot of the fractional change in the plasma HIV RNA load at week 2 versus the indinavir Cmin. Inclusion in study arm I (efavirenz at 600 mg every 24 h and indinavir at 1,000 mg q8h without abacavir) negatively influenced the fractional change in the plasma HIV RNA load at week 2 (P = 0.002), whereas a greater viral load at week 0 positively influence the fractional change in the plasma HIV RNA load at week 2 (P = 0.001). None of the parameters tested were predictive of a fractional change in the plasma HIV RNA load at week 4.
TABLE 4.
Multiple linear regression for fractional change in plasma HIV RNA concentrationa
| Predictor | Parameter estimate | 95% confidence interval | P value |
|---|---|---|---|
| Intercept | 0.97427 | 0.95650-0.99204 | <0.0001 |
| Indinavir AUC24 | −0.00000118 | −0.00000152-8.42612E-7 × 10−7 | <0.0001 |
| Indinavir Cmin | 0.00036837 | 0.00023708-0.00049967 | <0.0001 |
| Arm I | −0.03904 | −0.06122-−0.01686 | 0.0022 |
| Plasma HIV RNA load at wk 0 | 2.711975 × 10−7 | 1.327911 × 10−7-4.096039E × 10−7 | 0.0010 |
Eighteen subjects were included in the final model.
FIG. 1.
Partial regression plot of fractional change in plasma HIV RNA concentration at week 2 versus the indinavir Cmin. The final model included data for 18 subjects. The solid line represents the line of partial regression, and the dashed lines represent the 95% confidence ellipses about the line of partial regression. IDV, indinavir.
DISCUSSION
Since in vitro studies of abacavir have not shown an influence on the hepatic isozymes responsible for either indinavir or efavirenz metabolism, it is not surprising that abacavir failed to show evidence of influencing the pharmacokinetics of either drug (abacavir [Ziagen] product information, 2002; GlaxoSmithKline). Whether similar regimens were compared (Table 1) or data for all subjects were combined according to abacavir use (Table 2), abacavir failed to influence the CL/F, Vss, or t1/2 of either indinavir or efavirenz. Exposure parameters such as Cmin and Cmax were also similar regardless of abacavir use (Table 1). The pharmacokinetic parameters reported in this study are similar to those reported in prior studies of abacavir (4, 14, 15, 17, 19, 20, 25, 26; M. W. Kline, S. Blanchard, C. V. Fletcher, J. L. Shenep, R. E. McKinney, Jr., R. C. Brundage, M. Culnane, R. B. Van Dyke, W. M. Dankner, A. Kovacs, J. A. McDowell, S. Hetherington, et al., Pediatrics 103:E47, 1999 [abstract]).
Administration of indinavir less frequently but at a higher dose was expected to result in a higher Cmax and a lower Cmin. Indeed, indinavir at 1,200 mg q12h compared to indinavir at 1,000 mg q8h showed a trend toward a higher median Cmax (15,250 versus 10,172 nM) and a lower median Cmin (88.1 versus 139.3 nM). Failure to reach statistical significance may be due in large part to an inadequate sample size and a high degree of intersubject variability. A low rate of compliance may also have contributed to intersubject variability; however, indinavir has a relatively short t1/2, and the data for the times of administration of the last three doses were included in the model. There is concern that without the addition of a pharmacokinetic boosting agent such as ritonavir, q12h dosing with indinavir may be inadequate, especially as part of a regimen containing efavirenz. When indinavir dosings of q12h versus q8h in combination with zidovudine and lamivudine were compared, Haas et al. (11) concluded that q8h regimens are more effective and that q12h regimens should be considered only in situations in which a pharmacokinetic boosting agent is included. Haas et al. (12) have also shown that two NRTIs plus efavirenz at 600 mg q24h and indinavir at 1,000 mg q8h is an effective regimen in NRTI-experienced patients. A recently published guide to therapeutic drug monitoring (TDM) for antiretroviral agents (2) estimated the minimum Cmin range of indinavir for wild-type virus to be 80 to 120 ng/ml. Considering that the median Cmin of indinavir at 1,200 mg q12h was 54 ng/ml, this regimen does not appear to provide adequate exposure. The median Cmin of indinavir at 1,000 mg q8h was 85 ng/ml; therefore, 1,000 mg q8h may be more appropriate in a regimen containing efavirenz. When the information presented above is considered along with the growing amount of evidence that either the Cmin of a protease inhibitor or the ratio of the Cmin to IC50 for isolates of an individual phenotype may predict virologic outcome, indinavir at 1,200 mg q12h should be used with caution in regimens containing efavirenz [D. M. Burger, P. W. Hugen, J. Droste, and A. D. Huitema for the ATHENA Group, 2nd Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 6.2a and 6.2b, 2001; P. Clevenbergh, J. Durant, W. Verbiest, B. Larder, K. Hertogs, R. Garraffo, and P. Dellamonica, Antivir. Ther. 5(Suppl. 3):71, 2000, abstr. 90].
