Abstract
Linezolid, an oxazolidinone antibiotic, has 100% oral bioavailability and favorable activities against gram-positive pathogens including multidrug-resistant staphylococci, enterococci, and pneumococci. Safety assessments were conducted for 2,046 linezolid-treated patients and 2,001 comparator drug-treated patients from seven controlled clinical trials comparing the activities of linezolid and comparator drugs against nosocomial and community-acquired pneumonia, skin and skin structure infections, and methicillin-resistant staphylococcal infections. Drug-related adverse events were primarily transient. The most frequent (≥2%) adverse events caused by linezolid and the comparator drugs were diarrhea (4.3 and 3.2%, respectively; P = 0.074), nausea (3.4 and 2.3%, respectively; P = 0.036), and headache (2.2 and 1.3%, respectively; P = 0.047). Treatment discontinuations due to drug-related events (2.4 and 1.9%, respectively), serious adverse events (11.4 and 10.6%, respectively), and deaths (4.8 and 4.9%, respectively) were similar. No clinically significant drug-related hematologic events were reported, and laboratory safety data were comparable. In the first 6 months of postmarketing surveillance, hematologic abnormalities were reported in 0.1% of linezolid-treated patients, but no irreversible blood dyscrasias were documented. The risk for transient, reversible hematologic effects from treatment with linezolid should be considered together with the clinical benefits associated with its use.
Linezolid is the first available agent in the oxazolidinone class of antibiotics (6). It has significant in vitro activities against both drug-susceptible and multidrug-resistant gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), homogeneous and heterogeneous glycopeptide-resistant (and vancomycin-resistant) S. aureus, vancomycin-resistant S. aureus strains containing the vanA gene, vancomycin-resistant enterococcal species, and drug-resistant (penicillin- and macrolide-resistant) Streptococcus pneumoniae (7, 8, 9, 11, 29, 31, 32; data on file, Pharmacia Corp., 2000; S. K. Cammarata, L. K. Schueman, J. A. Timm, K. A. Hempsall, W. Chang, T. H. Oliphant, W. M. Todd, and B. Hafkin, Am. Thoracic Soc., abstr. E73, 2000; S. K. Cammarata, G. S. San Pedro, J. A. Timm, K. A. Hempsall, W. M. Todd, T. H. Oliphant, and B. Hafkin, Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. 237, 2000; S. E. Duvall, C. Seas, J. B. Bruss, M. A. McConnell-Martin, W. M. Todd, and B. Hafkin, 9th Int. Congr. Infect. Dis., abstr. 80.005, 2000). Linezolid has a unique mechanism of action via the inhibition of protein synthesis initiation at the 50S ribosome (27, 30, 32).
Linezolid is approved for use in more than 50 countries worldwide for the treatment of nosocomial and community-acquired pneumonia, complicated and uncomplicated skin and skin structure infections, and infections caused by MRSA and vancomycin-resistant Enterococcus faecium, including bacteremia associated with these infections (Zyvox package insert; Pharmacia & Upjohn Company, Kalamazoo, Mich., 2001). Preclinical toxicology studies showed mild reversible hematopoietic and hepatic alterations related to the dose and duration of exposure to linezolid. The most frequently reported adverse events in phase I studies included headache, rash, diarrhea, nausea, and tongue discoloration, all of which resolved after linezolid withdrawal (S. D. Pawsey, P. T. Daley-Yates, C. P. Wajszczuk, and D. J. Stalker, Eur. Congr. Antimicrob. Chemother., abstr. F151, 1996; D. J. Stalker, C. P. Wajszczuk, and D. H. Batts, 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-116, 1997; D. J. Stalker, C. P. Wajszczuk, and D. H. Batts, 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-115, 1997). Linezolid (up to 1,250 mg/day) was also well tolerated in four open-label, uncontrolled, phase II dose-finding trials, with diarrhea (4.7%), nausea (4.7%), headache (3.2%), and tongue discoloration (2.2%) being the most commonly reported drug-related adverse events. In two uncontrolled phase II studies with pediatric patients (≤12 years old), linezolid (10 mg/kg of body weight twice daily for 7 to 10 days) was well tolerated, with diarrhea, loose stools, vomiting, and rash being the most commonly reported adverse events (20; D. L. Fleishaker, D. C. Anderson, J. B. Bruss, W. H. Chang, W. M. Todd, and B. Hafkin, 38th Meet. Infect. Dis. Soc. Am., abstr. 65, 2000). There was no evidence of a dose-response relationship for adverse events when the data from phase II trials with adults who received doses of <1 g daily for 7 to 14 days and those who received 1 to 1.25 g daily for 7 to 14 days were compared. Therefore, on the basis of the improved efficacy observed with the 600-mg twice-daily dose, this dose regimen was selected for use in the pivotal clinical trials (S. K. Cammarata, B. Hafkin, W. M. Todd, and D. H. Batts, Am. Thoracic Soc., abstr. C98, 1998; S. K. Cammarata, B. Hafkin, D. M. Demke, S. M. Eckert, and D. H. Batts, 9th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. P181, 1999).
