Abstract
The penetration of ertapenem, a new carbapenem with a long half-life, reached 7.1 and 2.4% into inflamed and noninflamed meninges, respectively. Ertapenem had excellent antibacterial activity in the treatment of experimental meningitis due to penicillin-sensitive and -resistant pneumococci, leading to a decrease of 0.69 ± 0.17 and 0.59 ± 0.22 log10 CFU/ml · h, respectively, in the viable cell counts in the cerebrospinal fluid. The efficacy of ertapenem was comparable to that of standard regimens (ceftriaxone monotherapy against the penicillin-sensitive strain and ceftriaxone combined with vancomycin against the penicillin-resistant strain). In vitro, ertapenem in concentrations above the MIC was highly bactericidal against both strains. Even against a penicillin- and quinolone-resistant mutant, ertapenem had similar bactericidal activity in vitro.
The continuous global increase and spread of resistant pneumococci has jeopardized the treatment of pneumococcal infections (3). Furthermore, additional resistance to cephalosporins has limited the therapeutic options against penicillin-resistant isolates. Nevertheless, β-lactam antibiotics remain the first-line drugs for pneumococcal infections, except when penetration into infected tissues is compromised, as in meningitis. Currently the standard regimen for meningitis due to penicillin-resistant strains is the combination of an extended-spectrum cephalosporin with vancomycin (3, 12).
In addition, pneumococcal strains resistant to quinolones have been isolated, limiting the choice of alternative therapies (4). A regimen based on monotherapy would represent a clear advantage, especially when quinolone-resistant strains are suspected.
Ertapenem is a new carbapenem with a long half-life and activity against the majority of human bacterial pathogens, including penicillin-resistant pneumococci (11, 16, 17). On the other hand, little is known about the penetration and kinetics of ertapenem into the cerebrospinal fluid (CSF) and its efficacy in pneumococcal meningitis. The aim of this study was to investigate the penetration of ertapenem into inflamed and noninflamed meninges and to test its bactericidal properties against a penicillin-sensitive strain and a penicillin-resistant strain in experimental pneumococcal meningitis.
(This study was partially presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
MATERIALS AND METHODS
Strains.
The penicillin-resistant pneumococcal strain WB4 serotype 6 was isolated from a patient with pneumonia at the University Hospital of Bern, Bern, Switzerland. The MICs for this strain were as follows: penicillin, 4 mg/liter; ceftriaxone, 0.5 mg/liter; vancomycin, 0.12 to 0.25 mg/liter; and ertapenem 0.5 mg/liter. The penicillin-sensitive strain was kindly provided by the Institute for Infectious Diseases, University of Bern (MICs for this strain: penicillin, 0.05 mg/liter; ceftriaxone, 0.05 mg/liter; and ertapenem, 0.03 mg/liter). The penicillin- and quinolone-resistant mutant was obtained by sequential exposure of the penicillin-resistant strain to trovafloxacin in vitro. The MICs for this strain were as follows: penicillin, 4 mg/liter; ertapenem, 0.5 mg/liter; trovafloxacin, 4 mg/liter; and ciprofloxacin, 32 mg/liter.
Rabbit meningitis model.
The meningitis model, originally described by Dacey and Sande (8) was slightly modified. The experimental protocol was accepted by the Veterinäramt des Kantons Bern. In brief, young New Zealand White rabbits weighing 2 to 2.5 kg were anesthetized by intramuscular injections of ketamine (30 mg/kg) and xylazine (15 mg/kg) and were immobilized in stereotactic frames for induction of meningitis and CSF sampling. An inoculum containing approximately 1 × 105 to 106 CFU of penicillin-sensitive or penicillin-resistant pneumococci serotype 6, was directly injected into the cisterna magna. A long-acting anesthetic (ethyl carbamate [urethane], 3.5 g/rabbit) was injected subcutaneously, and animals were returned to their cages. Fourteen hours later, the cisterna magna was punctured again for periodic CSF sampling before and 1, 2, 4, 6, and 8 h after the initiation of therapy. A catheter was introduced into the femoral artery for serum sampling. Antibiotics were administered through a peripheral ear vein as bolus injections at the following doses: ceftriaxone, 125 mg/kg; vancomycin, 20 mg/kg; and ertapenem, 60 mg/kg. These dosages achieve serum concentrations similar to those observed in humans. Ceftriaxone was injected once at 0 h and vancomycin at 0 and 4 h according to Friedland et al. (9) and Cottagnoud et al. (6). Ertapenem was injected once at 0 h. Untreated controls received saline. Ertapenem was kindly provided by MSD. The other antibiotics and anesthetic drugs were commercially purchased.
