Abstract
Staphylococcus aureus is a prevalent cause of bacterial infections associated with indwelling medical devices. RNA III inhibiting peptide (RIP) is known to inhibit S. aureus pathogenesis by disrupting quorum-sensing mechanisms. RIP was tested in the present study for its ability to inhibit S. aureus biofilm formation in a rat Dacron graft model. The activity of RIP was synergistic with those of antibiotics for the complete prevention of drug-resistant S. aureus infections.
Staphylococci are a common cause of nosocomial infections related to bacterial biofilm formation on implanted devices (17). Central venous catheters, urinary catheters, prosthetic heart valves, orthopedic implants, and dialysis catheters are among the most common targets of infection (21, 28). Staphylococcus aureus and Staphylococcus epidermidis are often the predominant species found on biofilms after the removal of devices (20, 28). Infections related to bacterial biofilm formation may result in longer hospitalizations, a need for surgery, and even death (6). In hemodialysis patients, S. aureus is the leading cause of bacteremia, with an attributable mortality rate of 5 to 19%, often due to endocarditis (20). Peritoneal catheter-related infections (exit site and tunnel) are also often caused by S. aureus isolates and, as in hemodialysis patients, can be difficult to eradicate.
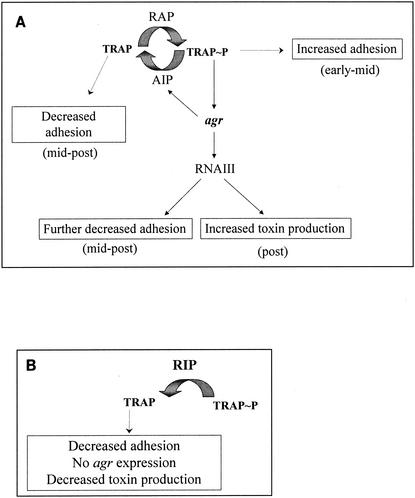
Infections characterized by bacterial biofilm formation can be described as follows. The biofilm structure is highly resistant to antibiotic treatment. Several explanations for the mechanism of biofilm resistance have been given, such as the failure of antimicrobial agents to penetrate the biofilm; the different metabolic states of the cells in the biofilm, which results in reduced susceptibilities to antimicrobial agents; and the expression of different genes by bacteria on a surface compared with those expressed by the planktonic counterparts (7, 10). One of the most intriguing explanations, however, is the possibility that antimicrobial resistance in the biofilm is acquired as a multicellular strategy, in which a “multicellular organism” collectively withstands antimicrobial treatments that would kill a lone cell (11, 12, 28). A novel way to prevent biofilm formation is to interfere with the bacterial cell-cell communication that leads to the virulence phenotype (4, 11, 27). The organization of the biofilm into complex structures is regulated by the exchange of chemical signals between cells in a process known as quorum sensing. Until now, two quorum-sensing mechanisms have been described in S. aureus. The first one is composed of the autoinducer RNA III-activating peptide (RAP) and its target protein, TRAP (3, 4). The second is composed of the peptide AIP and its receptor, AgrC (18, 19). When the amount of RAP reaches a threshold concentration, it induces the phosphorylation of TRAP, which, through an unknown mechanism, leads to increased cell adhesion and to the activation of the regulatory system agr, which encodes AIP and AgrC. AIP downregulates TRAP phosphorylation, leading to reduced cell adhesion, and induces the phosphorylation of its receptor, AgrC. This leads to the production of the regulatory RNA molecule RNA III, which triggers toxin production (23) (Fig. 1).
FIG. 1.
Proposed mechanism of regulation of cell adhesion and toxin production via TRAP and agr. (A) As the cells multiply, RAP accumulates in the supernatant, binds to its receptor, and induces the phosphorylation of TRAP. Cell adhesion is induced (at the early to midexponential phase of growth), as is the activation of agr (at the midexponential phase of growth). This leads to the production of AIP, which indirectly reduces the level of TRAP phosphorylation, and thus, cell adhesion is repressed (from the midexponential phase of growth). In parallel, RNA III is made, and the RNA III upregulates the production of toxins and the repression of surface proteins (postexponential phase of growth). (B) In the presence of RIP, TRAP is not phosphorylated, agr is not activated, AIP is not made, toxins are not produced, and biofilms do not form.