MATERIALS AND METHODS
Safety information was collected before, during, and after treatment in seven multicenter, multinational, comparator drug-controlled clinical trials with adult patients with gram-positive bacterial infections (Table 1). Safety data were pooled across these trials. Data for all patients in these trials who received at least one dose of study medication (intent-to-treat patient subgroup) were included in these analyses. Safety evaluations included overall adverse events, drug-related adverse events (relatedness was assessed by the investigator), drug-related adverse events leading to study medication discontinuation, serious adverse events, and deaths. Adverse events were defined as those reported by the patient, observed by the investigator, or elicited by a standard question at each visit (“Since the last study visit, have you had any health problems?”). Adverse events were recorded descriptively and were then coded systematically by using the Coding Symbols for Thesaurus of Adverse Reaction Terms nomenclature (26). Standard laboratory hematology and serum chemistry assays were performed at a central laboratory by using standardized reference ranges. Ranges of values for all laboratory assays were prospectively defined to identify patients with outlying values during the study, with an adjustment for abnormal baseline values. Data from eight laboratory assays are summarized here. For hematology assays, outliers were those patients whose normal baseline values fell to <75% of the lower limit of the normal range (LLN) for hemoglobin, platelets, or white blood cells (WBCs) or <50% of the LLN for neutrophils. For patients with abnormal baseline hematology values, outliers fell to <90% of the baseline values for hemoglobin, <75% of the baseline values for platelets or WBCs, or <50% of the baseline values for neutrophils. For serum chemistry assays, outliers were those patients whose alanine aminotransferase (ALT), aspartate aminotransferase (AST), amylase, or lipase values were normal at the baseline and rose to more than two times the upper limit of the normal range (ULN). For patients with abnormal baseline values, outliers were those whose assay values rose to more than two times their baseline values. P values were obtained for differences in proportions between the linezolid and comparator groups by using a stratified Cochran-Mantel-Haenszel test, with the seven studies serving as the strata.
TABLE 1.
Summary of comparator-controlled phase III clinical trials of linezolid
| Infection and trial description | Treatment regimen | No. of patients | Reference |
|---|---|---|---|
| Nosocomial pneumonia, double-blind, randomized; treatment duration, 7-21 daysa | Linezolid, 600 mg i.v. every 12 h | 203 | 27 |
| Vancomycin, 1 g i.v. every 12 h | 193 | ||
| Community-acquired pneumonia | |||
| Outpatient, investigator-blind, randomized; treatment duration, 10 to 14 days | Linezolid, 600 mg orally every 12 h | 274 | Cammarata et al. Am. Thoracic Soc., abstr. E73, 2000 |
| Cefpodoxime, 200 mg orally every 12 h | 266 | ||
| Inpatient, open-label, randomized, treatment duration 7-14 daysa | Linezolid, 600 mg i.v. BIDb and then 600 mg orally every 12 h | 381 | 28 |
| Ceftriaxone, 1 g i.v. BID and then cefpodoxime 200 mg orally every 12 h | 366 | ||
| Skin and soft tissue infections | |||
| Complicated, double-blind, randomized, treatment duration, 10-21 daysa | Linezolid, 600 mg i.v. every 12 h and then 600 mg orally 12 h | 400 | 31 |
| Oxacillin, 2 g every 6 h and then dicloxacillin 500 mg every 6 h | 419 | ||
| Uncomplicated, double-blind, randomized; treatment duration, 7-14 days | Linezolid, 400 mg orally every 12 h | 382 | Data on file, Pharmacia Corp., 2000 |
| Clarithromycin, 250 mg orally every 12 h | 371 | ||
| Uncomplicated, double-blind, randomized; treatment duration, 7-14 days | Linezolid, 400 mg orally every 12 h | 166 | Duvall et al. Int. Cong. Infect. Dis., abstr. 80.005, 2000 |
| Clarithromycin, 250 mg orally every 12 h | 166 | ||
| Methicillin-resistant staphylococcal species, open-label, randomized; treatment duration, 7 to 28 daysa | Linezolid, 600 mg i.v. every 12 h and then 600 mg orally every 12 h | 240 | 30 |
| Vancomycin, 1 g i.v. every 12 h | 220 |
The treatment regimen also included optional aztreonam at 1 to 2 g three times daily.
BID, twice a day.
RESULTS
Patient demographics.
Data for a total of 4,047 patients (2,046 linezolid-treated patients, 2,001 comparator drug-treated patients) from seven comparator-controlled phase III clinical studies (17, 27, 28, 30, 31; data on file, Pharmacia Corp., 2000; Cammarata et al., Am. Thoracic Soc., abstr. E73, 2000; Duvall et al., 9th Int. Congr. Infect. Dis., abstr. 80.005, 2000) are included in this analysis. Pooled data for baseline characteristics in the linezolid and comparator groups are summarized in Table 2. The linezolid and comparator groups were similar with respect to age, sex, race, presenting infection, and geographic distribution. Specifically, the distributions by indication (presenting infection) were similar for linezolid- and comparator drug-treated patients; overall, 44% of patients had pneumonia, 53% had skin and skin structure infections, and 3% had other infections, such as catheter-related infections and bacteremia of unknown source.
TABLE 2.
Demographic data for all patients
| Characteristic | Linezolid- treated patients (n = 2,046)
|
Comparator-treated patientsa (n = 2,001)
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Sex | ||||
| Male | 1,212 | 59.2 | 1,152 | 57.6 |
| Female | 834 | 40.8 | 849 | 42.4 |
| Age (yr)b | ||||
| <18 | 10 | 0.5 | 8 | 0.4 |
| 18 to 44 | 816 | 39.9 | 814 | 40.7 |
| 45 to 64 | 631 | 30.8 | 602 | 30.1 |
| ≥65 | 589 | 28.8 | 577 | 28.8 |
| Race | ||||
| White | 1,453 | 71.0 | 1,421 | 71.0 |
| Black | 207 | 10.1 | 223 | 11.1 |
| Asian or Pacific Islander | 125 | 6.1 | 136 | 6.8 |
| Otherc | 261 | 12.8 | 221 | 11.0 |
| Presenting infection | ||||
| Pneumonia | 908 | 44.4 | 874 | 43.7 |
| Skin or soft tissue | 1,070 | 52.3 | 1,064 | 53.2 |
| Other | 68 | 3.3 | 63 | 3.1 |
| Geographic regiond | ||||
| North America | 933 | 45.6 | 926 | 46.3 |
| Latin America | 343 | 16.8 | 321 | 16.0 |
| Europe | 652 | 31.9 | 635 | 31.7 |
| Asia and Pacific | 118 | 5.8 | 119 | 5.9 |
For all comparator-treated patients, the treatments consisted of ceftriaxone and cefpodoxime (n = 366), cefpodoxime (n = 266), vancomycin (n = 413), clarithromycin (n = 537), and oxacillin and dicloxacillin (n = 419).