Our results are consistent with those of previous studies of indinavir pharmacokinetics (3; E. P. Acosta, D. V. Havlir, D. D. Richman, X. J. Zhou, M. Hirsch, A. C. Collier, P. Tebas, and J. P. Sommadossi for the ACTG 343 Team, Program Abstr. 7th Conf. Retrovir. Opportunistic Infections, abstr. 455, 2000; D. M. Burger, R. M. W. Hoetelmans, J. W. Mulder, P. L. Meenhorst, P. W. H. Hugen, K. Brinkman, and P. P. Koopmans, 12th World AIDS Conf., abstr. 42275, 1998), which point out the large degree of interpatient variability in indinavir pharmacokinetic (CL/F, Vss/F, and t1/2) and exposure parameters (Cmax and Cmin) when indinavir is administered to HIV-1-infected patients. One possible explanation is the interindividual variability in expression of the hepatic and intestinal isozymes responsible for the metabolism of many protease inhibitors and NNRTIs (7, 9, 16). Although the value of TDM for ensuring adequate exposure remains controversial, wide interpatient pharmacokinetic variability is one of the characteristics that a drug must exhibit in order to be considered a candidate for TDM. Other requirements include a narrow therapeutic window and a validated, reproducible assay. Further study is necessary before TDM can be considered a standard part of antiretroviral therapy.
Efavirenz is a known inducer of the hepatic cytochrome P-450 isozyme primarily responsible for indinavir metabolism, CYP3A4 (efavirenz [Sustiva] product information, 2000; DuPont Pharmaceuticals, Wilmington, Del.) We did not observe a relationship between the efavirenz AUC24, an indicator of the level of daily efavirenz exposure, and the indinavir CL/F. One potential explanation for this finding is the fact that maximal induction of efavirenz occurs regardless of the degree of efavirenz exposure achieved. However, the level of efavirenz exposure during this study was within the range of clinical exposure levels, and it is possible that more induction would be seen at higher plasma efavirenz concentrations. Due to the small sample size and the lack of variability in the extent of efavirenz exposure, further study is needed before it can be concluded that the pharmacokinetics of indinavir are not influenced by the extent of efavirenz exposure.
Although the results of large prospective clinical trails are necessary before TDM becomes common practice, evidence supporting the use of TDM in antiretroviral therapy has been growing. Fletcher et al. (10) recently showed that a concentration-controlled approach to antiretroviral therapy, which included a target postdose indinavir q8h Cmin of 130 ng/ml, resulted in a greater proportion of HIV RNA levels <50 copies/ml after 52 weeks of therapy. The relationship that we observed between the fractional change in the plasma HIV RNA load at week 2 and the indinavir Cmin further supports the potential application of TDM for indinavir. As can be seen in Fig. 1, a change in the indinavir Cmin of 100 nM (61 ng/ml) results in an approximately 4% change in the HIV RNA load. However, it must be noted that adherence is paramount for clinical success, and TDM is neither an accurate measure of nor a substitute for adherence. Although the level of indinavir exposure at week 2 was not a predictor of a fractional change in the plasma HIV RNA load at week 4, the viral load at week 2 correlated positively with that at week 4 (r2 = 0.12; P = 0.048), and both the small sample size and the lack of collection of pharmacokinetic data at week 4 limit this analysis.