S. aureus virulence can be inhibited by the heptapeptide RNA III inhibiting peptide (RIP). RIP competes with RAP, thus inhibiting the phosphorylation of TRAP, leading to reduced adhesion and to the inhibition of RNA III synthesis, which leads to the suppression of toxin synthesis (1, 3, 4, 15). Synthetic RIP was designed in its amide form as YSPWTNF-NH2 and has been shown to be extremely effective in suppressing S. aureus infections in vivo, including cellulitis, septic arthritis, keratitis, osteomyelitis, and mastitis (1, 4).
In the present study we used a rat Dacron graft in vivo model (13) to test whether RIP can be used to coat medical devices and prevent graft-associated infections due to methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) isolates and to test whether its activity is synergistic with those of antibiotics.
The MICs of the drugs tested and the attachment of RIP to the Dacron graft were determined. The antimicrobial susceptibilities of the strains were determined by the broth microdilution method described by the National Committee for Clinical Laboratory Standards (22). Experiments were performed in triplicate. The MICs of cefazolin, teicoplanin, imipenem, levofloxacin, mupirocin, rifampin, and quinupristin-dalfopristin were 0.5, 0.25, 0.5, 0.25, 0.25, 0.25, and 0.25 μg/ml, respectively, for MSSA ATCC 29213 and 32, 0.25, 4, 0.5, 0.25, 0.5, and 0.25 μg/ml, respectively, for MRSA ATCC 43300. RIP did not demonstrate any in vitro activities against the two strains (MICs, >128 mg/liter), in accordance with its mechanism of action.
To determine how much RIP impregnates Dacron, 10 μg of fluorescein isothiocyanate (FITC)-labeled RIP [Cys(S, fluorescein)-YSPWTNF-NH2] per ml was applied to a 1-cm2 sterile collagen-sealed Dacron graft (Albograft; Sorin Biomedica Cardio, S.p.A., Saluggi VC, Italy) for 20 min at room temperature. The fluorescence in unbound solution was determined at optical densities of 485 and 530 nm in a Microplate fluorescence reader (FL 600; Bio-Tek, Winooski, Vt.) by using KC4 software. These experiments show that when 1 cm2 of Dacron is soaked in a 10-μg/ml RIP solution, 26 μg of RIP is bound to it.
Adult male Wistar rats (weight range, 250 to 300 g; Animal Facility, Istituto Nazionale Riposo e Cura Anziani, Istituto di Ricovero e Cura a Carattere Scientifico, Ancona, Italy) were used to test for the effects of RIP on graft-associated S. aureus infections. Each group included 15 animals.
During the experiments, the rats were anesthetized with ether (Sigma-Aldrich S.r.l., Milan, Italy), the hair on their backs was shaved, and the skin was cleansed with 10% povidone-iodine solution. A subcutaneous pocket was made on the side of the median line by use of a 1.5-cm incision. A 1-cm2 sterile collagen-sealed Dacron graft was aseptically implanted into the pocket. Immediately prior to implantation, the Dacron grafts were soaked for 20 min in a sterile solution of 10 μg of RIP (YSPWTNF-NH2; purified to 99% purity by high-pressure liquid chromatography; Neosystem, Strasbourg, France) per ml of saline or saline only as a control, with or without antibiotics. The antibiotics used were mupirocin (100 μg/ml; SmithKline Beecham Pharmaceuticals, Harlow, United Kingdom), quinupristin-dalfopristin (50 μg/ml; Centre de Recherches, Aventis Pharma, Vitry-Alfortville, France), levofloxacin (30 μg/ml; Hoechst Marion Roussel, Milan, Italy), and rifampin (5 μg/ml; Sigma-Aldrich S.r.l.). The pockets were closed with skin clips, and then 1 ml of sterile saline solution with or without 2 × 107 exponentially growing bacteria was inoculated onto the graft surface by using a tuberculin syringe to create a subcutaneous fluid-filled pocket. Strains MSSA ATCC 29213 and MRSA ATCC 43300 were used for quality control. The bacteria were grown at 37°C in Mueller-Hinton broth (Becton Dickinson Italia, Milan, Italy) prior to the experiment. Preparation of the animals and the surgical procedures were accomplished in less than 1 h.