The mean ± SD ages for the linezolid and comparator-treated patients were 51.0 ± 19.2 and 50.8 ± 19.5 years, respectively.
Includes “not allowed to ask,” “mixed,” and “missing” responses.
North America, United States and Canada; Latin America, Mexico and South America; Europe, Europe, Israel, South Africa, and Australia.
Studies designed to use only intravenous (i.v.) treatment enrolled more severely ill patients with nosocomial pneumonia; conversely, studies designed to use only oral treatment enrolled patients with uncomplicated skin infections or outpatients with community-acquired pneumonia. Three studies with hospitalized patients with suspected MRSA infections, community-acquired pneumonia, or complicated skin infections allowed initial i.v. treatment with a switch to oral follow-up therapy at the physician's discretion. Overall, 839 patients (41%) received i.v.-to-oral linezolid therapy, 822 patients (40%) received oral therapy only, and 385 patients (19%) received i.v. therapy only. The mean ± standard deviation (SD) durations of total treatment were comparable in both groups (11.6 ± 4.9 days for linezolid-treated patients, 11.6 ± 4.8 days for comparator drug-treated patients). The mean durations of i.v. treatment were 5.8 ± 4.1 days for patients receiving linezolid and 6.7 ± 4.9 days for the comparator group. The corresponding values for oral treatment were 10.4 ± 4.1 and 10.7 ± 3.9 days, respectively. A total of 75% of patients treated with linezolid received therapy for <14 days.
Reasons for discontinuation of study medication during the trial were similar between linezolid-treated and comparator drug-treated patients. The most frequently reported reasons for patients to discontinue the study medication in both the linezolid and comparator groups were nonserious adverse events (3.3 and 2.3%, respectively; P = 0.062), lack of efficacy (3.1 and 3.5%, respectively; P = 0.443), and ineligibility after starting the study medication (2.8 and 3.1%, respectively; P = 0.407).
Adverse events.
The frequency of adverse events by category is shown in Table 3. Adverse events and drug-related adverse events over the entire study period (treatment and follow-up) occurred at a higher frequency in the linezolid group than in the comparator group. The frequencies of adverse events resulting in study drug discontinuation, serious adverse events, and deaths were comparable between the linezolid and comparator groups.
TABLE 3.
Overall summary of adverse events by categorya
| Adverse event category | Linezolid- treated patients (n = 2,046)
|
Comparator- treated patients (n = 2,001)
|
P value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Adverse events | |||||
| Patients with one or more AEs | 1,137 | 55.6 | 988 | 49.4 | 0.001 |
| Drug discontinued due to AEs | 118 | 5.8 | 105 | 5.2 | 0.489 |
| Drug-related adverse events | |||||
| Patients with one or more DRAEs | 444 | 21.7 | 314 | 15.7 | 0.001 |
| Drug discontinued due to DRAEs | 50 | 2.4 | 38 | 1.9 | 0.230 |
| Serious adverse events | |||||
| Patients with one or more SAEs | 233 | 11.4 | 212 | 10.6 | 0.568 |
| Patients who died | 98 | 4.8 | 99 | 4.9 | 0.596 |
Abbreviations: AEs, adverse events; DRAEs, drug-related adverse events; SAEs, serious adverse events.
Irrespective of relationship to study medication, the most frequently reported adverse events in linezolid- and comparator drug-treated patients were diarrhea (8.3 and 6.3%, respectively; P = 0.018), headache (6.5 and 5.5%, respectively; P = 0.155), and nausea (6.2 and 4.6%, respectively; P = 0.024). However, when the adverse event was related to the investigator's assessment of the relationship to the study medication, these adverse event rates were reduced by half (Table 4). The most common drug-related adverse events associated with both linezolid and comparator agents remained diarrhea (4.3 and 3.2%, respectively; P = 0.074), nausea (3.4 and 2.3%, respectively; P = 0.036), and headache (2.2 and 1.3%, respectively; P = 0.047). Table 4 summarizes the drug-related adverse events reported for ≥1% of the patients in the linezolid and comparator groups, along with the corresponding frequency of drug discontinuation due to these events. Other potentially drug-related adverse events that were reported in >0.1% but <1% of linezolid-treated patients included abdominal pain, chills, fatigue, fungal infections (moniliasis), localized pain, injection or vascular catheter site pain, injection or vascular catheter site phlebitis or thrombophlebitis, increase in serum creatine phosphokinase levels, increase in ALT levels, hypertension, tongue complaints, dry mouth, dyspepsia, glossitis, stomatitis, tongue discoloration, eosinophilia, thrombocytopenia, increased amylase or lipase levels, dizziness, insomnia, paresthesia, diaphoresis, pruritus, and rash. None of the drug-related adverse events that led to drug discontinuation occurred in more than 1% of patients (Table 4).
TABLE 4.
Drug-related adverse events reported for ≥1% of patients in either group and associated rate of drug discontinuation
| Adverse event | Linezolid-treated patients (n = 2,046)
|
Comparator-treated patients (n = 2,001)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Drug-related adverse events
|
Rate of drug discontinuation
|
Drug-related adverse events
|
Rate of drug discontinuation
|
|||||
| No. | %a | No. | % | No. | %a | No. | % | |
| Patients with at least one | 444 | 21.7 | 50 | 2.4 | 314 | 15.7 | 38 | 1.9 |
| Diarrhea | 89 | 4.3 | 6 | 0.3 | 65 | 3.2 | 4 | 0.2 |
| Nausea | 69 | 3.4 | 11 | 0.5 | 46 | 2.3 | 3 | 0.1 |
| Headache | 44 | 2.2 | 8 | 0.4 | 27 | 1.3 | 1 | <0.1 |
| Taste perversionb | 24 | 1.2 | 0 | 0 | 14 | 0.7 | 1 | <0.1 |
| Moniliasis, vaginal | 24 | 1.2 | 0 | 0 | 13 | 0.6 | 0 | 0 |
| Vomiting | 23 | 1.1 | 6 | 0.3 | 8 | 0.4 | 3 | 0.1 |
| Liver function test abnormality | 21 | 1.0 | 3 | 0.1 | 7 | 0.3 | 1 | <0.1 |
Percentages are based on the number of patients reporting an adverse event. Patients are counted once per adverse event.
