Abstract
Mean fecal global yeast counts increased similarly during 7 days of treatment with telithromycin (800 mg once daily) or amoxicillin-clavulanic acid (amoxiclav) (1 g of amoxicillin and 125 mg of clavulanic acid 3 times daily) in human volunteers and decreased slowly thereafter. On skin, coagulase-negative staphylococci of decreased susceptibility (DS) to telithromycin increased in the telithromycin group, whereas those with DS to methicillin increased in the amoxiclav group. A similar antibiotic-related shift towards homologous DS was observed for oral nongroupable streptococci (NGS), but in addition, the prevalence of NGS resistant to both classes of antibiotics was significantly greater in the amoxiclav group at days 8 (P < 0.01) and 45 (P < 0.015).
Antibiotics induce the emergence of resistant microorganisms in oropharyngeal, cutaneous, and intestinal bacteria and modify the competitive balance between organisms (for a recent review, see reference 23). Resistance in commensal bacteria can then be transferred to pathogenic species (7, 8, 13, 18). Therefore, the emergence of resistance in the commensal flora can be included among the treatment-related adverse effects, but few studies (9, 11, 12) on this topic have been comparative between drugs which can be used alternately. Amoxicillin-clavulanic acid (amoxiclav) is an antibiotic widely used world-wide and well tolerated, except for intestinal side effects (17). Its impact on the intestinal (for a review, see reference 23), cutaneous (24), nasal (2, 4), and pharyngeal (11, 26) flora has been described. Telithromycin is a new ketolide antibiotic, designed for respiratory infections (14). It is well tolerated (1, 5) and seemed to have a favorable ecological profile in the oropharyngeal and intestinal microflora.
(This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
Here, we compared the emergence of resistance in the commensal microflora of 2 groups of 25 fully informed healthy (by history, physical examination, routine laboratory tests, and electrocardiogram) adult (19 to 44 years) male volunteers, treated with either amoxiclav or telithromycin in a single-center, randomized, open-label, parallel-group comparative study. The volunteers had not taken antibiotics for 3 months and had a normal intestinal transit and a fecal count of enterobacteria greater than 104 CFU/g of feces at enrollment. They received either 2 tablets of 400 mg of telithromycin (Aventis Pharma, Paris, France) once daily for 7 days or 1 g of amoxicillin and 125 mg of clavulanic acid (GlaxoSmithKline, Paris, France) three times daily for 7 days. They were not hospitalized, but they returned to the center for all drug intakes and for follow-up visits (days 8, 14, 21, and 45). They were asked to continue their usual life and diet and allowed no other medication. Safety was evaluated at days 0 and 8, and adverse events were recorded by medical examination daily during treatment and then at days 14, 21, and 45. Serum samples were assayed for telithromycin (9) or amoxiclav (10) before the morning dose on days 3, 5, and 8 and 1, 2, 3, and 4 h after the morning dose on day 5 in 20 subjects per group.
Fecal, cutaneous, and oropharyngeal samples were collected on days −1, 8, 14, 21, and 45 by using sterile containers for fecal samples, a VLM E0115 apparatus (Verre-Labo Mula, Corbas, France) for cutaneous washes (2 ml), and BBL Culturette swabs (Becton-Dickinson, Cockeysville, Md.) discharged in 2 ml of broth for oropharyngeal samples. All samples were immediately transferred to the laboratory and analyzed within 3 h of arrival and blind with regard to the treatment administered. The limit of detection of the quantitative microbiological assays was 102 CFU/ml. In each ecosystem, we focused on a specific microbiological species to analyze the ecological impact of treatments. In the feces, yeasts were chosen because their counts were reported to increase after amoxiclav treatment (14, 19, 26) but not after telithromycin treatment (9), and Clostridium difficile was chosen because of its role in antibiotic-associated intestinal side effects (3). Coagulase-negative staphylococci (CNS) and nongroupable streptococci (NGS) were chosen as target organisms in the cutaneous and pharyngeal flora because they can be the source of resistance in related pathogenic species such as Staphylococcus aureus (27) and Streptococcus pneumoniae (25), respectively, within these ecosystems. Fecal samples were assayed qualitatively and quantitatively for yeasts on Chromagar medium (Biomérieux, Marcy-l'Etoile, France) and qualitatively for Pseudomonas aeruginosa and S. aureus on Cetrimide (Biomérieux) and Chapman agar (Biomérieux), respectively. C. difficile was detected on CCFA agar (Biomérieux) and by toxin assay (toxins A and B; Premier Bioscience, Inc., Cincinnati, Ohio).