As parenteral prophylaxis, some of the animals were also injected intraperitoneally with a single dose of antibiotics, administered 30 min before graft implantation. A single dose was chosen as prophylaxis prior to surgery to prevent infection during the surgical procedure. Previous literature suggests that even when antibiotics with short half-lives are used, this treatment should offer protection from infection during rapid surgical interventions (8, 9, 26). The antibiotics used for parenteral prophylaxis were cefazolin (30 mg/kg of body weight; Sigma-Aldrich S.r.l.), imipenem (30 mg/kg; Merck Sharp & Dohme, Milan, Italy), and teicoplanin (10 mg/kg) and levofloxacin (10 mg/kg) (Hoechst Marion Roussel). Solutions of drugs were made fresh on the day of assay or were stored at −70°C in the dark for short periods, in accordance with the recommendations of the manufacturers.
Initial experiments showed that the amount of bacteria removed from the grafts immediately after injection (time zero) varied between 104 and 106 CFU/ml with no slime formation and that biofilm formation with slime formation was fully achieved at 7 days (data not shown).
After 7 days, the grafts were explanted and placed in sterile tubes, washed in sterile saline solution, placed in tubes containing 10 ml of phosphate-buffered saline solution, and sonicated at 22,000 Hz for 5 min to remove the adherent bacteria from the grafts (these conditions have been shown to be adequate for complete removal of the bacteria with no adverse effects on the bacteria). Quantitation of viable bacteria was performed by culturing serial 10-fold dilutions (0.1 ml) of the bacterial suspension on blood agar plates. All plates were incubated at 37°C for 48 h and evaluated for the presence of the strain. The bacteria were quantitated by counting the number of CFU per plate. To determine if the bacteria were efficiently removed from the graft, washed and sonicated grafts were observed under an Eclipse E 600 optical microscope (Nikon, Kawasaki, Japan).
For statistical analysis, quantitative culture results for the in vivo experiments were presented as means ± standard deviations (SDs) of the means. Comparisons of the results were performed by analysis of variance of the log-transformed data. Significance was accepted when the P value was ≤0.05.
The results obtained at 7 days are summarized in Table 1 (parenteral prophylaxis) and Table 2 (local prophylaxis). None of the animals included in the uncontaminated control group had microbiological evidence of graft infection. All 15 rats included in the MSSA- and MRSA-infected but untreated control group demonstrated evidence of graft infection, with quantitative culture results showing 3.1 × 107 ± 5.4 × 106 and 5.4 × 107 ± 1.3 × 107 CFU/ml, respectively.
TABLE 1.
Prevention of S. aureus infection with RIP-coated Dacron grafts in the presence or absence of parenteral antibiotic prophylaxis
| Antibiotic(s) | Bacterial concn (log CFU/ml [mean ± SD])a
|
|
|---|---|---|
| MRSA | MSSA | |
| Control (untreated) | 7.73 ± 0.11 | 7.49 ± 0.07 |
| RIP | 4.58 ± 0.09b,c | 4.64 ± 0.06b,c |
| Cefazolin | 7.49 ± 0.04 | 5.64 ± 0.25b,d |
| Cefazolin + RIP | 4.40 ± 0.09b,d | 3.40 ± 0.13b,d |
| Teicoplanin | 2.90 ± 0.05b | 1.93 ± 0.03b |
| Teicoplanin + RIP | <1b,e,f | <1b,e,f |
| Imipenem | 4.91 ± 0.19b | 3.89 ± 0.12b |
| Imipenem + RIP | 3.84 ± 0.09b | 2.86 ± 0.09b |
| Levofloxacin | 5.84 ± 0.13b | 4.59 ± 0.16b |
| Levofloxacin + RIP | 4.68 ± 0.06b,g | 3.69 ± 0.08b,g |
n = 15 rats per group.