Bitter taste reported.
Drug-related adverse events in linezolid-treated patients were also assessed by route of administration. The most common events (≥2% of patients) were diarrhea, nausea, and headache regardless of the route of administration. Linezolid was well tolerated when it was administered i.v., with low rates (<1%) of individual i.v. catheter-related adverse events such as phlebitis, inflammation, and edema, which were comparable to those seen in patients treated i.v. with the comparator drugs (ceftriaxone, oxacillin, and vancomycin) (S. K. Cammarata, V. Le, K. A. Hempsall, T. H. Oliphant, and B. Hafkin, 38th Meet. Infect. Dis. Soc, abstr. 69, 2000).
Oral antibiotic treatment is frequently associated with higher rates of gastrointestinal adverse events compared with the rates of adverse events after i.v. treatment, including an increased risk of Clostridium difficile overgrowth and resulting complications. Since more linezolid-treated patients received oral antibiotic therapy, higher rates of diarrhea were expected in the linezolid groups than in the comparator groups (8.3 and 6.3%, respectively), and thus, adverse event data were examined by using Coding Symbols for Thesaurus of Adverse Reaction Terms terminology for C. difficile colitis, C. difficile diarrhea, pseudomembranous colitis, and evidence of C. difficile toxin in stool. Overall, the rates of C. difficile-related complications were similar between the linezolid and the comparator groups. C. difficile was reported in 0.2% of the linezolid-treated patients and 0.4% of the comparator drug-treated patients. C. difficile diarrhea or colitis was reported in six comparator drug-treated patients and none of the linezolid-treated patients. Pseudomembranous colitis was reported for 1 (0.05%) patient in each of the linezolid and comparator groups. C. difficile toxin was detected in the stools of three (0.15%) linezolid-treated patients and one (0.05%) comparator drug-treated patient (S. K. Cammarata, V. H. Le, T. H. Oliphant, W. M. Todd, and B. Hafkin, 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 947, 2000).
The difference in the rates of serious adverse events between the linezolid and comparator groups was not statistically significant across the seven studies (Table 3). By definition, serious adverse events were life-threatening, required prolonged hospitalization, resulted in persistent or significant disability, or required intervention to prevent one of these outcomes. The only serious adverse event reported in 1% or more of the patients in the linezolid and comparator groups was pneumonia, which was reported in 1.3% (26 of 2,046) of linezolid-treated patients and 1.2% (24 of 2,001) of comparator drug-treated patients. Deaths occurred in 5% of patients in each group; none of the deaths in linezolid-treated patients were related to treatment with study medication but were the result of underlying comorbidities, as assessed by the investigator.
In the linezolid and comparator groups, 0.4% of all patients had a serious adverse event that was judged by the investigator to be potentially related to the study medication (8 of 2,046 linezolid-treated patients, 9 of 2,001 comparator drug-treated patients). The drug was discontinued in five linezolid-treated patients as a result of the events (thrombocytopenia, hypertension, severe vomiting, transient ischemic attack, and abnormal liver function test [one patient each]); these events resolved in all five patients after drug discontinuation. The remaining events judged to be potentially related to linezolid without resulting in drug discontinuation were pancreatitis in two patients and renal failure in one patient. One of the patients with pancreatitis recovered; the other two patients died as a result of underlying comorbidities (aspiration in an intubated patient with MRSA pneumonia; hydronephrosis and severe hypotension in a patient with a complicated MRSA skin infection and a history of acute renal failure). In the comparator group, nine patients had 11 serious drug-related adverse events. These included three patients with allergic reaction (vancomycin), anaphylaxis (vancomycin), or drug fever and abdominal cramps (oxacillin-dicloxacillin) and two patients with hepatitis (oxacillin-dicloxacillin). In these five patients, the study drug was discontinued and the events resolved. Study medication was not discontinued in the remaining four comparator drug-treated patients. One patient treated with cephalosporins had pseudomembranous colitis, which resolved. Three patients treated with vancomycin had reports of thrombocytopenia, kidney failure, or nephritis and C. difficile infection; these events were ongoing at the time that each patient died as a result of sepsis or respiratory failure.
Laboratory abnormalities.
Clinical chemistry and hematology data were assessed by comparing mean values and the change from the baseline value during treatment and follow-up for each group. There were no significant differences between the linezolid and the comparator groups when these assay data were analyzed overall or by age, sex, race, or change from the baseline. In addition, patients with outlying values were identified by using prospectively defined limits for each assay that included an adjustment for abnormal baseline values. Data for patients who had outlying values at any time during the study were further analyzed by using cumulative percentage plots.
Clinical chemistry findings overall were similar between the linezolid and comparator groups. Liver function assays, particularly mean ALT and AST values, remained within the normal range throughout the course of the studies (Table 5). The proportions of patients with outlying values (i.e., more than two times the ULN) were similar between the linezolid and comparator groups (Table 6). Changes in laboratory parameters were reversible after completion of therapy, and few patients discontinued therapy due to drug-related laboratory abnormalities (linezolid group, three patients [0.1%]; comparator group, four patients [0.2%]).
TABLE 5.