Bacterial titers were measured by 10-fold serial dilutions of CSF samples plated on blood agar plates containing 5% sheep blood and incubated overnight at 37°C. In parallel, 20 μl of undiluted CSF samples was plated (limit of detectability, 50 CFU/ml). Comparisons between different dilutions of CSF were used to exclude significant carryover effects during therapy. The antimicrobial activity of the regimens during the 8-h treatment was calculated by linear regression analysis and expressed as a decrease in log10 CFU per milliliter per hour and as a killing rate over 8 h. A value of 1.7 log10 CFU/ml (the limit of detectability) was assigned to the first sterile CSF sample, and a value of 0 log10 CFU/ml was assigned to any following sterile sample. The results were expressed as means ± standard deviations. The statistical significance was determined by the Newman-Keuls test.
In the same experimental setting, ertapenem (60 mg/kg) was injected into uninfected animals in order to measure the penetration of ertapenem through uninflamed meninges. Sampling was performed as described above. Ten rabbits were used in each ertapenem monotherapy group, and 4 rabbits were in each group for the standard regimens and control groups.
Measurement of antibiotic levels in CSF.
Antibiotic concentrations in serum and CSF were determined by bioassay with the agar diffusion method. For serum levels, standard curves were determined in rabbit serum and in saline with 5% rabbit serum in order to mimic CSF protein concentration (15). Bacillus subtilis (ATCC 6633) was used as test strain for ertapenem (20). The intra- and interday variability of this method was less than 10%. The limit of detection was 0.1 mg/liter for ertapenem.
In vitro assays.
The pneumococcal strains (a penicillin-sensitive strain and a penicillin-resistant strain) were grown in C+Y medium (13) to an optical density of 0.3 at 590 nm and then diluted 40-fold to 106 CFU/ml, corresponding approximately to the CSF bacterial titer in rabbits before the initiation of therapy. Ertapenem was added in concentrations corresponding to 1, 5, and 10 times the MIC (0.03, 0.15, and 0.3 mg/liter for the penicillin-sensitive strain and 0.5, 2.5, and 5 mg/liter for the penicillin-resistant strain). Bacterial titers were determined at 0, 2, 4, 6, and 8 h by serial dilution of samples plated on agar plates containing 5% sheep blood and incubated at 37°C for 24 h. Experiments were performed in triplicate, and results were expressed as means ± standard deviations.
Pharmacokinetic (PK) analysis.
A zero-order input (bolus injection), first-order elimination, and expanded three-compartment model is used to describe the time course of serum and CSF drug concentrations. The three-compartment model consists of a central compartment (serum), which is linked to a peripheral compartment, and the CSF compartment (5, 18). Influx and efflux clearance in the CSF compartment are modeled as CLin = CLdiff, CLout = CLdiff + CLbulk + CLmetab, and Pcsf = CLin/CLout, where CLin is the total clearance from the central compartment to the CSF compartment (in milliliters/minute) and is assumed to be equal to CLdiff, the passive transcellular diffusion clearance (i.e., the active influx clearance is absent). CLout is the total clearance from the CSF compartment to the central compartment (in milliliters/minute). CLbulk is the clearance by CSF bulk flow, or active efflux (14). CLmetab, the clearance by drug metabolism in the CSF, is assumed to be negligible (14). Pcsf equals the CSF-to-serum area under the concentration-time curve ratio and is a measure of CSF penetration.
The population kinetic model was fit to all measured concentrations of ertapenem in serum and CSF from all rabbits by using ADVAN5 TRANS1 and the FOCE method of the computer program NONMEM (nonlinear mixed effects modeling; NONMEM user's guide, NONMEM Project Group, University of California at San Francisco, San Francisco) (1). The NONMEM output, when the program is used to fit a population model, among other things, consists of the value of the objective function at convergence (approximately minus twice the maximized log likelihood of the data). This can be used to test the merit of a more-complex model (larger [more parameters]) over that of a less-complex submodel (2).
