Abstract
The molecular mechanisms underlying the clinical effects of alpha interferon (IFN) and ribavirin are not understood. Elimination of infected cells occurs in part by cytotoxic T lymphocytes (CTLs) expressing CD95 ligand and thereby attacking target cells which are positive for the death receptor CD95. Since many viruses have evolved mechanisms to inhibit apoptosis, the opposite, namely, promotion of apoptosis, could be a strategy to strengthen the host antiviral response. In the present study, we have asked whether the antiviral substances IFN and ribavirin could support CD95-mediated apoptosis by interfering with the activation of caspases, a family of proteases known for their essential role in apoptosis. HepG2 cells, stimulated with the agonistic anti-CD95 antibody, served as a minimal model to mimic the CD95 stimulation ocurring during a CTL attack of target cells in vivo. Apoptosis was quantitated by flow cytometric detection of hypodiploid nuclei. Caspase activity was measured by cytofluorometry, immunocytochemistry, and immunoblot analysis. IFN and ribavirin sensitized HepG2 cells for CD95-mediated apoptosis. This effect was correlated with an increase in CD95-mediated caspase activation and enhanced cleavage of the caspase substrate poly(ADP-ribose) polymerase. Furthermore, the positive effect on CD95-mediated caspase activation by IFN and ribavirin was confirmed by immunocytochemistry for activated caspase-3 and by immunoblot detection of activated caspase-3, caspase-7, and caspase-8. Our data demonstrate that the antiviral substances IFN and ribavirin are able to sensitize for CD95-mediated apoptosis. IFN and ribavirin also enhance CD95-mediated caspase activation, which might in part be responsible for the apoptosis-promoting effect of these antiviral compounds.
Hepatitis C virus is an RNA virus that causes predominantly persistent infection with subsequent chronic liver disease. For many years, alpha interferon (IFN) was the only approved therapy for chronic hepatitis C. Nevertheless, sustained elimination of the virus could be achieved in only 10 to 15% of patients treated with IFN. Recently, combination therapy with IFN and ribavirin was established as the new standard treatment (7, 24, 29). The virus can be eliminated in nearly 40% of patients treated with this regimen. However, the molecular mechanisms underlying this clinical success are poorly understood.
The guanosine analogue ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) is transported into almost all cell types of the body by sodium-dependent nucleoside transporters (13). The exact molecular alterations induced by ribavirin are unclear (27). There are some reports indicating that ribavirin might also be able to modulate cytokine profiles and the differentiation pattern of lymphocytes (26, 35).
IFN belongs to the group of acid-stable interferons that are produced mainly by leukocytes and lymphoblasts. IFN binds to its cell surface receptor, which consists of the two chains IFN-R1 and IFN-R2. Following receptor occupation, the intracellular signal chain via Janus kinases (JAK) and signal-transducing and activating factors of transcription (STAT) (16) is responsible for the expression of a set of proteins that mediate the biological actions of IFN. These can be direct effects on infected or uninfected cells or indirect effects by interfering with the regulation of the immune system (28). A prominent example is the serine-threonine protein kinase PKR (38), which leads to the inhibition of mRNA translation and a concomitant block in viral replication. In addition, cells overexpressing PKR undergo apoptosis (8, 34, 36) and are more sensitive to apoptosis induced by viruses (1) or by tumor necrosis factor (40). This suggests that promotion of apoptosis is an alternative pathway by which IFN, in addition to its inhibitory effects on viral replication, counteracts the virus. In addition to direct effects on infected cells, antiviral drugs may act indirectly by supporting the intrinsic host response to viral infections. Cytotoxic T-lymphocyte (CTL) recognition of virus-infected cells results in rapid apoptotic death of the target cells, providing an important element of the intrinsic host antiviral response. CTLs use two major mechanisms to induce apoptosis in their target cells, the perforin-granzyme B system and the CD95 pathway. The death receptor ligand CD95 ligand (CD95L) is expressed on activated CTLs. Binding of CD95L to the death receptor CD95 on target cells leads to the destruction of infected cells by activation of intracellular caspases. Caspases, the central mediators of apoptosis, comprise a family of at least 14 members in mammalian cells (5, 19). Once activated, they evoke the morphological and biochemical aspects of apoptosis either by cleaving other caspases in a proteolytic cascade or by degrading cellular target proteins. The initial event in apoptosis induced by ligation of the death receptor CD95 is thought to be procaspase-8 recruitment to CD95 (33), which leads to activation of downstream caspases. Alternatively, caspases can be activated via the so-called mitochondrial death pathway, which is activated by a variety of stimuli including chemotherapeutic drugs (39) and irradiation (2, 20). Interestingly, a variety of viruses have developed mechanisms to directly interfere with the caspase machinery of cells. Examples are viral FLIPs (caspase-8/Flice inhibitory proteins produced by herpesviruses) (15), CrmA (cytokine response modifier A) produced by cowpox virus (37), and p35 as well as IAPs (inhibitors of apoptosis), both produced by baculovirus (3). In all these examples, the viral products are specific inhibitors of the proapoptotic caspases. Thus, expression of these viral proteins has antiapoptotic effects on the host cell and might prevent the killing of target cells otherwise induced by either CD95 stimulation or internal signals. Current concepts assume that tissue damage in chronic hepatitis C is caused predominantly by the immune response, targeting infected cells (4, 31), and not by the virus itself. Destruction of virally infected cells is in part mediated by the major histocompatibility complex (MHC)-restricted action of CTLs with subsequent activation of caspases in the target cells. Antiapoptotic effects have also been described for hepatitis C virus NS5A (12) and core proteins (11, 23, 30). However, the mechanisms of this effect remain controversial since other groups could not confirm these data or came to opposite conclusions, namely, that proapoptotic effects were occurring (9, 32, 41). Nevertheless, the present state of knowledge suggests that inhibition of host cell apoptosis is a mechanism by which many viruses create an environment favoring viral replication or persistence (34). At present it is unknown whether IFN, ribavirin, or, especially, the combination of the two substances has an impact on apoptosis and the regulation of caspase activation. To investigate this, we asked whether the clinically successful antiviral substances IFN and ribavirin, in addition to specific effects on viral replication, can modulate apoptosis induced by stimulation of the death receptor CD95.