Selected laboratory assay values over time from comparator-controlled phase III clinical trials of linezolida
| Assay (units) | Baseline | Treatment days 1-7 | Posttreatment days:
|
|
|---|---|---|---|---|
| 1-7 | 15-21 | |||
| ALT (U/liter) | ||||
| Linezolid | 33.0 ± 59.9 | 38.4 ± 115.2 | 37.7 ± 60.7 | 28.4 ± 31.8 |
| Comparators | 34.4 ± 58.8 | 37.0 ± 74.4 | 35.1 ± 92.2 | 27.2 ± 29.4 |
| AST (U/liter) | ||||
| Linezolid | 33.3 ± 66.4 | 36.9 ± 180.8 | 32.2 ± 76.7 | 25.5 ± 21.2 |
| Comparators | 32.4 ± 45.2 | 34.4 ± 121.6 | 34.5 ± 152.2 | 25.7 ± 19.4 |
| Amylase (U/liter) | ||||
| Linezolid | 50.0 ± 36.7 | 54.9 ± 45.7 | 60.4 ± 31.8 | 58.5 ± 30.0 |
| Comparators | 52.9 ± 55.7 | 56.4 ± 39.5 | 61.5 ± 34.1 | 59.1 ± 32.9 |
| Lipase (U/liter) | ||||
| Linezolid | 83.6 ± 85.1 | 96.5 ± 103.4 | 99.6 ± 99.4 | 88.6 ± 70.1 |
| Comparators | 90.5 ± 134.0 | 105.0 ± 120.9 | 105.6 ± 120.3 | 88.3 ± 73.5 |
| Hemoglobin (g/dl) | ||||
| Linezolid | 12.93 ± 2.16 | 12.73 ± 2.12 | 13.06 ± 1.89 | 12.96 ± 1.94 |
| Comparators | 12.89 ± 2.26 | 12.75 ± 2.11 | 13.05 ± 2.03 | 13.18 ± 1.94 |
| Neutrophils (103/mm3) | ||||
| Linezolid | 7.86 ± 5.30 | 5.85 ± 4.11 | 4.93 ± 3.07 | 4.89 ± 3.04 |
| Comparators | 7.83 ± 5.04 | 5.77 ± 3.59 | 5.20 ± 3.68 | 4.71 ± 2.72 |
| Platelets (103/mm3) | ||||
| Linezolid | 260 ± 102 | 290 ± 116 | 284 ± 121 | 292 ± 99 |
| Comparators | 264 ± 111 | 292 ± 116 | 312 ± 121 | 261 ± 94 |
| WBCs (103/mm3) | ||||
| Linezolid | 10.75 ± 6.07 | 8.42 ± 4.45 | 7.54 ± 3.61 | 7.63 ± 3.38 |
| Comparators | 10.54 ± 5.78 | 8.44 ± 4.05 | 7.87 ± 4.03 | 7.47 ± 3.19 |
The values are means ± SDs.
TABLE 6.
Frequency of patients with outlying valuesa by selected laboratory assays for safety (corrected for baseline abnormalities)
| Laboratory assay | Linezolid- treated patients
|
Comparator- treated patientsb
|
P value | ||
|---|---|---|---|---|---|
| Frequencyc | % | Frequencyc | % | ||
| ALT (U/liter) | 145/1,959 | 7.4 | 139/1,919 | 7.2 | 0.874 |
| AST (U/liter) | 80/1,959 | 4.1 | 102/1,920 | 5.3 | 0.063 |
| Amylase (U/liter) | 36/2,024 | 1.8 | 30/1,983 | 1.5 | 0.548 |
| Lipase (U/liter) | 79/2,018 | 3.9 | 74/1,976 | 3.7 | 0.858 |
| Hemoglobin (g/dl) | 110/2,020 | 5.4 | 95/1,974 | 4.8 | 0.450 |
| Neutrophils (103/mm3) | 15/1,954 | 0.8 | 17/1,909 | 0.9 | 0.540 |
| Platelets (103/mm3) | 48/2,010 | 2.4 | 30/1,966 | 1.5 | 0.066 |
| WBCs (103/mm3) | 33/2,020 | 1.6 | 21/1,974 | 1.1 | 0.115 |
Outlying laboratory values were as follows: <0.75 times the LLN for hemoglobin, platelets, and WBCs; < 0.5 times the LLN for neutrophils; and more than two times the ULN for ALT, AST, amylase, and lipase.
For all comparator-treated patients, the treatments consisted of ceftriaxone and cefpodoxime (n = 366), cefpodoxime (n = 266), vancomycin (n = 413), clarithromycin (n = 537, and oxacillin and dicloxacillin (n = 419).
Total number of patients with an outlying value/total number of patients with at least one observation of the given laboratory parameters while in the study.
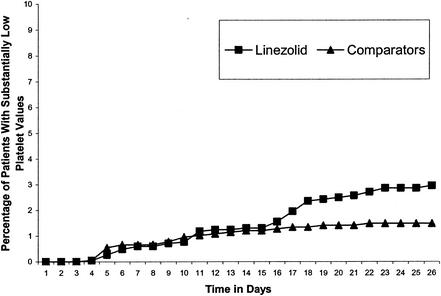
Hematology findings overall were also comparable between the linezolid and comparator groups. Mean values remained within normal ranges (Table 5), and there were no statistically significant differences between the linezolid and comparator groups with regard to the frequency of outlying values (Table 6) (G. Fierlbeck, S. Duvall, J. Bruss, M. Molinari, B. Winterhalter, and C. Grassi, Eur. Soc. Med. Oncol., abstr. 686P, 2000). Analyses showed essentially no difference between the linezolid and comparator groups in the cumulative percentage of patients with outlying hemoglobin, neutrophil, or platelet counts over the first 14 days of therapy. With longer treatment durations, there appeared to be a small increased risk for decreased platelet counts in linezolid-treated patients compared with the risk for the comparator group, but the difference was not statistically significant (Fig. 1). Low platelet counts in conjunction with bleeding-related adverse events (epistaxis, purpura, or ecchymosis) were reported for three patients in the linezolid group and none in the comparator group. These events were judged by the investigator to be mild to moderate in intensity, none were categorized as drug related, and all were noted in patients receiving concomitant anticoagulation therapy (all three patients received warfarin, two patients received aspirin, and one patient received heparin) (14).
FIG. 1.
Cumulative percentage over time of patients in linezolid and comparator groups with at least one substantially low platelet count. Reprinted from reference 14.
Drug interactions.