PK parameters for the ith individual are estimated as
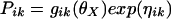 |
Where Pik is the kth element of Pi, gik is a model for its (log) expectation, and ηik is a normally distributed mean zero random effect. The vector θX consists of mean population PK parameters (X = central and peripheral volume of distribution, clearance, CLdiff, and CLbulk).
The inflammation effect (IE) on passive transcellular diffusion clearance (CLdiff) is modeled as
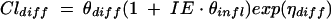 |
where θdiff is the population CLdiff in rabbits without meningitis, θinfl is the proportional increase of population CLdiff in rabbits with meningitis versus rabbits without meningitis, and ηdiff is the interindividual variance of CLdiff. IE is an indicator variable taking the value 1 if rabbits have meningitis, otherwise it is 0.
RESULTS
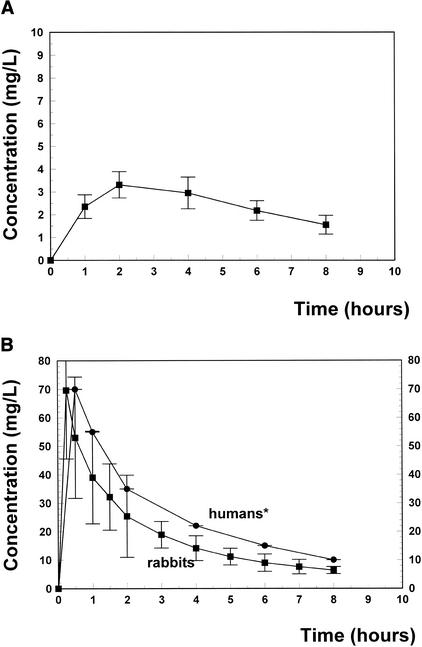
Figure 1 shows the kinetics of ertapenem after one dose of 60 mg/kg of body weight. The peak serum level ranged around 70 mg/liter, declining slowly to 6.5 mg/liter 8 h later. The peak CSF level around 3.4 mg/liter appeared 2 h after intravenous injection, decreasing slowly to 1.5 mg/liter at the end of the treatment period. Ertapenem levels remained above the MIC (0.03 and 0.5 mg/liter for the penicillin-sensitive and -resistant strains, respectively) during the entire treatment period. The CSF/MIC ratios ranged between 6.8 and 3 for the resistant strain and between 113 and 50 for the penicillin-sensitive strain.
FIG. 1.
(A) Ertapenem concentrations in CSF for 8 h after one intravenous injection of 60 mg of ertapenem/kg. The concentrations of ertapenem in CSF remained above the MIC during the entire treatment period (MICs, 0.06 and 0.5 mg/liter for the penicillin-sensitive and -resistant strains, respectively). (B) Ertapenem concentrations in serum of rabbits after one injection of 60 mg/kg and in serum of human adults after one injection of 500 mg (MSD, personal communication [∗]).
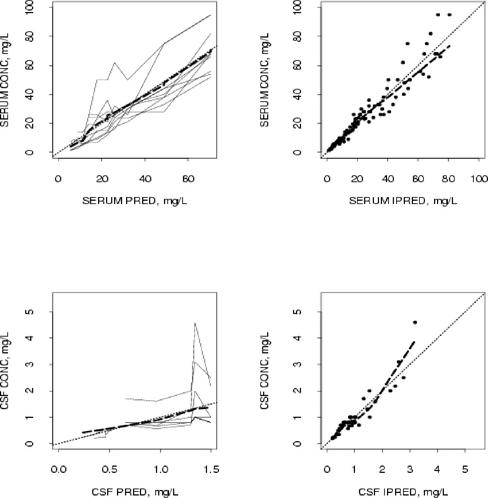
Table 1 presents the estimates of the population parameters. The population average of passive diffusion clearance was increased by 2.9 in rabbits with meningitis compared to rabbits without meningitis. The population average value for CSF penetration in rabbits with meningitis was 7.1%. Using the NONMEM computer program, the expanded three-compartment model for ertapenem kinetics demonstrates excellent goodness of fit (Fig. 2).
TABLE 1.