P < 0.05 versus the control (untreated group).
P < 0.05 versus cefazolin treatment (MRSA) and teicoplanin treatment.
P < 0.05 versus cefazolin treatment.
P < 0.05 versus teicoplanin treatment.
P < 0.05 versus lerofloxacin-RIP treatment and rifampin-RIP treatment.
P < 0.05 versus levofloxacin treatment.
TABLE 2.
Prevention of S. aureus infection using RIP-coated Dacron grafts in the presence or absence of local antibiotic prophylaxis
| Antibiotic(s) | Bacterial concn (log CFU/ml [mean ± SD])a
|
|
|---|---|---|
| MRSA | MSSA | |
| Control (untreated) | 7.73 ± 0.11 | 7.49 ± 0.07 |
| RIP | 4.52 ± 0.09b,c | 4.64 ± 0.06b,c |
| Mupirocin | 2.91 ± 0.16b | 2.84 ± 0.17b |
| Mupirocin + RIP | <1b,d,e | <1b,d,e |
| Rifampin | 4.85 ± 0.15b | 4.92 ± 0.14b |
| Rifampin + RIP | 2.96 ± 0.09b,f | 3.57 ± 0.04b |
| Quinupristin-Dalfopristin | 1.68 ± 0.08b | 1.64 ± 0.19b |
| Quinupristin-Dalfopristin + RIP | <1b,e | <1b,e |
| Levofloxacin | 5.9 ± 0.15b | 4.89 ± 0.14b |
| Levofloxacin + RIP | 4.18 ± 0.19b,g | 3.93 ± 0.09b |
n = 15 rats per group.
P < 0.05 versus untreated control group.
P < 0.05 versus mupirocin treatment, and Quinipristin-dalfopristin treatment.
P < 0.05 versus mupirocin treatment.
P < 0.05 versus levofloracin-RIP treatment and rifampin-RIP treatment.
P < 0.05 versus rifampin treatment.
P < 0.05 versus levofloxacin treatment.
Groups treated with Dacron grafts soaked with 10 μg of RIP per ml showed evidence of MSSA infection (4.4 × 104 ± 6.8 × 103 CFU/ml) and MRSA infection (3.3 × 104 ± 6.8 × 103 CFU/ml), as did the groups treated intraperitoneally with cefazolin (MSSA, 4.4 × 105 ± 2.3 × 105 CFU/ml; MRSA, 3.1 × 107 ± 0.3 × 107 CFU/ml), teicoplanin (MSSA, 8.5 × 101 ± 0.6 × 101 CFU/ml; MRSA, 7.9 × 102 ± 0.9 × 102 CFU/ml), imipenem (MSSA, 7.7 × 103 ± 2.1 × 103 CFU/ml; MRSA, 8.1 × 104 ± 3.3 × 104 CFU/ml), or levofloxacin (MSSA, 3.9 × 104 ± 1.4 × 104 CFU/ml; MRSA, 6.9 × 105 ± 2.0 × 105 CFU/ml) without pretreatment of the graft with RIP. There were significant differences (P < 0.05) between the results obtained for all the antibiotic-treated groups and the results obtained for the contaminated control group, with the exception of the cefazolin-treated group.
Rats that received Dacron grafts soaked with 10 μg of RIP per ml plus cefazolin, teicoplanin, imipenem, and levofloxacin intraperitoneally showed MSSA infections with 2.5 × 103 ± 7.1 × 102, <1 × 10, 7.2 × 102 ± 1.5 × 102, and 4.9 ×103 ± 8.7 × 102 CFU/ml, respectively, and MRSA infections with 2.5 × 104 ± 0.5 × 104, <1 × 10, 6.9 × 103 ± 1.4 × 103, and 4.8 ×104 ± 7.2 × 103 CFU/ml, respectively. All groups treated with a combination of antibiotic and RIP showed decreases in the levels of MSSA and MRSA infection compared to those after prophylaxis with antibiotic alone. Statistical significance (P < 0.05) was detected in particular for the combination of cefazolin and RIP versus cefazolin and for the combination of levofloxacin and RIP versus levofloxacin for the MSSA-infected grafts and for the same combinations as well as the combination of teicoplanin and RIP versus teicoplanin for the MRSA-infected grafts (Table 1).