Skin staphylococci were counted on Chapman agar, either free of antibiotics for total counts or containing 1 mg of erythromycin/liter, 1 mg of telithromycin/liter, or 2 mg of methicillin/liter for counts of subpopulations with decreased susceptibility (DS) to the corresponding antibiotics.
The presence of S. pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae in oropharyngeal swabs was detected on blood and chocolate agar. Total oropharyngeal NGS were counted on 5% sheep blood Columbia agar containing 10 mg of colimycin/liter and 15 mg of nalidixic acid/liter (CNA agar), and those with DS to erythromycin, telithromycin, or amoxiclav were counted on the same plates but supplemented with 1, 1, or 4 mg of the corresponding antibiotic/liter, respectively. The prevalence of streptococci coresistant to erythromycin, telithromycin, and amoxicillin was determined by testing the susceptibility of clones isolated from plain CNA agar by using the disk diffusion technique, as recommended previously (6). We used this technique in that particular case because the detection of coresistance with screening plates containing several antibiotics has never been used in the literature.
The sample size was calculated to demonstrate a difference of at least 2 decimal logarithms between yeast counts at day 8 (standard error, 2.3; power, 80%; type I error, 5%), by using the two-tailed Wilcoxon exact test, adjusted for the presence of yeasts at day −1. Secondary comparisons between the 2 groups were restricted to 6 variables: the area under the curve (trapezoidal rule) for the fecal yeasts between days −1 and 45 (Wilcoxon exact test stratified on the presence of yeast at day −1), the prevalence of C. difficile at day 8, and the prevalence of colonization by cutaneous staphylococci and oropharyngeal streptococci at days 8 and 45 (Fisher's exact test or Cochran-Mantel-Haenzel exact test if the tested factor [presence or absence of strains] was present at day 1). We used a global type I error of 4.5%, controlled for multiple testing by the Simes procedure (21). The incidence of treatment-related digestive adverse events was compared by using Fisher's exact test.
Effects of treatments on intestinal flora.
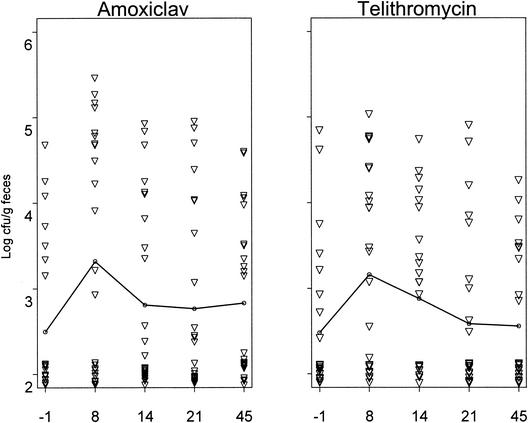
The mean fecal density of yeasts increased during treatment between days −1 and 8 (2.50 ± 0.85 versus 3.32 ± 1.39 CFU/g of feces for amoxiclav, and 2.48 ± 0.86 versus 3.16 ± 1.16 CFU/g of feces for telithromycin) (Fig. 1), decreased progressively, and were close to the baseline at day 21 with no significant between-group differences in counts at day 8 or in areas under the curves. Pretreatment prevalences of fecal yeast colonization were of 7 of 25 (28%) and 8 of 25 (32%) in the amoxiclav and telithromycin groups, respectively, increased to 14 of 25 (56%) and 15 of 25 (60%) at day 8, and then returned to baseline (9 of 25, 36%) by day 21 in the telithromycin group but were still elevated at day 45 in the amoxiclav group (13 of 24, 54%) (Table 1). C. difficile was isolated only in 4 volunteers (one with severe intestinal symptoms) from the amoxiclav group (data not significant). No P. aeruginosa or S. aureus was isolated.
FIG. 1.
Effect of 7 days of oral treatment of human volunteers with either amoxiclav (1 g of amoxicillin and 125 mg of clavulanic acid 3 times/day) or telithromycin (800 mg 1 time/day) on fecal yeast counts (25 volunteers/treatment group). The line represents the mean counts. ▿, individual data.
TABLE 1.