Population PK parameters for ertapenem in serum and CSF in the rabbit meningitis model
| Parameter (unit) | Mean | CVa |
|---|---|---|
| PK parameters | ||
| Central volume of distribution (ml) (Vs) | 1,170 | |
| Peripheral volume of distribution (ml) (Vp) | 1,630 | 16b |
| Intercompartmental clearance between Vs and Vp (ml/min) | 30.4 | |
| Total clearance (ml/min) | 12.6 | 24 |
| CSF-serum barrier transfer parameters | ||
| Passive diffusion clearance (CLdiff) (ml/min) in rabbits with (without) meningitis | 0.00084 (0.00029) | 49 |
| Clearance by bulk flowc (CLbulk) (ml/min) | 0.0116 | 58 |
| CSF penetration (%)d in rabbits with (without) meningitis | 7.1 (2.4) |
CV, interindividual variability shown as a percentage.
Fixed CV.
Clearance by active efflux and or bulk flow.
CSF penetration, calculated as the ratio CLin/CLout, where CLout = CLdiff + CLbulk.
FIG. 2.
Goodness-of-fit plots of the three-compartment PK model for ertapenem. The upper panels show measured serum concentrations (CONC) versus population and individual predictions (PRED and IPRED, respectively). The lower panels show measured CSF concentrations versus PRED and IPRED. Each solid line in the left panels represents one animal. Fine dashed lines are the lines of identity. The heavy dashed line represents the smoothness of measured concentrations versus PRED and IPRED, respectively.
Predicted and measured ertapenem concentrations in serum and CSF agreed reasonably well by using population mean PK parameters (R2 = 0.91, P < 0.0001 [serum]; R2 = 0.72, P < 0.0001 [CSF]). The agreement between predicted and measured concentrations in serum and CSF was excellent when using individual PK parameters (R2 = 0.97, P < 0.0001 [serum]; R2 = 0.95, P < 0.0001 [CSF]).
The efficacies of the different treatment groups are summarized in Table 2. In untreated controls, the bacterial titers increased slowly over 8 h (+0.30 ± 0.06 log10 CFU/ml). Before the initiation of treatment, the initial bacterial titer did not differ significantly between all treatment groups. Against the penicillin-sensitive strain, ertapenem was highly bactericidal in comparison to the standard regimen (ceftriaxone monotherapy), and it sterilized the CSF of all rabbits. Against the penicillin-resistant strain, ertapenem was also highly efficacious (−0.59 ± 0.22 log10 CFU/ml), producing killing rates comparable to the standard regimen and sterilizing the CSF of 8 of 10 rabbits after 8 h.
TABLE 2.
Activities of single drug and combination therapies against penicillin-sensitive and penicillin-resistant Streptococcus pneumoniae in experimental meningitis
| Antibiotic (infecting strain susceptibility)a | n | Mean initial titer ± SD (log10 CFU/ml) | Mean killing rate ± SD (Δlog10 CFU/ml · hr) | Mean killing rate/8 h ± SD (log10 CFU/ml) |
|---|---|---|---|---|
| None (controls) (Pens) | 4 | 6.02 ± 0.49 | +0.08 ± 0.15b | +0.30 ± 0.08b |
| Ertapenem (Pens) | 10 | 5.12 ± 0.10 | −0.69 ± 0.17c | −5.63 ± 0.97c |
| Ceftriaxone (Pens) | 4 | 5.50 ± 0.63 | −0.80 ± 0.25c | −5.65 ± 0.72c |
| None (controls) (Penr) | 4 | 6.35 ± 0.61 | +0.10 ± 0.06 | +0.35 ± 0.32 |
| Ertapenem (Penr) | 10 | 5.30 ± 0.48 | −0.59 ± 0.22d | −4.60 ± 1.10d |
| Ceftriaxone + vancomycin (Penr) | 4 | 5.80 ± 0.73 | −0.50 ± 0.20d | −4.05 ± 0.68d |
Pens, penicillin sensitive; Penr, penicillin resistant.
P < 0.05 versus all groups.
P not significant.
P not significant.
In time-killing assays over 8 h, in vitro ertapenem was highly bactericidal (data not shown). Against the penicillin-sensitive strain, ertapenem in concentrations above the MIC (5 and 10 times the MIC) sterilized the cultures within 4 h. Even with concentrations around the MIC, ertapenem was highly bactericidal and sterilized the cultures after 6 h. Ertapenem was similarly efficacious against the penicillin-resistant strain in vitro, although the rapid decrease of the viable cell counts was less pronounced. The MIC led to a negligible decrease of the viable cell count after 8 h (0.9 log10 CFU/ml). Concentrations of ertapenem above the MIC (5 and 10 times the MIC) managed to sterilize the cultures after 4 and 6 h, respectively. Even against the penicillin- and quinolone-resistant mutant, ertapenem produced a pronounced decrease of the viable cell count over 8 h in concentrations above the MIC (−6.4 and −5.1 log10 CFU/ml for 10 and 5 times the MIC, respectively).