The groups that received local prophylaxis with mupirocin, rifampin, quinupristin-dalfopristin, and levofloxacin on Dacron grafts showed MSSA infections with 6.9 × 102 ± 2.1 × 102, 8.4 × 104 ± 2.6 × 104, 4.4 × 101 ± 1.8 × 101, and 7.7 × 104 ± 2.5 × 104 CFU/ml, respectively, and MRSA infections with 8.2 × 102 ± 2.9 × 102, 7.1 × 104 ± 2.4 × 104, 4.8 × 101 ± 0.9 × 101, and 7.9 × 105 ± 2.6 × 105 CFU/ml, respectively. The results for all treated groups were statistically significant (P < 0.05) compared to those for the infected controls (Table 2). The same groups that received Dacron grafts soaked with 10 μg of RIP per ml showed <1 × 10 CFU/ml (RIP and mupirocin), 3.7 × 103 ± 3.8 × 102 CFU/ml (RIP and rifampin), <1 × 10 CFU/ml (RIP and quinopristin-dalfopristin), and 8.5 × 103 ± 1.7 × 103 CFU/ml (RIP and levofloxacin) for the MSSA-infected grafts and <1 × 10 CFU/ml (RIP and mupirocin), 9.1 × 102 ± 1.8 × 102 CFU/ml (RIP and rifampin), <1 × 10 CFU/ml (RIP and quinopristin-dalfopristin), and 1.5 × 104 ± 6.3 × 103 CFU/ml (RIP and levofloxacin) for the MRSA-infected grafts. Although all groups treated with the combination of RIP and an antibiotic showed decreases in the levels of MSSA infections compared to those after treatment with the antibiotic alone, statistical significance (P < 0.05) was detected for the groups treated with RIP plus mupirocin versus mupirocin alone and RIP plus rifampin versus rifampin alone.
Of note is the fact that none of the agents used showed any toxicity, and none of the animals included in any group died or had clinical evidence of drug-related adverse effects, such as local signs of perigraft inflammation, anorexia, vomiting, diarrhea, or behavioral alterations. It must be noted, however, that no histopathological tests were done.
RIP is emerging as a highly effective peptide for the prevention of infections caused by drug-sensitive or drug-resistant staphylococci. RIP acts by inhibiting TRAP phosphorylation, leading both to reduced cell adhesion and to inhibition of agr-regulated toxin production. Until recently, it was not clear how RIP has such a pleiotropic effect. The dogma has been that agr upregulates toxin production and downregulates adhesion. It was expected that any peptide that inhibits agr would cause an increase in cell adhesion. It was then discovered that TRAP plays a role not only in agr expression but also in cell adhesion. In the absence of TRAP phosphorylation (by RIP), the bacteria do not adhere or form a biofilm (5) and do not express agr (3). Consequently, a peptide that prevents TRAP from being phosphorylated would be effective in reducing biofilm formation, but a peptide that acts only on agr would have an opposite effect.
In our in vivo model, pretreatment of grafts with a solution containing RIP led to a 1,000-fold decrease in the load of any bacterial strain tested. The effect of RIP was similar to those of the most effective antibiotics used at present. Streptogramins (quinopristin-dalfopristin), glycopeptides (teicoplanin), and mupirocin were the most effective of the antibiotics tested, as predicted by previous reports in the literature (24), but did not eradicate the infection by 100%. RIP in combination with these antibiotics was able to achieve 100% inhibition. Carbapenems (imipenem), cephalosporins (cefazolin), and quinolones (levofloxacin) were less effective, but the addition of RIP strongly increased their inhibition potentials. Nevertheless, we cannot exclude the possibility that the lack of inhibition by cefazolin treatment in the MRSA-infected animals could also have been due to the small amount of the single dose used as prophylaxis for this group (8, 9).