MATERIALS AND METHODS
Cell line.
The human hepatoma cell line HepG2 was obtained from Deutsche Sammlung von Mikroorganismen and Zellkulturen GmbH (Braunschweig, Germany) (ACC 180) and maintained in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum 100 U of penicillin/ml, 0.1 mg of streptomycin/ml, and 1% nonessential amino acids 100× (all from Gibco BRL, Eggenstein, Germany). The cells were grown at 37°C in a 5% CO2 atmosphere and maintained in log phase.
Stimulation of HepG2 cells.
For determination of apoptosis and quantitative caspase activation, 2 × 104 HepG2 cells per well were seeded in a 96-well microtiter plate. For immunocytochemistry, 4 × 104 cells per well were seeded onto an eight-well chamber slide (Nunc, Wiesbaden, Germany). For immunoblot analysis, 3 × 105 cells per well were cultured in a 12-well microtiter plate. Subsequently, cells were stimulated as indicated in figure legends. Control samples were prepared with the respective diluent control. Cells were stimulated for 4 h for assessment of caspase activation by fluorometry or immunoblot analysis and for 12 h for flow cytometric determination of apoptosis.
Fluorometric measurement of caspase activity.
Cytosolic cell extracts were prepared by lysing cells in a buffer containing 1% NP-40, 200 mM NaCl, 20 mM Tris-HCl (pH 7.4), 2 μg of aprotinin per ml, 2 μg of leupeptin per ml, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Caspase activities were determined by incubation of cell lysates with 50 μM N-acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin (DEVD-AMC) (Bachem, Heidelberg, Germany), a fluorogenic substrate, in 200 μl of buffer containing 10 mM HEPES (pH 7.4), 220 mM mannitol, 68 mM sucrose, 2 mM NaCl, 2.5 mM KH2PO4, 0.5 mM EGTA, 2 mM MgCl2, 5 mM pyruvate, 1 mM PMSF, and 1 mM 1,4-dithiothreitol. The release of aminomethylcoumarin was measured by fluorometry using an excitation wavelength of 360 nm and an emission wavelength of 480 nm.
Measurement of apoptosis.
The leakage of fragmented DNA from apoptotic nuclei was measured by the method of Nicoletti et al. (25). Briefly, apoptotic nuclei were prepared by lysing cells in a hypotonic buffer (1% sodium citrate, 0.1% Triton X-100, 50 μg of propidium iodide per ml) and subsequently analyzed by flow cytometry. Nuclei to the left of the 2N peak containing hypodiploid DNA were considered as apoptotic. All flow cytometry analyses were performed on a FACScalibur apparatus (Becton Dickinson, Heidelberg, Germany) using CellQuest analysis software.
Immunocytochemical detection of active caspase-3.
Cells were fixed by adding 4% paraformaldehyde at room temperature to each chamber slide well (Nunc). After four washes with phosphate-buffered saline (PBS), nonspecific binding was blocked for 20 min at room temperature with 2% goat serum. Active caspase-3 was detected by using an antibody specific for the cleaved form of caspase-3. Rabbit anti-caspase-3 polyclonal antibody (1:2,000; R&D Sytems, Wiesbaden, Germany) was used for 1 h as primary antibody. Subsequently, cells were incubated with 3% H2O2. Following multiple washes with PBS, biotinylated goat anti-rabbit immunoglobulin G (1:200; Linaris, Wiesbaden, Germany) was used for 30 min as the secondary antibody. After four washes, the reaction products were developed using a standard peroxidase method (Vectastain ABC kit; Vector, Burlingame, Calif.).
Immunoblotting.
Cleavage of poly(ADP-ribose) polymerase (PARP), caspase-3,caspase-7, and caspase-8 was detected by immunoblotting. Following stimulation, cells were harvested with a rubber policeman, washed in cold PBS, and lysed in lysis buffer (1% Triton X-100, 50 mM Tris [pH 7.6], 150 mM NaCl, 3 μg of aprotinin per ml, 3 μg of leupeptin per ml, 3 μg of pepstatin A per ml, 2 mM PMSF). Subsequently, proteins were separated under reducing conditions by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8 to 15% polyacrylamide). Following SDS-PAGE, proteins were electroblotted to a polyvinylidene difluoride membrane (Amersham, Braunschweig, Germany). The loading and transfer of equal amounts of protein was confirmed by staining the nitrocellulose membrane with Ponceau S. The membranes were blocked for 1 h with 5% nonfat dry milk powder in Tris-buffered saline (TBS) and then immunoblotted for 1 h with either rabbit anti-PARP polyclonal antibody (1:2,000; Roche Diagnostics, Mannheim, Germany), mouse anti-caspase-3 monoclonal antibody (1:500; Transduction Laboratory, Heidelberg, Germany), mouse anti-caspase-7 monoclonal antibody (1:500; Pharmingen, Heidelberg, Germany), or mouse anti-caspase-8 monoclonal antibody (1:10 dilution of hybridoma supernatant; Biocheck, Münster, Germany). The membranes were washed four times with TBS-0.05% Tween 20 and incubated for 1 h with peroxidase-conjugated affinity-purified goat anti-rabbit (once) or rabbit anti-mouse (three times) IgG. Following extensive washing, the reaction was developed by enhanced chemiluminescence staining using enhanced chemiluminescence reagents (Amersham).
Reagents and drugs.
The broad-range peptide inhibitor of caspases benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (zVAD-fmk) was purchased from Enzyme Systems (Dublin, Calif.). The agonistic anti-CD95 antibody was obtained from BioCheck (Münster, Germany). Ribavirin and cycloheximide (CHX) were purchased from Sigma (Deisenhofen, Germany), and IFN was purchased from Essex (München, Germany).