DISCUSSION
The continuous increasing spread of penicillin-resistant pneumococci has endangered the use of β-lactam antibiotics for the treatment of pneumococcal infections, especially in meningitis, for which the therapeutic options are limited. In the search for potential alternatives, it was recently shown that the combination of ceftriaxone with quinolones acts synergistically and was very effective against penicillin-resistant pneumococci in the same experimental model (6), although monotherapy would represent a considerable advantage. Among the potential candidates, new carbapenems could play a central role in the treatment of pneumococcal meningitis due to resistant strains.
Ertapenem, a new carbapenem with a long half-life and resistance to degradation by renal dehydropeptidase I, has a broad antimicrobial spectrum, covering the majority of human bacterial pathogens, including Listeria monocytogenes. In this study, we have investigated the efficacy of ertapenem in pneumococcal meningitis due to penicillin-sensitive and -resistant strains and its penetration into inflamed and noninflamed meninges.
The dose of ertapenem used (60 mg/kg) produced levels in serum corresponding to one intravenous injection of 500 mg in humans (peak level, 70 mg/liter in rabbits versus 70.3 mg/liter in humans; after 8 h, 6.5 mg/liter in rabbits versus 9.5 mg/liter in humans) (Fig. 1B). As the recommended dose in humans is 1 g once a day, the data reported in this model are likely to represent the minimum effectiveness possible.
As expected with a β-lactam agent, inflammation at the serum-CSF barrier affects the CSF PKs of ertapenem. In rabbits with experimental meningitis, passive diffusion clearance of ertapenem is significantly increased. The large variability of passive diffusion clearance in rabbits with inflamed meninges is reflected by the large variability in CSF concentration (Fig. 2A). The average CSF penetration was estimated to be 7.1% in rabbits with inflamed meninges and 2.4% in rabbits with uninflamed meninges.
The difference between influx and efflux clearance was estimated to be 0.0116 ± 0.0088 ml/min, which is close to previously reported values for clearance by CSF bulk flow (19, 21). Although this finding indicates that the difference between influx and efflux clearance can be primarily explained by CSF bulk flow (14), we cannot rule out other transport mechanisms at the serum-CSF barrier such as active efflux transport (e.g., via P-glycoprotein). However, based on our results, these active efflux mechanisms probably play a minor (if any) role in the CSF PKs of ertapenem.
In our experimental meningitis model, ertapenem was very efficacious and sterilized the CSF of all rabbits infected with the penicillin-sensitive strain and 8 of 10 rabbits' CSF in the penicillin-resistant group, confirming the highly bactericidal activity of ertapenem observed in time-killing assays in vitro. Higher doses of ertapenem, corresponding to an injection of 1 g in humans, would probably sterilize the CSF of all rabbits in the penicillin-resistant group. Although the doses used against the penicillin-resistant strain were not optimal, ertapenem produced CSF levels and killing rates similar to those observed with high doses of meropenem, recently tested in the same experimental model (meropenem peak level, 3.76 mg/liter; meropenem killing rate, −0.48 ± 0.14 Δlog10 CFU/ml) (10).
Interestingly, the bactericidal efficacy of ertapenem was not affected by quinolone resistance in vitro, as is the case for other β-lactam antibiotics (7).
In conclusion, the sufficient penetration into inflamed meninges in rabbits, the antimicrobial spectrum against common meningeal pathogens, and the efficacy against penicillin-sensitive and -resistant pneumococci might qualify ertapenem for the empirical treatment of bacterial meningitis, especially when penicillin-resistant strains are suspected. These data deserve further investigations in humans.
REFERENCES
- 1.Beal, S., and L. B. Sheiner. 1980. The NONMEM system. Am. Stat. 34:118-119. [Google Scholar]
- 2.Bickel, P. J., and K. A. Doksum. 1976. Likehood tests and related procedures in mathematical statistics: basic ideas and selected topics, p. 192-247. In E. L. Lehmann (ed.), Holden-Day, Inc., San Francisco, Calif.