In other in vivo experiments, the results for RIP eradication of S. epidermidis biofilm formation were similar to those reported here when RIP was tested against methicillin-susceptible, methicillin-resistant, and glycopeptide-intermediate S. epidermidis (2), suggesting the possibility that RIP is a global inhibitor of staphylococci. This is not surprising in view of the fact that TRAP, which is the target molecule of RIP, is highly conserved among staphylococcal strains and species. Therefore, RIP can inhibit infections caused by any strain or species of staphylococci (1, 15, 30).
RIP was not able to totally eradicate biofilm formation when it was used alone. It may be possible that the amount of RIP used to precoat the graft before implantation was not sufficient, as suggested by the FITC-RIP incubation experiments described above. Experiments with increased RIP concentrations are ongoing. Although other strategies have been developed as alternatives to antibiotic prophylaxis to fight bacterial biofilm formation, such as the design of graft material resistant to colonization (14, 15, 29) or coating of the graft with antibodies with activities against bacterial adhesins (25), these strategies are limited because they decrease bacterial adhesion but do not influence the production of toxins. In agreement with previous opinions (16), we believe that direct inhibition of key genes in the quorum-sensing mechanism is a promising approach, which, instead of killing the bacteria, attenuates or inhibits their pathogenicities. Such an approach, however, must be used with caution, because as has been shown for RIP (which inhibits RAP and TRAP quorum sensing) and AIP (which inhibits agr quorum sensing), not all inhibitors of any quorum-sensing system are effective. In conclusion, we have shown in this paper an alternative approach to the inhibition of drug-resistant S. aureus biofilm formation in vivo by a quorum-sensing inhibition mechanism and have shown that the activity of RIP is synergistic with those of antibiotics.
REFERENCES
- 1.Balaban, N., L. V. Collins, J. S. Cullor, E. B. Hume, E. Medina-Acosta, O. Vieira da Motta, R. O'Callaghan, P. V. Rossitto, M. E. Shirtliff, L. Serafim da Silveira, A. Tarkowski, and J. V. Torres. 2000. Prevention of diseases caused by Staphylococcus aureus using the peptide RIP. Peptides 21:1301-1311. [DOI] [PubMed] [Google Scholar]
- 2.Balaban, N., A. Giacometti, O. Cirioni, Y. Gov, R. Ghiselli, F. Mocchegiani, C. Viticchi, M. S. Del Prete, V. Saba, G. Scalise, and G. Dell'Acqua. 2003. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 187:625-630. [DOI] [PubMed] [Google Scholar]
- 3.Balaban, N., T. Goldkorn, Y. Gov, M. Hirshberg, N. Koyfman, H. R. Matthews, R. T. Nhan, B. Singh, and O. Uziel. 2001. Regulation of Staphylococcus aureus pathogenesis via target of RNAIII-activating protein (TRAP). J. Biol. Chem. 276:2658-2667. [DOI] [PubMed] [Google Scholar]
- 4.Balaban, N., T. Goldkorn, R. T. Nhan, L. B. Dang, S. Scott, R. M. Ridgley, A. Rasooly, S. C. Wright, J. W. Larrick, R. Rasooly, and J. R. Carlson. 1998. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science 280:438-440. [DOI] [PubMed] [Google Scholar]
- 5.Balaban, N., Y. Gov, A. Bitler, and J. R. Boelaert. 2003. The quorum sensing inhibitor RIP prevents adherence and biofilm formation of Staphylococcus aureus to human keratinocytes and dialysis catheters. Kidney Int. 63:340-345. [DOI] [PubMed] [Google Scholar]
- 6.Barie, P. S. 1998. Antibiotic-resistant gram-positive cocci: implications for surgical practice. World J. Surg. 22:118-126. [DOI] [PubMed] [Google Scholar]