RESULTS
IFN and ribavirin induce apoptosis.
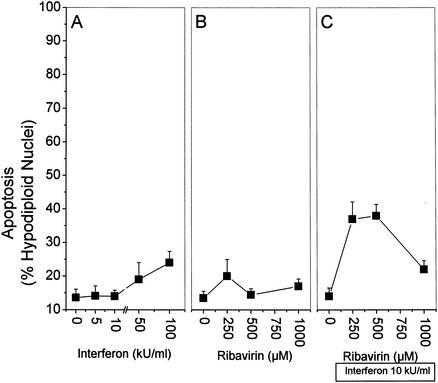
First, we assessed whether the antiviral substances IFN and ribavirin given alone or in combination are able to induce apoptosis independently of additional stimulation with anti-CD95. The quantitative measurement of hypodiploid nuclei by fluorescence-activated cell sorter analysis was used to detect apoptosis. IFN at high doses produced a slight (25%) increase in apoptosis (Fig. 1A). Ribavirin alone, at a dose of 250 μM, tended to enhance apoptosis by 20% (Fig. 1B). The two substances together had a more pronounced effect on apoptosis, with maximal frequencies of 40% (Fig. 1C). No apoptosis was observed in the presence of the broad-range caspase inhibitor zVAD (data not shown).
FIG. 1.
IFN and ribavirin induce apoptosis in HepG2 cells. HepG2 cells were incubated for 36 h with the indicated stimuli. Subsequently, nuclei were isolated by hypotonic lysis, stained with propidium iodide, and analyzed by flow cytometry for hypodiploid DNA content as an indicator of apoptosis. A total of 8,000 nuclei was counted per data point. The incidence of nuclei with hypodiploid DNA content is expressed as a percentage of the total number of nuclei. (A) IFN only; (B) ribavirin only; (C) 10 kU of IFN per ml plus ribavirin as indicated on the x axis. Values are means and standard deviations of triplicate determinations. For comparison, 0.25 μg of anti-CD95 per ml in combination with CHX (an inhibitor of translation) would result in 90 to 100% apoptosis after 24 h.
IFN and ribavirin sensitize to anti-CD95-induced apoptosis.
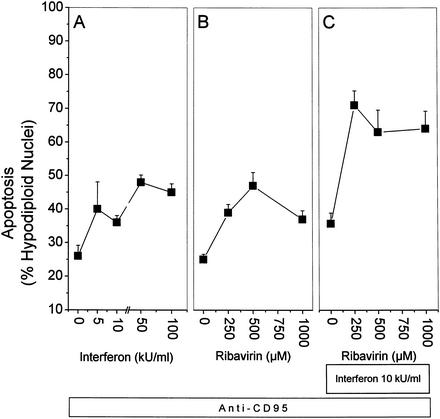
Virally infected cells will eventually express viral proteins on the cell surface and thereby induce a T-cell-receptor-mediated activation of CTLs. Activated CTLs employ two major pathways to destroy their target cells, the perforin-granzyme B system and the C95L-CD95 interaction. We used HepG2 cells stimulated with the agonistic anti-CD95 antibody as a minimal model to mimic this CD95L-CD95 interaction. Based on the assumption that infected hepatocytes in vivo are simultaneously exposed to the antiviral drugs IFN and ribavirin as well as to CD95L from attacking CTLs, we wanted to know whether anti-CD95-induced apoptosis can be modulated by pretreatment with IFN and ribavirin. Stimulation with anti-CD95 alone for 12 h resulted in 25% apoptosis (0 kU/ml [Fig. 2A ]). Preincubation with IFN alone sensitized the cells for CD95-mediated apoptosis in a dose-dependent manner, resulting in up to 48% apoptosis (Fig. 2A). In a similar fashion, ribavirin alone could also sensitize HepG2 cells for anti-CD95-induced apoptosis, resulting in up to 47% apoptosis (Fig. 2B). When the two substances were used together for preincubation, the cells were even more strongly sensitized, resulting in up to 70% apoptosis.
FIG. 2.
IFN and ribavirin enhance anti-CD95-induced apoptosis in HepG2 cells. HepG2 cells were pretreated for 24 h with the indicated stimuli and then stimulated for 12 h with 0.25 μg of anti-CD95 per ml. Subsequently, nuclei were isolated by hypotonic lysis, stained with propidium iodide, and analyzed by flow cytometry for hypodiploid DNA content as an indicator of apoptosis. A total of 8,000 nuclei was counted per data point. The incidence of nuclei with hypodiploid DNA content is expressed as a percentage of the total number of nuclei. (A) IFN only; (B) ribavirin only; (C) 10 kU of IFN per ml plus ribavirin as indicated on the x axis. Values are means and standard deviations of triplicate determinations. For comparison, the same dose of anti-CD95 in combination with CHX would result in 90 to 100% apoptosis after 24 h.
These data show that pretreatment with either ribavirin or IFN can significantly sensitize HepG2 cells for anti-CD95-induced apoptosis. Sensitization for apoptosis is further enhanced after pretreatment with the combination of ribavirin and IFN (Fig. 2C).
IFN and ribavirin activate caspases.
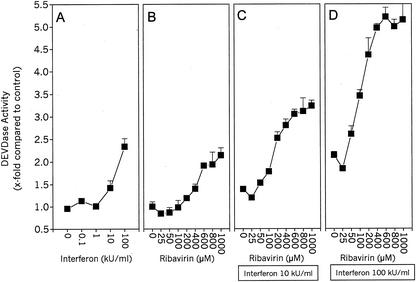
We have observed mild induction of apoptosis by IFN and ribavirin and a pronounced increase in CD95-mediated apoptosis induction by these substances. The activation of caspases is thought to be a prerequisite for apoptosis. Therefore, we examined whether the observed apoptosis is correlated with an increase in caspase activity. Caspase activity was quantitated by measuring the release of fluorogenic aminomethylcoumarin (AMC) from DEVD-AMC, a substrate for caspase-3-like caspases. Both IFN and ribavirin, when given alone, induced a mild but significant increase in caspase activity after an incubation period of 24 h (Fig. 3A and B). However, when ribavirin and IFN were combined, they were more effective in caspase activation than was either single substance alone (Fig. 3C and D). Together, they evoked a more than 5-fold increase in caspase activity, whereas IFN or ribavirin alone induced a maximal 2.4- or 2.2-fold increase, respectively. These data demonstrate that the antiviral substances IFN and ribavirin induce a mild but significant increase in caspase activity.