Effect of 7 days of oral treatment of human volunteers with either amoxiclav (1 g of amoxicillin and 125 mg of clavulanic acid 3 times/day) or telithromycin (800 mg 1 time/day) on the prevalence of fecal yeast colonization (25 volunteers/treatment group)
| Day | No. (%) of subjects colonized before or after treatment with:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amoxiclav
|
Telithromycin
|
|||||||||
| Any yeasta | Candida albicans | Other Candida sp. | Geotricum | Other | Any yeasta | Candida albicans | Other Candida sp. | Geotricum | Other | |
| −1 | 7 (28) | 5 (20) | 2 (8)b | 7 (28) | 0 | 8 (32) | 4 (16) | 2 (8)c | 4 (16) | 0 |
| 8 | 14 (56) | 11 (45) | 4 (16)d | 2 (8) | 3 (12)o | 15 (60) | 8 (32) | 2 (8)e | 6 (24) | 3 (12)f |
| 14 | 13 (52) | 8 (32) | 1 (4)g | 10 (40) | 0 | 14 (56) | 9 (36) | 2 (8)h | 9 (36) | 1 (4)i |
| 21 | 12 (48) | 10 (40) | 2 (8)j | 6 (24) | 0 | 9 (36) | 7 (28) | 1 (4) | 4 (16) | 1 (4)k |
| 45l | 13 (54) | 7 (29) | 2 (8)m | 10 (42) | 1 (4) | 9 (38) | 7 (29) | 2 (8)n | 5 (21) | 0 |
Some volunteers were colonized by multiple species.
C. tropicalis, 1 subject; C. krusei, 1 subject.
C. lusitaniae, 1 subject; C. glabrata, 1 subject.
C. tropicalis, 1 subject; C. Kefyr, 1 subject; C. krusei, 1 subject.
C. norvegensis, 1 subject; C. glabrata, 1 subject.
Trichosporon, 2 subjects; Rhodotorula rubra, 1 subject.
C. tropicalis, 1 subject.
C. glabrata, 2 subjects.
R. rubra, 1 subject.
C. kefyr, 1 subject; C. krusei, 1 subject.
R. rubra, 1 subject.
There were 24 volunteers in each treatment group only.
C. kefyr, 1 subject; C. inconspicua, 1 subject.
C. krusei, 1 subject; C. glabrata, 1 subject; C. candida sp., 1 subject.
Saccharomyces cerevisiae, 3 subjects.
Effects of treatments on cutaneous flora.
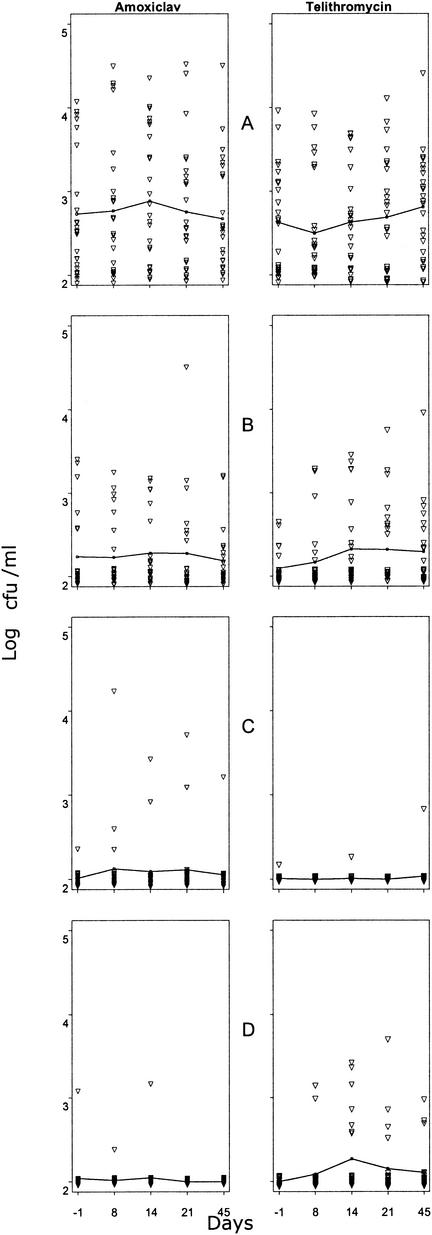
Thirty-nine volunteers (78%) were colonized by CNS before treatment, but only 11 (22%), 2 (4%), and 1 (2%) were colonized by CNS with DS to erythromycin (11 of 50, 22%), methicillin, or telithromycin, respectively. The prevalence of colonization by CNS with DS to erythromycin was close in the 2 groups. Colonization by CNS with DS to methicillin or telithromycin tended to be higher in the amoxiclav and telithromycin groups, respectively (Table 2). This specific treatment-related effect was also evidenced by the changes in counts of CNS with DS to the antibiotic received at the end of treatment or thereafter (Fig. 2C and D). By contrast, total CNS counts (Fig. 2A) and counts of CNS with DS to erythromycin (Fig. 2B) did not differ much between the 2 groups. No S. aureus was isolated.