- 3.Bradley, J., and W. M. Scheld. 1997. Penicillin-resistant Streptococcus pneumoniae meningitis: current antibiotic therapy in the 1990s. Clin. Infect. Dis. 24(Suppl. 2):213-217. [DOI] [PubMed] [Google Scholar]
- 4.Chen, D. K., A. McGeer, J. C. de Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 5.Cottagnoud, P., F. Acosta, M. Cottagnoud, M. Pfister, and M. G. Täuber. 2002. Efficacies of BMS 284756 against penicillin-sensitive, penicillin-resistant, and quinolone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 46:184-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cottagnoud, P., F. Acosta, M. Cottagnoud, K. Neftel, and M. G. Täuber. 2000. Synergy between trovafloxacin and ceftriaxone against penicillin-resistant pneumococci in the rabbit meningitis model and in vitro. Antimicrob. Agents Chemother. 44:2179-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottagnoud, P., F. Acosta, M. Cottagnoud, and M. G. Täuber. 2002. Cefepime is efficacious against penicillin- and quinolone-resistant pneumococci in experimental meningitis. J. Antimicrob. Chemother. 49:327-330. [DOI] [PubMed] [Google Scholar]
- 8.Dacey, R. G., and M. A. Sande. 1974. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob. Agents Chemother. 6:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedland, I., M. Paris, S. Ehret, S. Hickey, K. Olsen, and G. H. McCracken, Jr. 1993. Evaluations of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob. Agents Chemother. 37:1630-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber, C. M., M. Cottagnoud, K. Neftel, M. G. Täuber, and P. Cottagnoud. 1999. Meropenem alone and in combination with vancomycin in experimental meningitis caused by a penicillin-resistant pneumococcal strain. Eur. J. Clin. Microbiol. Infect. Dis. 18:866-870. [DOI] [PubMed] [Google Scholar]
- 11.Hoellman, D. B., L. M. Kelly, K. Credito, L. Anthony, L. M. Ednie, M. R. Jacobs, and P. C. Appelbaum. 2002. In vitro antianaerobic activity of ertapenem (MK-0826) compared to seven other compounds. Antimicrob. Agents Chemother. 46:220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan, S. L., and E. O. Mason. 1998. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin. Microbiol. Rev. 11:628-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lack, S., and R. D. Hotchkiss. 1960. A study of genetic material determining a enzyme activity in pneumococcus. Biochem. Biophys. Acta 39:508-518. [DOI] [PubMed] [Google Scholar]
- 14.Nau, R., F. Sorgel, and H. W. Prange. 1998. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin. Pharmacokinet. 35:223-246. [DOI] [PubMed] [Google Scholar]
- 15.Nau, R., M. Sachdeva, M. A. Sande, and M. G. Täuber. 1995. Rifampin for the therapy of experimental meningitis in rabbits. Antimicrob. Agents Chemother. 38:1186-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odenholt, I. 2001. Ertapenem: a new carbapenem. Expert Opin. Investig. Drugs 10:1157-1166. [DOI] [PubMed] [Google Scholar]
- 17.Pankuch, G. A., T. A. Davies, M. R. Jacobs, and P. C. Appelbaum. 2002. Antipneumococcal activity of ertapenem (MK-0826) compared to those of other agents. Antimicrob. Agents Chemother. 46:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfister, M., C. M. Gerber, M. Hammarlund-Udenaes, L. B. Sheiner, L. Zhang, M. G. Täuber, and P. Cottagnoud. 2003. Modeling of transfer kinetics at the serum-cerebrospinal fluid barriers in rabbits with experimental meningitis: application to grepafloxacin. Antimicrob. Agents Chemother. 47:138-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollay, M., and H. Davson. 1963. The passage of certain substances out of the cerebrospinal fluid. Brain 86:137-150. [DOI] [PubMed] [Google Scholar]
- 20.Simon, H. J., and E. Y. Yin. 1970. Microbioassay of antimicrobial agents. Appl. Microbiol. 19:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welch, K. 1963. Secretion of cerebrospinal fluid by choroid plexus of the rabbit. Am. J. Physiol. 205:617-624. [DOI] [PubMed] [Google Scholar]