FIG. 3.
IFN and ribavirin induce DEVDase activity in HepG2 cells. Lysates of HepG2 cells were assayed with DEVD-AMC after incubation for 24 h with the indicated stimuli. The slope of the AMC release was measured fluorometrically and expressed as fold AMC release in unstimulated cells. (A) IFN only; (B) ribavirin only; (C) 10 kU of IFN per ml plus ribavirin as indicated on the x axis; (D) 100 kU of IFN per ml plus ribavirin as indicated on the x axis. Values are means and standard deviations of triplicate determinations. Representative plots from two independent experiments are shown.
IFN and ribavirin sensitize to anti-CD95-induced caspase activation.
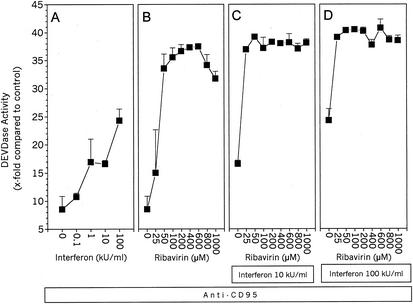
Stimulation with anti-CD95 alone for 4 h caused an eight fold increase in caspase activity (Fig. 4A [0 kU/ml] and Fig. 4B [0 μM]). Preincubation with IFN alone sensitized the cells to CD95-mediated caspase activation in a dose-dependent manner, resulting in an up to 25-fold increase in caspase activity (Fig. 4A). In a similar fashion, ribavirin alone could also sensitize HepG2 cells to anti-CD95-induced caspase activation, resulting in a more than 35-fold increase (Fig. 4B). When the two substances were used together in the preincubation, the cells were even more strongly, sensitized, rendering lower doses of each substance more efficient to induce a certain level of caspase activation (Fig. 4C and D). Thus, 25 μM ribavirin alone caused a 15-fold increase in anti-CD95-induced caspase activity (Fig. 4B), whereas in combination with 10 kU of IFN per ml, the increase was 37-fold (Fig. 4C), and in combination with 100 kU of IFN per ml, the increase was 39-fold (Fig. 4D). These data show that low doses of ribavirin which are clinically relevant can significantly sensitize HepG2 cells to anti-CD95-induced caspase activation. In addition, IFN can sensitize HepG2 cells, and when it is used together with ribavirin, the sensitization is further enhanced.
FIG. 4.
IFN and ribavirin enhance anti-CD95-induced caspase activity in HepG2 cells. Lysates of HepG2 cells were assayed for caspase-3-like activity by using DEVD-AMC after preincubation for 24 h with the indicated stimuli and subsequent stimulation for 4 h with 0.25 μg of anti-CD95 per ml. The slope of the AMC release was measured fluorometrically and is expressed as fold AMC release in unstimulated cells. (A) IFN only; (B) ribavirin only; (C) 10 kU of IFN per ml plus ribavirin as indicated on the x axis; (D) 100 kU of IFN per ml plus ribavirin as indicated on the x axis. Values are means and standard deviations of triplicate determinations. Representative plots from two independent experiments are shown.
IFN and ribavirin enhance anti-CD95-induced PARP cleavage.
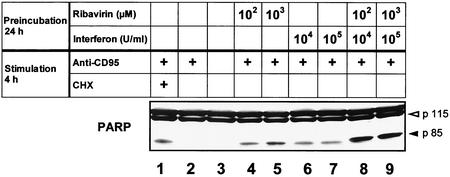
To further confirm the functional significance of enhanced caspase activation as measured by fluorometry, we performed immunoblot experiments for the DNA repair enzyme PARP, a classical substrate of caspases. Stimulation with anti-CD95 alone was able to induce AMC release from DEVD-AMC as measured by fluorometry and 25% apoptosis as measured by fluorescence-activated cell sorter analysis (see above). However, no cleavage product of PARP was observed by immunoblot analysis after stimulation with anti-CD95 alone (Fig. 5, lane 2). Addition of the protein synthesis inhibitor CHX to anti-CD95 served as a positive control. CHX, when coincubated for 4 h with anti-CD95, dramatically increased the extent of AMC release and the rate of apoptosis (40-fold and 90%, respectively [data not shown]), and only then could the PARP cleavage product p85 be detected by immunoblot analysis (lane 1). In contrast to stimulation with anti-CD95 alone, preincubation with both IFN and ribavirin led to the appearance of the p85 cleavage product (lanes 4 to 7). An even stronger enhancement of this cleavage product was observed after pretreatment with the combination of the two antiviral substances (lanes 8 and 9). However, no PARP cleavage was detected after incubation with IFN, ribavirin, or the combination for 24 h in the absence of anti-CD95 (data not shown).
FIG. 5.
Pretreatment with IFN and ribavirin enhances anti-CD95-induced cleavage of PARP. HepG2 cells were pretreated for 24 h with medium (lanes 1 to 3), ribavirin (lanes 4 and 5), IFN (lanes 6 and 7), or the combination of ribavirin and IFN (lanes 8 and 9). Subsequently the cells were stimulated for 4 h with medium (lane 3), 0.25 μg of anti-CD95 per ml (lanes 1, 2, and 4 to 9), or anti-CD95 plus CHX (lane 1) as a positive control. Cell lysates were then assayed for PARP by immunoblot analysis. Uncleaved PARP is indicated by the open arrowhead, and cleavage products are indicated by the solid closed arrowhead. Note that the nonspecific band above 115 kDa serves as the loading control.