In preclinical studies, linezolid was shown to be a mild, reversible competitive, nonselective inhibitor of the monoamine oxidase (MAO) enzymes; thus, it has a small potential to interact with adrenergic and serotonergic agents (19). A series of phase I drug-drug interaction studies conducted to further assess this potential showed that linezolid alone has no intrinsic hypertensive effects (18). In other studies conducted with healthy volunteers, no adverse events related to the inhibition of MAO leading to a drug-drug interaction were reported when linezolid was used in combination with paroxetine, meperidine, or oral and inhaled albuterol (data on file, Pharmacia Corp., 2000; Zyvox package insert, Pharmacia & Upjohn Company, 2001).
There were no contraindicated drugs in the phase III trials. During these trials, patients did receive classes of medications such as analgesics, vasopressors, cyclic antidepressants, selective serotonin-reuptake inhibitors, and indirectly acting sympathomimetics that could potentially interact with MAO-inhibiting medications. Only one patient had a report of a possible drug interaction. This patient, who had a history of hypertension, was receiving fluoxetine treatment and was admitted to the hospital with hypoxia and pneumonia. He had one transient episode of asymptomatic hypertension after one dose of linezolid, but this resolved without sequelae. This single episode was not accompanied by other manifestations of a serotonin syndrome (i.e., hyperpyrexia, diaphoresis, excitation, restlessness, tremor, myoclonus) and was, therefore, unlikely to be due to an MAO-related interaction. However, as linezolid has the potential to cause serotonin syndrome when used in combination with selective serotonin-reuptake inhibitors, it should be used cautiously in patients concomitantly receiving these agents (24, 34).
To further evaluate the potential for drug-drug interactions related to inhibition of MAO with linezolid use, patients who were treated at any time during the comparator-controlled trials with medication that could potentially interact with an MAO inhibitor (i.e., sympathomimetics, vasopressors, antidepressants, stimulants, antitussives, and analgesics) were identified. Adverse events that could potentially be attributed to an MAO inhibition-related interaction, such as hypertension, hyperthermia, arrhythmias, confusion, myoclonus, or palpitations, were reviewed for these patients. In the seven comparator-controlled trials, 30.9% (632 of 2,046) of linezolid-treated patients and 30.3% (605 of 1,999) of comparator drug-treated patients also received potentially MAO-interacting medications. The overall incidence of potentially MAO-related adverse events was similar between groups. The only reports of potentially drug-related events for linezolid- and comparator drug-treated patients were hypertension (0.3 and 0.2%, respectively), hyperthermia (0.9 and 0.8%, respectively), and palpitations (0.2 and 0.2%, respectively). Importantly, the comparator agents in these studies did not possess MAO-inhibitory properties. Furthermore, when specific drug classes were examined, there was no clear pattern suggestive of an MAO-inhibitory interaction effect with linezolid (C. S. Hartman, T. S. Leach, V. H. Le, W. M. Todd, and B. Hafkin, 10th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. 258, 2000).
DISCUSSION
Linezolid is a novel agent in a new class of antibiotics—the oxazolidinones—with excellent activity against gram-positive bacteria. It is important to establish a comprehensive picture of its safety profile based on the available preclinical, clinical, and postmarketing data.
Data from preclinical and phase I and II clinical trials noted the potential for hematologic effects with linezolid use. The pooled analysis presented here includes data for over 2,000 linezolid-treated patients from seven comparator-controlled phase III clinical trials and shows that linezolid was well tolerated when given as recommended, by i.v. and/or oral routes at doses of up to 600 mg twice daily for periods of up to 28 days. The most frequently reported drug-related adverse events associated with linezolid were diarrhea, nausea, and headache. These adverse events were generally mild to moderate in intensity and limited in duration, with few patients (≤0.5%) requiring discontinuation of the study medication (Table 4). Four of these seven trials were double blinded, and the adverse events reported were similar, in both character and magnitude, between the linezolid and comparator groups; no significant differences in adverse events were noted between groups in these four trials. Across the seven trials, the overall rates of adverse events and drug-related adverse events were usually higher for linezolid. This was generally due to transient gastrointestinal events commonly observed with many antibiotics, especially those administered orally. In contrast, the frequencies of C. difficile-related complications were slightly lower in the linezolid groups than in the comparator groups. The rates of treatment-related adverse events resulting in study drug discontinuation, serious adverse events, and deaths were each comparable between the linezolid and comparator groups across all studies.
Laboratory safety results from the pooled data from phase III clinical trials were comparable over time for patients receiving linezolid and comparator antibiotics. The majority of changes in serum chemistry and hematology values were reversible, self-limited, and not associated with treatment discontinuation. There was no evidence to suggest any clinically significant untoward effects of linezolid on the liver. The incidence of drug-related hematologic adverse events from the data reported for linezolid-treated patients was less than 1% and was similar to that for the comparator group. The character of the hematologic abnormalities, the time of onset, and the reversibility upon withdrawal of study drug in these trials suggest a risk of reversible, duration-dependent myelosuppression due to linezolid treatment that is more commonly seen with therapy beyond 14 days (1, 2, 4, 10, 12-15, 21, 22, 23).
In the first 6 months of approved use of linezolid, the postmarketing surveillance program sponsored by the manufacturer received 72 reports of hematologic abnormalities in 55,000 treated patients, for an overall reported hematologic event rate of 0.13% (approximately 1 in 750) for exposed patients, with thrombocytopenia occurring in 0.06% of patients and anemia occurring in 0.03% of patients (24). Leukopenia was reported in two patients (0.0036%). Neutropenia was reported in three patients (0.0055%) in combination with either thrombocytopenia (one patient) or anemia (two patients).