TABLE 2.
Effects of 7 days of oral treatment of human volunteers with either amoxiclav (1 g of amoxicillin and 125 mg of clavulanic acid 3 times/day) or telithromycin (800 mg 1 time/day) on the prevalence of cutaneous colonization by CNS (25 volunteers/treatment group)
| Day | No. (%) of subjects colonized before or after treatment with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Amoxiclav
|
Telithromycin
|
|||||||
| Any CNS | CNS with DS to:
|
Any CNS | CNS with DS to:
|
|||||
| Erythro- mycin | Methi- cillin | Telithro- mycin | Erythro- mycin | Methi- cillin | Telithro- mycin | |||
| −1 | 21 (84) | 6 (25) | 1 (4) | 1 (4) | 18 (72) | 5 (20) | 1 (4) | 0 |
| 8 | 19 (76) | 7 (28) | 3 (12) | 1 (4) | 16 (64) | 6 (24) | 0 | 2 (8) |
| 45a | 18 (75) | 9 (38) | 2 (8) | 0 | 20 (83) | 10 (42) | 2 (8) | 5 (21) |
There were 24 volunteers only in each treatment group.
FIG. 2.
Effect of 7 days of oral treatment of human volunteers with either amoxiclav (1 g of amoxicillin and 125 mg of clavulanic acid 3 times/day) or telithromycin (800 mg 1 time/day) on cutaneous counts of CNS (25 volunteers/treatment group). Lines represent mean counts. ▿, individual data. (A) Total CNS; (B) CNS with DS to erythromycin; (C) CNS with DS to methicillin; (D) CNS with DS to telithromycin.
Effect of treatments on oropharyngeal flora.
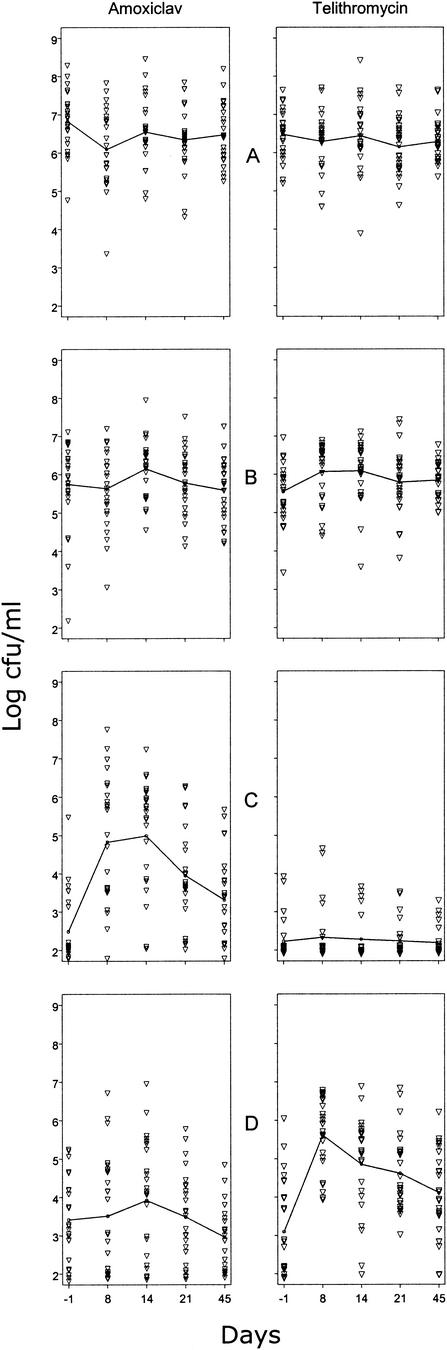
Before treatment, 98, 68, and 24% of the volunteers were colonized by NGS with DS to erythromycin, telithromycin, or amoxicillin, respectively (Table 3). During and after treatments, colonization by NGS with DS to amoxicillin increased more in the amoxiclav-treated group, whereas colonization by those with DS to telithromycin increased more in the telithromycin group, both in terms of prevalence of colonization (Table 3) and in terms of bacterial counts (Fig. 3B and C). In neither case was the return to baseline obtained by day 45. By contrast, total streptococcus counts (Fig. 3A) and counts of streptococci with DS to erythromycin (Fig. 3B) differed little between groups.