These results demonstrate that the IFN- and/or ribavirin-induced enhancement of caspase activation, as measured by fluorometry, supports cleavage of the caspase substrate PARP and is thus of functional significance in the cellular context.
Ribavirin and IFN enhance the activation of the effector caspase-3 and caspase-7.
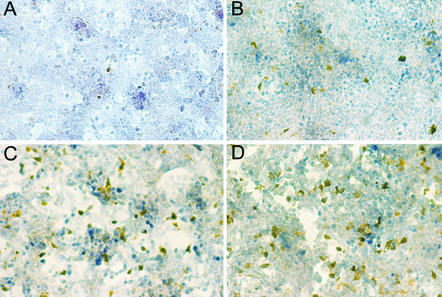
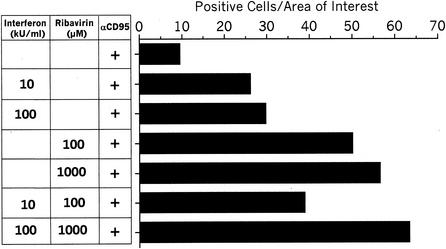
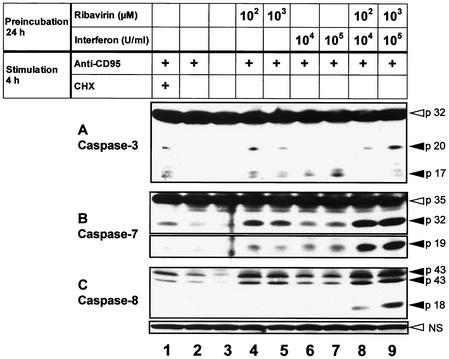
Measurement of caspase activity by determining the release of AMC is a very sensitive and easily quantifiable method. However, this method detects all caspase-3-like activities and cannot distinguish between different caspases of this group. To confirm the fluorometric data by an alternative approach and to further identify specific caspase activities that are affected by IFN and ribavirin, we performed immunocytochemistry experiments using an antibody which is specific for active caspase-3. Caspase-3 is a central effector caspase which serves as a converging point for many different stimuli. Following its own activation by proteolytic cleavage, caspase-3 destroys a variety of cellular substrates (like PARP), which eventually leads to the demise of the cell. Representative photomicrographs are shown in Fig. 6, and the results are summarized in a semiquantitative way in Fig. 7. Stimulation with anti-CD95 alone induced caspase-3 activation in only 10 cells per field (Fig. 6A and 7). The rate of detection of positive cells was enhanced by preincubation with either IFN (Fig. 6B and Fig. 7), to 30 positive cells per field, or ribavirin (Fig. 6C and Fig. 7), to 55 positive cells per field. The combination of the two substances further enhanced this effect to 60 positive cells per field (Fig. 6D and Fig. 7). The effect of IFN and ribavirin on caspase-3 activation was further confirmed by immunoblot analysis (Fig. 8A). Uncleaved procaspase-3 is detected as a 32-kD protein. Activation results in an intermediate 20-kD fragment with minor activity and a 17-kD fragment with full proteolytic activity. Stimulation with anti-CD95 alone did not evoke any visible caspase-3 cleavage products (Fig. 8A, lane 2). In contrast, stimulation with anti-CD95 in the presence of CHX induced cleavage into the typical cleavage products of caspase-3 (lane 1, p20 + p17). Preincubation with IFN alone led to the appearance of the 17-kDa fragment (lanes 6 and 7), whereas preincubation with ribavirin alone led to the appearance of the 20- and 17-kDa fragments (lanes 4 and 5). The combination of the two antiviral substances preferentially enhanced the appearance of the 20-kDa fragment (lanes 8 and 9). No cleavage of caspase-3 was detected after incubation with IFN, ribavirin, or the combination for 24 hs in the absence of anti-CD95 (data not shown).
FIG. 6.
Pretreatment with IFN and ribavirin enhances anti-CD95-induced caspase-3 activation. Representative photomicrographs of HepG2 cells following stimulation with 0.25 μg of anti-CD95 per ml for 4 h and subsequent immunocytochemical detection of active caspase-3 are shown. (A) No pretreatment. (B) Pretreatment with 105 U of IFN per ml for 24 h. (C) Pretreatment with 103 μM ribavirin for 24 h. (D) Pretreatment with 105 U of IFN per ml plus 103 μM ribavirin for 24 h.
FIG. 7.
Pretreatment with IFN and ribavirin enhances anti-CD95-induced caspase-3 activation. This figure shows a semiquantitation of the photomicrographs in Fig. 6. Eight randomly selected microscopic fields (area of interest) per treatment were selected, and brown cells corresponding to cells stained with the specific antibody against active caspase-3 were counted.
FIG. 8.
Pretreatment with IFN and ribavirin enhances anti-CD95-induced cleavage of caspase-3, caspase-7, and caspase-8. HepG2 cells were pretreated for 24 h with medium (lanes 1 to 3), ribavirin (lanes 4 and 5), IFN (lanes 6 and 7), or the combination of ribavirin and IFN (lanes 8 and 9). Subsequently the cells were stimulated for 4 h with medium (lane 3), 0.25 μg of anti-CD95 (lanes 1, 2, and 4 to 9), or anti-CD95 plus CHX (lane 1) as a positive control. Cell lysates were then assayed by immunoblot analysis for caspase-3 (A), caspase-7 (B), and caspase-8 (C). Uncleaved procaspases are indicated by open arrowheads, and cleavage products are indicated by solid arrowheads. Note that procaspase-8 is not shown; instead, another nonspecific band from the same blot (NS) is shown; this band serves as a loading control.