Data on the long-term use of linezolid are available from the compassionate use program. A total of 796 patients received a total of 828 courses of linezolid. The duration of linezolid therapy was <28 days in 556 treatment courses, with a mean duration of therapy of 14 days. The duration of linezolid therapy was ≥28 days in 272 treatment courses, with a mean duration of therapy of 54 days. The following hematologic events were reported as possibly related to linezolid during the 828 courses: thrombocytopenia (7.2%; 62 events), a decrease in hemoglobin or hematocrit levels (4.2%; 35 events), leukopenia (2.2%; 18 events), pancytopenia (0.24%; 2 events), and red blood cell hypoplasia (0.12%; 1 event). Of the patients with thrombocytopenia, 12 (19.3%) were treated for <14 days, 26 (41.9%) were treated for 14 to 28 days, and 24 (38.7%) were treated for >28 days. The hematologic events reported were mild to moderate in severity, transient in nature, related to treatment duration, and reversible when therapy was discontinued (5).
Recent reports suggest that linezolid is associated with reversible myelosuppression (3, 16, 33; K. Hicks, R. Y. Hachem, A. Huen, and I. I. Raad, 39th Meet. Infect. Dis. Soc. Am., abstr. 467, 2001; J. S. Lewis and J. E. Patterson, 39th Meet. Infect. Dis. Soc. Am., abstr. 470, 2001; R. L. Patron, 39th Meet. Infect. Dis. Soc. Am., abstr. 466, 2001). Attassi et al. (3) reported decreased (range, 30 to 79%) platelet counts in 9 of 19 patients receiving linezolid for 10 to 42 days, with platelet counts <100,000/mm3 in 7 patients treated for more than 10 days. Follow-up platelet counts as well as concurrent illnesses and medications were not reported (3). Lewis and Patterson (39th Meet. Infect. Dis. Soc. Am., abstr. 470, 2001) reported similar findings, such that 12 of 27 courses of linezolid therapy evaluated resulted in a 25% decrease in platelet count within 2 weeks of therapy. However, severity of illness and concomitant medications may have been contributing factors and were not controlled for in either of the two studies. Green et al. (16) described three adult patients who experienced reversible myelosuppression manifested as transient thrombocytopenia or anemia after 2 weeks of linezolid therapy. Each of the three patients (ages 43 to 70 years) had MRSA infections (graft infection, chronic facial sinus infection, and osteomyelitis) and received linezolid at 600 mg twice daily for 2 to 16 weeks. In each of the patients, hemoglobin, platelet, and reticulocyte counts decreased and treatment was discontinued. Following linezolid discontinuation, values returned to normal. The investigators noted that increasing levels of iron in serum and iron saturation preceded the decrease in peripheral blood cell counts in these three patients. There appeared to be temporal associations between linezolid use and the hematologic changes and resolution observed; none of these three cases was compatible with a diagnosis of pancytopenia or aplastic anemia (16). Nevertheless, monitoring of complete blood counts in patients receiving linezolid for more than 2 weeks is advised.
In summary, the data available to date suggest that linezolid is safe in patients with serious and potentially serious infections caused by gram-positive organisms when the recommended dosage regimen of up to 600 mg every 12 h for up to 28 days is used. More linezolid-treated patients than comparator drug-treated patients received oral antibiotic therapy; however, the rates of mild to moderate gastrointestinal side effects and C. difficile-related complications were similar between treatments. Less commonly, there were changes in laboratory assay values suggestive of myelosuppression (i.e., thrombocytopenia or anemia) in association with linezolid treatment; however, these changes were infrequent, were reversible upon discontinuation of the drug, and occurred in patients who were treated with linezolid for more than 14 days (14). The mechanism by which myelosuppression may occur has not been elucidated. Other oxazolidinones have been shown to inhibit mitochondrial protein synthesis, and a similar mechanism may play a role in linezolid-related reversible myelosuppression. With the reversible hematologic effects being more common with prolonged use, adherence to the recommended duration should be encouraged and hematologic risk should be managed through periodic monitoring of complete blood counts, particularly in patients with preexisting bone marrow suppression or those who received drugs known to cause hematopoietic effects. Given the unique antibacterial activity of linezolid against gram-positive bacterial pathogens, including resistant organisms such as MRSA, penicillin-resistant S. pneumoniae, and vancomycin-resistant enterococci, the risk for transient, reversible, predictable hematologic effects with linezolid should be considered together with the clinical benefits associated with its use.
Acknowledgments
This work was supported by Pharmacia Corp.
At the time that this work was completed, B.H. was employed by Pharmacia Corp., Peapack, N.J.
REFERENCES
- 1.Alanis, A., and A. J. Weinstein. 1983. Adverse reactions associated with the use of oral penicillins and cephalosporins. Med. Clin. N. Am. 67:113-129. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, D. P., M. E. Russo, D. E. Fohrman, and G. Rothstein. 1983. Nafcillin-induced platelet dysfunction and bleeding. Antimicrob. Agents Chemother. 23:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attassi, K., E. Hershberger, R. Alam, and M. J. Zervos. 2002. Thrombocytopenia associated with linezolid therapy. Clin. Infect. Dis. 34:695-698. [DOI] [PubMed] [Google Scholar]
- 4.Beris, P., and P. A. Miescher. 1988. Hematological complications of antiinfectious agents. Semin. Hematol. 25:123-139. [PubMed] [Google Scholar]
- 5.Birmingham, M. C., C. R. Rayner, A. K. Meagher, S. M. Flavin, D. H. Batts, and J. J. Schentag. 2003. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate use program. Clin. Infect. Dis. 36:159-168. [DOI] [PubMed]