One limitation of this analysis is that the efavirenz dosing regimens used in this trial resulted in similar levels of efavirenz exposure between groups, which limits the ability of the level of efavirenz exposure to display an influence on the fractional change in the viral load. Unlike indinavir, efavirenz has a long t1/2, resulting in less fluctuation between Cmax and Cmin once steady state is achieved (Table 1). Also, the median Cmax (8,968 nM for indinavir versus 8,317 nM for efavirenz) and Cmin (4,286 nM for indinavir versus 4,757 nM for efavirenz) were similar regardless of the efavirenz regimen, which limits the ability to find an association between Cmin and a fractional change in the viral load. Because other data suggest that efavirenz may also be a candidate for TDM, further investigation with efavirenz regimens designed to reach different levels of efavirenz exposure is necessary before conclusions concerning the relationship between the level of efavirenz exposure and clinical outcome can be drawn (18, 23, 24; C. V. Fletcher, T., Fenton, C. Powell, et al., Program Abstr. 8th Conf. Retrovir. Opportunistic Infect., abstr. 259, 2001; A. S. Joshi, J. S. Barrett, W. D. Fiske, et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1201, 1999).
The study design also limited the analysis of the indinavir AUC. The selection of indinavir q12h and q8h dosing regimens to achieve similar AUC24s precludes us from drawing conclusions concerning the relationship between the indinavir AUC24 and the fractional change in the viral load. The indinavir AUC24 had a very small negative relationship with the fractional change in the viral load, which conflicts with the findings of previous trials, which showed that exposure parameters such as AUC and Cmin are significant predictors of antiviral activity (8, 10, 13, 21, 22; Burger et al., 12th World AIDS Conf., abstr. 42275, 1998). A potential reason why AUC24 and Cmin have opposite coefficients may be due to the effect of abacavir on antiviral activity in two regimens that otherwise have overlapping AUC24s but some separation in Cmins. This is shown in our model results as a negative effect of arm I, which lacked abacavir yet which included indinavir q8h. Further limitations to the analyses include the small number of subjects in each dosing arm and the use of a short time frame to determine the change in viral load.
Other limitations of this trial include the fact that indinavir is now commonly combined with ritonavir to inhibit indinavir metabolism, decrease the frequency of administration, and potentially increase antiviral potency (Burger et al., 12th World AIDS Conf., abstr. 42275, 1998). However, there is little evidence to suggest that the use of a pharmacokinetic booster, such as ritonavir, results in less interpatient variability in exposure. In fact, recent TDM studies have noted the need to reduce indinavir doses to avoid excessive exposure (Burger et al., 2nd Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 6.2a and 6.2b, 2001).
In summary, we determined that indinavir at 1,200 mg q12h did not achieve an adequate Cmin when given as part of an efavirenz-containing highly active antiretroviral therapy regimen and that the variation in the levels of efavirenz exposure may not be an important determinant of indinavir induction. Considering the short t1/2 of indinavir and the high degree of interpatient variability in exposure levels, indinavir is a prime candidate for TDM, especially when it is given in combination with efavirenz. These findings are consistent with those presented in other reports. However, it should be noted that the addition of ritonavir may eliminate many of the criteria that make indinavir an attractive candidate for TDM, regardless of the presence of efavirenz (1). We also found that abacavir did not influence the pharmacokinetic or exposure parameters of either indinavir or efavirenz. Lastly, efavirenz exposure levels were similar in subjects receiving efavirenz q12h or q24h.
Acknowledgments
This work was supported by funding from the National Institute of Allergy and Infectious Diseases, Adult AIDS Clinical Trials Group Pharmacology Support Laboratory grant 200PC006.