Caspase-7 is another effector caspase that recognizes the DEVD tetrapeptide sequence and could therefore be responsible for “caspase-3-like activity” in the fluorometric assay. Accordingly, we performed immunoblot experiments for caspase-7. Uncleaved procaspase-7 is detected as a 35-kDa protein. Activation results in an intermediate 32-kDa fragment and subsequently in a 19-kDa fragment. In agreement with the results for caspase-3, anti-CD95 alone resulted in only a very moderate cleavage of caspase-7 into the intermediate cleavage product p32 (Fig. 8B, lane 2), which was clearly enhanced in the presence of CHX (lane 1). Preincubation with IFN increased the cleavage of caspase-7 into the 32- and 19-kDa fragments (lanes 6 and 7), whereas preincubation with ribavirin promoted the appearance mainly of the 32-kDa fragment (lanes 4 and 5). Preincubation with IFN and ribavirin together enhanced the activation of caspase-7 more strongly than did the single substances (lanes 8 and 9). No cleavage of caspase-7 was detected after incubation with IFN, ribavirin, or the combination for 24 h in the absence of anti-CD95 (data not shown).
These data demonstrate that anti-CD95-induced activation of the effectors caspase-3 and caspase-7 is enhanced by preincubation with either IFN, ribavirin, or the combination of the two.
Effect of IFN and ribavirin on the cleavage of the initiator caspase-8.
Stimulation of CD95 leads to the recruitment and subsequent activation of the initiator procaspase-8. Caspase-8, in turn, cleaves a number of other caspases including the executioner caspase-3 and caspase-7. Therefore, we examined, whether the extent of caspase-8 cleavage is also influenced by preincubation with IFN and ribavirin by using immunoblot experiments for caspase-8. Uncleaved procaspase-8 is detected as a 53/55-kDa protein. Activation results in the processing into an intermediate 41/43-kDa fragment that is subsequently cleaved into an 18-kDa fragment. Stimulation with anti-CD95 alone induced only a moderate activation of caspase-8 (Fig. 8C, lane 2). In agreement with the activation of caspase-3 and caspase-7, addition of CHX increased the processing of caspase-8 (lane 1). Preincubation with both IFN and ribavirin also enhanced the appearance of the intermediate cleavage products p41/p43 (lanes 4 to 7) and further increased the activity of caspase-8, as indicated by the additional cleavage product p18 (lanes 8 and 9). No cleavage of caspase-8 was detected after incubation with IFN, ribavirin, or the combination for 24 h in the absence of anti-CD95 (data not shown).
These data demonstrate that the activation of the initiator caspase-8 is enhanced by preincubation with either IFN or ribavirin and that the enhancement is more pronounced by the combination of the two. Thus, preincubation with IFN and/or ribavirin promotes the activation of the initiator caspase-8 as well as the activation of the executioner caspase-3 and caspase-7. These effects on caspase activity correlate with enhanced degradation of the caspase substrate PARP and with enhanced apoptosis.
DISCUSSION
Combination therapy with ribavirin and IFN is the current standard treatment of chronic hepatitis C. Compared to monotherapy with IFN, this regimen has increased the virus elimination rate from 15 to 40%. However, the molecular mechanisms of this effect are poorly understood. With respect to ribavirin, direct antiviral effects have been described; nevertheless, they do not seem to fully explain the range of biological effects being observed. Apart from direct antiviral effects, antiviral compounds could also interfere with host factors and thereby determine the ability of the host to eliminate the virus. Elements that influence the host response to a virus include not only cellular and humoral components of the immune system but also the functional state of the infected target cells when they are attacked by the immune system. Therefore the question posed in the present study was whether—independently of the presence or absence of viral infection—the antiviral substances IFN and ribavirin are able to modulate the functional state of cells with respect to inducibility of apoptosis. Since many viruses have evolved mechanisms that inhibit apoptosis, the opposite, namely, promotion of apoptosis, could be a successful mechanism by which a pharmaceutical compound strengthens the host antiviral response. IFN has a clinically proven antiproliferative potency in hairy cell leukemia, melanoma, chronic myeloic leukemia, and lymphomas (28) and eventually also in hepatocellular carcinoma and other neoplasias (18). However, it is not established whether modulation of apoptosis is part of the “antiproliferative” potency of IFN. In cells, infected with RNA viruses, IFN induces the double-stranded RNA-dependent serine/threonine protein kinase PKR, a key molecule with respect to the antiviral potency of IFN (10). Apart from this direct antiviral potency, cells overexpressing PKR are more sensitive to apoptosis (1), and it has been demonstrated that PKR can induce PARP cleavage (17) and an upregulation of CD95 mRNA in NIH 3T3 cells (8). Following this line and considering that infected cells are likely to be attacked by CD95L-expressing immune cells, we have asked whether the antiviral substances IFN and ribavirin could modulate CD95-mediated apoptosis in the absence of virus infection and PKR expression.
In our experiments, we have shown that IFN and ribavirin induce apoptosis to a moderate extent and, furthermore, that they are able to sensitize hepatoma cells for apoptosis induction by anti-CD95. Thus, we demonstrate an additional apoptosis-promoting effect of IFN and ribavirin that might be functional in host cells. How can IFN and ribavirin induce or promote apoptosis? Based on the very sensitive fluorometric assay, we provide evidence for caspase activation in response to IFN and ribavirin in the absence of CD95 stimulation. The caspase activity was higher when IFN and ribavirin were given in combination than when each substance was used separately. However, neither incubation with the single substances nor incubation with the combination led to visible cleavage products in the less sensitive Western blot assay. Therefore, we consider the caspase activity under these conditions to be moderate. Stimulation with anti-CD95 alone induced also only limited apoptosis and levels of caspase activity that were detectable fluorometrically but not in the immunoblot assay. In contrast, when anti-CD95 was applied after pretreatment with either IFN, ribavirin, or the combination of the two, caspase activity was elevated to a level that was detectable by immunoblot analysis, particularly when the combination was used. The positive effect of IFN and ribavirin on caspase cleavage and activation correlated with increased cleavage of the caspase substrate PARP and with an increased frequency of apoptosis, demonstrating that the activated caspases are functional in the cellular context. In the presence of zVAD, an unselective irreversible caspase inhibitor, neither caspase activity nor apoptosis was detected. Thus, moderate activation of caspases might be a mechanism by which IFN and ribavirin support apoptosis induced by other moderate caspase activators such as anti-CD95 in our example.