- 6.Brickner, S. J., D. K. Hutchinson, M. R. Barbachyn, P. R. Manninen, D. A. Ulanowicz, S. A. Garmon, K. C. Grega, S. K. Hendges, D. S. Toops, C. W. Ford, and G. E. Zurenko. 1996. Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections. J. Med. Chem. 39:673-679. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902.. [PubMed] [Google Scholar]
- 8.Chien, J. W., M. L. Kucia, and R. A. Salata. 2000. Use of linezolid, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clin. Infect. Dis. 30:146-151. [DOI] [PubMed] [Google Scholar]
- 9.Dresser, L. D., and M. J. Rybak. 1998. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 18:456-462. [PubMed] [Google Scholar]
- 10.Ehmann, W. C. 1992. Cephalosporin-induced hemolysis: a case report and review of the literature. Am. J. Hematol. 40:121-125. [DOI] [PubMed] [Google Scholar]
- 11.Eliopoulos, G. M., C. B. Wennersten, H. S. Gold, and R. C. Moellering, Jr. 1996. In vitro activities of new oxazolidinone antimicrobial agents against enterococci. Antimicrob. Agents Chemother. 40:1745-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fass, R. J., E. A. Copelan, J. T. Brandt, M. L. Moeschberger, and J. J. Ashton. 1987. Platelet-mediated bleeding caused by broad-spectrum penicillins. J. Infect. Dis. 155:1242-1248. [DOI] [PubMed] [Google Scholar]
- 13.George, J. N., G. E. Raskob, S. R. Shah, M. A. Rizvi, S. A. Hamilton, S. Osborne, and T. Vondracek. 1998. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann. Intern. Med. 129:886-890. [DOI] [PubMed] [Google Scholar]
- 14.Gerson, S. L., S. L. Kaplan, J. B. Bruss, V. Le, F. M. Arellano, B. Hafkin, and D. J. Kuter. 2002. Hematologic effects of linezolid: summary of clinical experience. Antimicrob. Agents Chemother. 46:2723-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girdwood, R. H. 1976. Drug-induced anaemias. Drugs 11:394-404. [DOI] [PubMed] [Google Scholar]
- 16.Green, S. L., J. C. Maddox, and E. D. Huttenbach. 2001. Linezolid and reversible myelosuppression. JAMA 285:1291.. [DOI] [PubMed] [Google Scholar]
- 17.Hau, T. 2002. Efficacy and safety of linezolid in the treatment of skin and soft tissue infections. Eur. J. Clin. Microbiol. Infect. Dis. 21:491-498. [DOI] [PubMed] [Google Scholar]
- 18.Hendershot, P. E., E. J. Antal, I. R. Welshman, D. H. Batts, and N. K. Hopkins. 2001. Linezolid: pharmacokinetic and pharmacodynamic evaluation of coadministration with pseudoephedrine HCl phenylpropanolamine HCl, and dextromethorphan HBr. J. Clin. Pharmacol. 41:563-572. [DOI] [PubMed] [Google Scholar]
- 19.Humphrey, S. J., J. T Curry, C. N. Turman, and R. P. Stryd. 2001. Cardiovascular sympathomimetic amine interactions in rats treated with monoamine oxidase inhibitors and the novel oxazolidinone antibiotic linezolid. J. Cardiovasc. Pharmacol. 37:548-563. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan, S. L., L. Patterson, K. M. Edwards, P. H. Azimi, J. S. Bradley, J. L. Blumer, T. O. Tan, F. G. Lobeck, and D. C. Anderson. 2001. Linezolid for the treatment of community-acquired pneumonia in hospitalized children. Pediatr. Infect. Dis. J. 20:488-494. [DOI] [PubMed] [Google Scholar]
- 21.Kueh, Y. K. 1991. Haematological adverse drug reactions in hospital practice. Ann. Acad. Med. Singapore 20:106-113. [PubMed] [Google Scholar]
- 22.Kuruppu, J. C., T. P. Le, and C. Tuazon. 1999. Vancomycin-associated thrombocytopenia: case report and review of the literature. Am. J. Hematol. 60:249-250. [DOI] [PubMed] [Google Scholar]
- 23.Kuter, D. J., and G. S. Tillotson. 2001. Hematologic effects of antimicrobials: focus on the oxazolidinone linezolid. Pharmacotherapy 21:1010-1013. [DOI] [PubMed] [Google Scholar]
- 24.Lavery, S., R. H. Ravi, W. W. McDaniel, and Y. R. Pushkin. 2001. Linezolid and serotonin syndrome. Psychosomatics 42:432-434. [DOI] [PubMed] [Google Scholar]
- 25.Matassova, N. B., M. V. Rodnina, R. Endermann, H. P. Krol, U. Pleiss, H. Wild, and W. Wintermeyer. 1999. Ribosomal RNA is the target for oxazolidinones, a novel class of translational inhibitors. RNA 5:939-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Technical Information Service. 1991. Coding symbols for thesaurus of adverse reaction terms, 3rd ed. National Technical Information Service, Springfield, Va.
- 27.Rubinstein, E., S. K. Cammarata, T. H. Oliphant, and R. G. Wunderink. 2001. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin. Infect. Dis. 32:402-412. [DOI] [PubMed] [Google Scholar]
- 28.San Pedro, G. S., S. K. Cammarata, T. H. Oliphant, and T. Todisco. 2002. Linezolid versus ceftriaxone/cefpodoxime in patients hospitalized for the treatment of Streptococcus pneumoniae pneumonia. Scand. J. Infect. Dis. 10:720-728. [DOI] [PubMed] [Google Scholar]
- 29.Shinabarger, D. L., K. R. Marotti, R. W. Murray, A. H. Lin, E. P. Melchior, S. M. Swaney, D. S. Dunyak, W. F. Demyan, and J. M. Buysse. 1997. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens, D. L., D. Herr, H. Lampiris, J. L. Hunt, D. H. Batts, B. Hafkin, and the Linezolid MRSA Study Group. 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. Clin. Infect. Dis. 34:1481-1490. [DOI] [PubMed] [Google Scholar]
- 31.Stevens, D. L., L. G. Smith, J. B. Bruss, M. A. McConnell-Martin, S. E. Duvall, W. M. Todd, and B. Hafkin. 2000. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 44:3408-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaney, S. M., H. Aoki, M. C. Ganoza, and D. L. Shinabarger. 1998. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldrep, T. W., and D. J. Skiest. 2002. Linezolid-induced anemia and thrombocytopenia. Pharmacotherapy 22:109-112. [DOI] [PubMed] [Google Scholar]
- 34.Wigen, C. L., and M. B. Goetz. 2002. Serotonin syndrome and linezolid. Clin. Infect. Dis. 34:1651-1652. [DOI] [PubMed] [Google Scholar]
- 35.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kiburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]