- 7.Becker, P., W. Hufnagle, G. Peters, and M. Herrmann. 2001. Detection of differential gene expression in biofilm-forming versus planktonic populations of Staphylococcus aureus using micro-representational-difference analysis. Appl. Environ. Microbiol. 67:2958-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergamini, T. M., J. C. Peyton, and W. G. Cheadle. 1992. Prophylactic antibiotics prevent bacterial biofilm graft infection. J. Surg. Res. 52:101-105. [DOI] [PubMed] [Google Scholar]
- 9.Citak, M. S., J. I. Cue, J. C. Peyton, and M. A. Malangoni. 1992. The critical relationship of antibiotic dose and bacterial contamination in experimental infection. J. Surg. Res. 52:127-130. [DOI] [PubMed] [Google Scholar]
- 10.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 11.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 12.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiselli, R., A. Giacometti, L. Goffi, O. Cirioni, F. Mocchegiani, F. Orlando, A. M. Paggi, E. Petrelli, G. Scalise, and V. Saba. 2001. Prophylaxis against Staphylococcus aureus vascular graft infection with mupirocin-soaked, collagen-sealed Dacron. J. Surg. Res. 99:316-320. [DOI] [PubMed] [Google Scholar]
- 14.Gottenbos, B., D. W. Grijpma, H. C. van der Mei, J. Feijen, and H. J. Busscher. 2001. Antimicrobial effects of positively charged surfaces on adhering gram-positive and gram-negative bacteria. J. Antimicrob. Chemother. 48:7-13. [DOI] [PubMed] [Google Scholar]
- 15.Gov, Y., A. Bitler, G. Dell'Acqua, J. V. Torres, and N. Balaban. 2001. RNAIII inhibiting peptide (RIP), a global inhibitor of Staphylococcus aureus: structure and function analysis. Peptides 22:1609-1620. [DOI] [PubMed] [Google Scholar]
- 16.Hartman, G., and R. Wise. 1998. Quorum sensing: potential means of treating gram-negative infections? Lancet 351:848-849. [DOI] [PubMed] [Google Scholar]
- 17.Huebner, J., and D. A. Goldman. 1999. Coagulase-negative staphylococci: role as pathogens. Annu. Rev. Med. 50:233-236. [DOI] [PubMed] [Google Scholar]
- 18.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 19.Lina, G., S. Jarraud, G. Ji, T. Greenland, A. Pedraza, J. Etienne, R. P. Novick, and F. Vandenesch. 1998. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol. Microbiol. 28:655-662. [DOI] [PubMed] [Google Scholar]
- 20.Marr, K. A. 2000. Staphylococcus aureus bacteremia in patients undergoing hemodialysis. Semin. Dial. 13:23-29. [DOI] [PubMed] [Google Scholar]
- 21.Nassar, G. M., and J. C. Ayus. 2001. Infectious complications of the hemodialysis access. Kidney Int. 60:1-13. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 2001. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard. M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paradisi, F., G. Corti, and D. Messeri. 2001. Antistaphylococcal (MSSA, MRSA, MSSE, MRSE) antibiotics. Med. Clin. N. Am. 85:1-17. [DOI] [PubMed] [Google Scholar]
- 25.Pei, L., and J. I. Flock. 2001. Functional study of antibodies against a fibrinogen-binding protein in Staphylococcus epidermidis adherence to polyethylene catheters. J. Infect. Dis. 184:52-55. [DOI] [PubMed] [Google Scholar]
- 26.Sher, K. S. 1997. Studies on the duration of antibiotic administration for surgical prophylaxis. Am. Surg. 63:59-62. [PubMed] [Google Scholar]
- 27.Smith, K. M., Y. Bu, and H. Suga. 2003. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 10:81-89. [DOI] [PubMed] [Google Scholar]
- 28.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 29.Tiller, J. C., C. J. Liao, K. Lewis, and A. M. Klibanov. 2001. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 98:5981-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viera-da-Motta, O., P. D. Ribeiro, W. Dias da Silva, and E. Medina-Acosta. 2001. RNAIII inhibiting peptide (RIP) inhibits agr-regulated toxin production. Peptides 22:1621-1627. [DOI] [PubMed] [Google Scholar]