REFERENCES
- 1.Aarnoutse, R. E., K. J. Grintjes, D. S. Telgt, M. Stek, Jr., P. W. Hugen, P. Reiss, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2002. The influence of efavirenz on the pharmacokinetics of a twice-daily combination of indinavir and low-dose ritonavir in healthy volunteers. Clin. Pharmacol. Ther. 71:57-67. [DOI] [PubMed] [Google Scholar]
- 2.Acosta, E. P., J. G. Gerber, and the Adult Pharmacology. Committee of the AIDS Clinical Trials Group. 2002. Position paper on therapeutic drug monitoring of antiviral agents. AIDS Res. Hum. Retrovir. 18:825-834. [DOI] [PubMed]
- 3.Acosta, E. P., T. N. Kakuda, R. C. Brundage, P. L. Anderson, and C. V. Fletcher. 2000. Pharmacodynamics of HIV-1 protease inhibitors. Clin. Infect. Dis. 30(Suppl. 2):S151-S159. [DOI] [PubMed] [Google Scholar]
- 4.Cremieux, A. C., C. Katlama, C. Gillotin, D. Demarles, G. J. Yuen, F. Raffi, and the AZl10002 Study Group. 2000. A comparison of the steady-state pharmacokinetics and safety of abacavir, lamivudine, and zidovudine taken as a triple combination tablet and as abacavir plus a lamivudine-zidovudine double combination tablet by HIV-1-infected adults. Pharmacotherapy 21:424-430. [DOI] [PubMed] [Google Scholar]
- 5.D'Argenio, D. Z., and A. Schumitzky. 1979. A program package for simulation and parameter estimation in pharmacokinetic systems. Comp. Programs Biomed. 9:115-134. [DOI] [PubMed] [Google Scholar]
- 6.D'Argenio, D. Z., and A. Schumitzky. 1997. Adapt II users guide, release 4. Biomedical Simulations Resource, University of Southern California, Los Angeles.
- 7.de Wildt, S. N., G. L. Kearns, J. S. Leeder, and J. N. van den Anker. 1999. Cytochrome P450 3A: ontogeny and drug disposition. Clin. Pharmacokinet. 37:485-505. [DOI] [PubMed] [Google Scholar]
- 8.Durant, J., P. Clevenbergh, R. Garraffo, P. Halfon, S. Icarc, P. DelGiudice, et al. 2000. Importance of protease inhibitor plasma levels in HIV-infected patients treated with genotypic-guided therapy: pharmacological data from the Viradapt study. AIDS 14:1333-1339. [DOI] [PubMed] [Google Scholar]
- 9.Felix, C. A., A. H. Walker, B. J. Lange, T. M. Williams, N. J. Winick, N. K. V. Cheung, B. D. Lovett, P. C. Nowell, I. A. Blair, and T. R. Rebbeck. 1998. Association of CYP3A4 genotype with treatment-related leukemia. Proc. Natl. Acad. Sci. USA 95:13176-13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher, C. V., P. L. Anderson, T. N. Kakuda, T. W. Schack, K. Henry, C. R. Gross, and R. C. Brundage. 2002. Concentration-controlled compared with conventional antiretroviral therapy for HIV infection. AIDS 16:551-560. [DOI] [PubMed] [Google Scholar]
- 11.Haas, D. W., E. Arathoon, M. A. Thompson, R. de Jesus Pedro, J. E. Gallant, D. E. Uip, J. Currier, L. M. Noriega, D. S. Lewi, P. Uribe, L. Benetucci, P. Cahn, D. Paar, A. C. White, Jr., A. C. Collier, C. H. Ramirez-Ronda, C. Harvey, M. O. Chung, D. Mehrotra, J. Chodakewitz, and B. Y. Nguyen. 2000. Comparative studies of two-times-daily versus three-times-daily indinavir in combination with zidovudine and lamivudine. AIDS 14:1973-1978. [DOI] [PubMed] [Google Scholar]
- 12.Haas, D. W., W. J. Fessel, R. A. Delapenha, H. Kessler, D. Seekins, M. Kaplan, et al. 2001. Therapy with efavirenz plus indinavir in patients with extensive prior nucleoside reverse-transcriptase inhibitor experience: a randomized double-blind, placebo-controlled trial. J. Infect. Dis. 183:392-400. [DOI] [PubMed] [Google Scholar]