Starting at doses of 25 to 50 μM ribavirin, the response to anti-CD95 was clearly increased with respect to caspase activation. These doses are close to ribavirin levels in the serum of patients treated with ribavirin (6). Moreover, since ribavirin is actively transported into cells (13), the intracellular concentrations might be even higher. With respect to IFN, rather high doses were required in our experiments to induce biological effects. On the other hand, the density and sensitivity of IFN receptors might be suboptimal under our culture conditions and extended incubation times could possibly lower the required IFN dose.
We further analyzed single elements of the death receptor-dependent pathway of caspase activation. The experiments revealed that the enhanced appearance of caspase cleavage products is similar for the executioner caspase-3 and caspase-7 and for the initiator caspase-8. This suggests that the initial event induced by pretreatment with IFN and ribavirin is located upstream of caspase activation. However, the exact nature of the initial event remains to be established. Recent data support the possibility that IFN acts on the death-inducing signaling complex, which is composed of the cytoplasmic part of CD95, Fas-associated death domain, and procaspase-8 (1). Ribavirin might affect signal transduction pathways by exerting nonspecific effects on cell proliferation and protein synthesis. However, the minimal ribavirin dose needed to significantly affect cell proliferation and protein synthesis was 500 μM after 24 h and 60 μM after 72 h in primary human hepatocytes (14). In our experiments, we observed effects after 24 h when starting at doses of 25 μM, indicating that these effects might be independent of antiproliferative effects. Furthermore, other reports support the concept that low doses of ribavirin can influence intracellular signal transduction. Thus, ribavirin promotes a shift toward a Th1 cytokine profile at doses between 1 and 10 μM (22, 26, 35) and ribavirin at 20 μM was able to induce mediator release from mast cells (21).
In summary, our data demonstrate that the antiviral substances IFN and ribavirin are able to induce apoptosis and to sensitize for apoptosis mediated by the death receptor CD95. The sensitization to CD95 stimulation by the antiviral agents might be of especial importance since binding of CD95L to CD95 is one of the two major pathways used by CTLs to kill their target cells. Therefore, such a mechanism could enhance the lymphocytic response of an organism to virus-infected or malignant cells.
Acknowledgments
We thank B. Yurbas for excellent technical assistance.
This work was supported in part by grants from the DFG to S. F. Schlosser (Schl 403/2-1) and from the Federal Ministry of Education, Science, Research and Technology (Fö. 01KS9602) and the Interdisciplinary Center of Clinical Research (IZKF) of the Universities of Tübingen to M. Schuler and K. Lauber.
REFERENCES
- 1.Balachandran, S., P. C. Roberts, T. Kipperman, K. N. Bhalla, R. W. Compans, D. R. Archer, and G. N. Barber. 2000. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J. Virol. 74:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belka, C., J. Rudner, S. Wesselborg, A. Stepczynska, P. Marini, A. Lepple-Wienhues, H. Faltin, M. Bamberg, W. Budach, and K. Schulze-Osthoff. 2000. Differential role of caspase-8 and BID activation during radiation- and CD95-induced apoptosis. Oncogene 19:1181-1190. [DOI] [PubMed] [Google Scholar]
- 3.Bump, N. J., M. Hackett, M. Hugunin, S. Seshagiri, K. Brady, P. Chen, C. Ferenz, S. Franklin, T. Ghayur, P. Li, et al. 1995. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science 269:1885-1888. [DOI] [PubMed] [Google Scholar]
- 4.Cerny, A., and F. V. Chisari. 1999. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 30:595-601. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326: 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor, J. D., M. Hintz, R. VanDyke, J. B. McCormick, and K. McIntosh. 1984. Ribavirin pharmacokinetics in children and adults during therapeutic trials, p. 107-123. In R. A. Smith, V. Knight, and J. A. D. Smith (ed.), Clinical applications of ribavirin. Academic press, Inc., Orlando, Fla.