- 13.Harris, M., C. Durakovic, S. Rae, J. Raboud, S. Fransen, A. Shillington, B. Conway, and J. S. Montaner. 1998. A pilot study of nevirapine, indinavir and lamivudine among patients with advanced human immunodeficiency virus disease who had failure of combination nucleoside therapy. J. Infect. Dis. 177:1514-1520. [DOI] [PubMed] [Google Scholar]
- 14.Hughes, W., J. A. McDowell, J. Shenep, P. Flynn, M. W. Kline, R. Yogev, W. Symonds, Y. Lou, and S. Hetherington. 1999. Safety and single-dose pharmacokinetics of abacavir (1592U89) in human immunodeficiency virus type 1-infected children. Antimicrob. Agents Chemother. 43:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izzedine, H., V. Launay-Vacher, G. Aymard, M. Legrand, and G. Deray. 2001. Pharmacokinetics of abacavir in HIV-1-infected patients with impaired renal function. Nephron 89:62-67. [DOI] [PubMed] [Google Scholar]
- 16.Kuehl, P., J. Zhang, L. Lamba, J. Assem, M. Schuetz, J. Watkins, P. B. Daly, A. Wrighton, S. A. Hall, S. D. Maurel, R. Relling, M. Brimer, C. Yasuda, K. Venkataramanan, R. Strom, S. Thummel, and M. S. Boguski. 2001. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 27:383-391. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, P. N., D. E. Sweet, J. A. McDowell, W. Symonds, Y. Lou, S. Hetherington, and S. LaFon. 1999. Safety and pharmacokinetics of abacavir (1592U89) following oral administration of escalating single doses in human immunodeficiency virus type 1-infected adults. Antimicrob. Agents Chemother. 43:603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzolini, C., A. Telenti, L. A. Decosterd, G. Greub, J. Biollaz, and T. Buclin. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effect in HIV-1 infected patients. AIDS 15:71-75. [DOI] [PubMed] [Google Scholar]
- 19.McDowell, J. A., G. E. Chittick, J. R. Ravitch, R. E. Polk, T. M. Kerkering, and D. S. Stein. 1999. Pharmacokinetics of [14C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob. Agents Chemother. 43:2855-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDowell, J. A., Y. Lou, W. S. Symonds, and D. S. Stein. 2000. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 44:2061-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy, R., J. P. Sommadossi, M. Lamson, D. B. Hall, M. Myers, and A. Dusek. 1999. Antiviral effect and pharmacokinetic interaction between nevirapine and indinavir in persons with human immunodeficiency virus type 1. J. Infect. Dis. 179:1116-1123. [DOI] [PubMed] [Google Scholar]
- 22.Sadler, B. M., C. Gillotin, and D. S. Stein. 2001. Pharmacokinetic and pharmacodynamic study of the human immunodeficiency virus protease inhibitor amprenavir after multiple oral dosing. Antimicrob. Agents Chemother. 45:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, P. F., R. DiCenzo, and G. D. Morse. 2001. Clinical pharmacokinetics of non-nucleoside reverse transcriptase inhibitors. Clin. Pharmacokinet. 40:893-905. [DOI] [PubMed] [Google Scholar]
- 24.Villani, P., M. B. Regazzi, F. Castelli, P. Viale, C. Torti, E. Seminari, and R. Maserati. 1999. Pharmacokinetics of efavirenz (EFV) alone and in combination therapy with nelfinavir (NFV) in HIV-1 infected patients. Br. J. Clin. Pharmacol. 48:712-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, L. H., G. E. Chittick, and J. A. McDowell. 1999. Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection. Antimicrob. Agents Chemother. 43:1708-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller, S., K. M. Radomski, Y. Lou., and D. S. Stein. 2000. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 44:2052-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]