- 7.Davis, G. L., R. Esteban-Mur, V. Rustgi, J. Hoefs, S. C. Gordon, C. Trepo, M. L. Shiffman, S. Zeuzem, A. Craxi, M. H. Ling, J. Albrecht, and the International Hepatitis Interventional Therapy Group. 1998. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N. Engl. J. Med. 339:1493-1499. [DOI] [PubMed] [Google Scholar]
- 8.Donze, O., J. Dostie, and N. Sonenberg. 1999. Regulatable expression of the interferon-induced double-stranded RNA dependent protein kinase PKR induces apoptosis and fas receptor expression. Virology 256:322-329. [DOI] [PubMed] [Google Scholar]
- 9.Dumoulin, F. L., A. van dem Bussche, J. Sohne, T. Sauerbruch, and U. Spengler. 1999. Hepatitis C virus core protein does not inhibit apoptosis in human hepatoma cells. Eur. J. Clin. Investig. 29:940-946. [DOI] [PubMed] [Google Scholar]
- 10.Gale, M., Jr., and M. G. Katze. 1998. Molecular mechanisms of interferon resistance mediated by viral- directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol. Ther. 78:29-46. [DOI] [PubMed] [Google Scholar]
- 11.Gale, M., Jr., B. Kwieciszewski, M. Dossett, H. Nakao, and M. G. Katze. 1999. Antiapoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase. J. Virol. 73:6506-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 13.Glue, P. 1999. The clinical pharmacology of ribavirin. Semin. Liver Dis. 19(Suppl. 1):17-24. [PubMed] [Google Scholar]
- 14.Ilyin, G. P., S. Langouet, M. Rissel, J. G. Delcros, A. Guillouzo, and C. Guguen-Guillouzo. 1998. Ribavirin inhibits protein synthesis and cell proliferation induced by mitogenic factors in primary human and rat hepatocytes. Hepatology 27:1687-1694. [DOI] [PubMed] [Google Scholar]
- 15.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 16.Lee, C. K., H. A. Bluyssen, and D. E. Levy. 1997. Regulation of interferon-alpha responsiveness by the duration of Janus kinase activity. J. Biol. Chem. 272:21872-21877. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. B., D. Rodriguez, J. R. Rodriguez, and M. Esteban. 1997. The apoptosis pathway triggered by the interferon-induced protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2, and involves ICE-like proteases. Virology 231:81-88. [DOI] [PubMed] [Google Scholar]
- 18.Llovet, J. M., M. Sala, L. Castells, Y. Suarez, R. Vilana, L. Bianchi, C. Ayuso, V. Vargas, J. Rodes, and J. Bruix. 2000. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology 31:54-58. [DOI] [PubMed] [Google Scholar]
- 19.Los, M., S. Wesselborg, and K. Schulze-Osthoff. 1999. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity 10:629-639. [DOI] [PubMed] [Google Scholar]
- 20.Lowe, S. W., E. M. Schmitt, S. W. Smith, B. A. Osborne, and T. Jacks. 1993. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362:847-849. [DOI] [PubMed] [Google Scholar]
- 21.Marquardt, D. L., H. E. Gruber, and L. L. Walker. 1987. Ribavirin inhibits mast cell mediator release. J. Pharmacol. Exp. Ther. 240:145-149. [PubMed] [Google Scholar]
- 22.Martin, J., S. Navas, J. A. Quiroga, M. Pardo, and V. Carreno. 1998. Effects of the ribavirin-interferon alpha combination on cultured peripheral blood mononuclear cells from chronic hepatitis C patients. Cytokine 10:635-644. [DOI] [PubMed] [Google Scholar]
- 23.Marusawa, H., M. Hijikata, T. Chiba, and K. Shimotohno. 1999. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-κB activation. J. Virol. 73:4713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, J. K. Albrecht, and the Hepatitis Interventional Therapy Group. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 25.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 26.Ning, Q., D. Brown, J. Parodo, M. Cattral, R. Gorczynski, E. Cole, L. Fung, J. W. Ding, M. F. Liu, O. Rotstein, M. J. Phillips, and G. Levy. 1998. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J. Immunol. 160:3487-3493. [PubMed] [Google Scholar]
- 27.Patterson, J. L., and R. Fernandez-Larsson. 1990. Molecular mechanisms of action of ribavirin. Rev. Infect. Dis. 12:1139-1146. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer, L. M., C. A. Dinarello, R. B. Herberman, B. R. Williams, E. C. Borden, R. Bordens, M. R. Walter, T. L. Nagabhushan, P. P. Trotta, and S. Pestka. 1998. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 58:2489-2499. [PubMed] [Google Scholar]
- 29.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, J. Albrecht, and the International Hepatitis Interventional Therapy Group. 1998. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 30.Ray, R. B., K. Meyer, R. Steele, A. Shrivastava, B. B. Aggarwal, and R. Ray. 1998. Inhibition of tumor necrosis factor (TNF-alpha)-mediated apoptosis by hepatitis C virus core protein. J. Biol. Chem. 273:2256-2259. [DOI] [PubMed] [Google Scholar]
- 31.Rehermann, B., K. M. Chang, J. McHutchinson, R. Kokka, M. Houghton, C. M. Rice, and F. V. Chisari. 1996. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J. Virol. 70:7092-7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruggieri, A., T. Harada, Y. Matsuura, and T. Miyamura. 1997. Sensitization to Fas-mediated apoptosis by hepatitis C virus core protein. Virology 229:68-76. [DOI] [PubMed] [Google Scholar]
- 33.Schulze-Osthoff, K., D. Ferrari, M. Los, S. Wesselborg, and M. E. Peter. 1998. Apoptosis signaling by death receptors. Eur. J. Biochem. 254:439-459. [DOI] [PubMed] [Google Scholar]
- 34.Spriggs, M. K. 1996. One step ahead of the game: viral immunomodulatory molecules. Annu. Rev. Immunol. 14:101-130. [DOI] [PubMed] [Google Scholar]
- 35.Tam, R. C., B. Pai, J. Bard, C. Lim, D. R. Averett, U. T. Phan, and T. Milovanovic. 1999. Ribavirin polarizes human T cell responses towards a type 1 cytokine profile. J. Hepatol. 30:376-382. [DOI] [PubMed] [Google Scholar]
- 36.Tan, S. L., and M. G. Katze. 1999. The emerging role of the interferon-induced PKR protein kinase as an apoptotic effector: a new face of death? J. Interferon Cytokine Res. 19:543-554. [DOI] [PubMed] [Google Scholar]
- 37.Tewari, M., L. T. Quan, K. O'Rourke, S. Desnoyers, Z. Zeng, D. R. Beidler, G. G. Poirier, G. S. Salvesen, and V. M. Dixit. 1995. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81:801-809. [DOI] [PubMed] [Google Scholar]
- 38.Thomis, D. C., and C. E. Samuel. 1992. Mechanism of interferon action: autoregulation of RNA-dependent P1/eIF-2 alpha protein kinase (PKR) expression in transfected mammalian cells. Proc. Natl. Acad. Sci. USA 89:10837-10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wesselborg, S., I. H. Engels, E. Rossmann, M. Los, and K. Schulze-Osthoff. 1999. Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood 93:3053-3063. [PubMed] [Google Scholar]
- 40.Yeung, M. C., J. Liu, and A. S. Lau. 1996. An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc. Natl. Acad. Sci. USA 93:12451-12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, N., A. Khoshnan, R. Schneider, M. Matsumoto, G. Dennert, C. Ware, and M. M. Lai. 1998. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J. Virol. 72:3691-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]