TABLE 3.
Influence of 7 days of oral treatment of human volunteers with either amoxiclav (1 g of amoxicillin and 125 mg of clavulanic acid 3 times/day) or telithromycin (800 mg 1 time/day) on the prevalence of oral colonization by NGS (25 volunteers/treatment group)
| Day | No. (%) of subjects colonized before or after treatment with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Amoxiclav
|
Telithromycin
|
|||||||
| Any NGS | NGS with DS to:
|
Any NGS | NGS with DS to:
|
|||||
| Erythro- mycin | Amoxi- cillin | Telithro- mycin | Erythro- mycin | Amoxi- cillin | Telithro- mycin | |||
| −1 | 25 (100) | 24 (96) | 7 (28) | 20 (80) | 25 (100) | 25 (100) | 5 (20) | 14 (56) |
| 8 | 25 (100) | 25 (100) | 24 (96) | 16 (64) | 25 (100) | 25 (100) | 6 (24) | 25 (100) |
| 45a | 24 (100) | 24 (100) | 19 (79) | 19 (79) | 24 (100) | 24 (100) | 7 (29) | 22 (92) |
There were 24 volunteers in each treatment group only.
FIG.3.
Effect of 7 days of oral treatment of human volunteers with either amoxiclav (1 g of amoxicillin and 125 mg of clavulanic acid 3 times/day) or telithromycin (800 mg 1 time/day) on oropharyngeal counts of NGS (25 volunteers/treatment group). Lines represent mean counts. ▿, individual data. (A) Total NGS; (B) NGS with DS to erythromycin; (C) NGS with DS to amoxicillin; (D) NGS with DS to telithromycin.
The prevalence of NGS coresistant to erythromycin, telithromycin, and amoxicillin, when tested by the disk diffusion technique, did not differ much between groups before treatment (20 versus 12%) but increased more in the amoxiclav group at day 8 (64 versus 24%, P < 0.01) and at day 45 (63 versus 25%, P < 0.015).
One volunteer (from the amoxiclav group) was colonized by S. pneumoniae (day −1), and 9 volunteers (5 from the amoxiclav group and 4 from the telithromycin group) were colonized by Haemophilus spp. No M. catarrhalis was isolated.
Adverse events.
Twelve and 17 adverse events (all and 12, respectively, were treatment related) were reported by 8 and 13 subjects (32 and 52%) in the telithromycin and amoxiclav groups, respectively. None led to the discontinuation of treatment. Most affected the digestive system, and these were significantly less frequent in the telithromycin group than in the amoxiclav group (1 [4%] versus 8 [32%]; P = 0.02). Treatments had no significant effects on clinical or laboratory parameters, vital signs, physical examination, or electrocardiogram.
Residual drug concentrations at days 3, 5, and 8 were undetectable for amoxicillin (except for one subject at day 5) and clavulanic acid, whereas they remained stable and above the quantification limit for telithromycin at 0.033 ± 0.018 mg/liter (0.01 to 0.08), 0.039 ± 0.016 mg/liter (0.02 to 0.08), and 0.037 ± 0.016 mg/liter (0.02 to 0.07) at days 3, 5, and 8, respectively. The maximum concentrations of telithromycin, amoxicillin, and clavulanic acid in serum were 1.89 ± 1.24, 11.16 ± 4.32, and 1.99 ± 0.8 mg/liter, respectively. The times to the maximum concentrations of telithromycin, amoxicillin, and clavulanic acid in serum were 1.29 ± 0.28, 1.40 ± 0.21, and 1.04 ± 0.22 h, respectively. The results for telithromycin are from 20 subjects, and the results for amoxicillin and clavulanic acid are from 16 subjects (clavulanic acid was undetectable in 4 subjects, even at 1.5 h).
In all, we described here the emergence of bacteria with DS to the antibiotic administered in the pharynx and on the skin of humans during treatments with amoxiclav or telithromycin, together with the emergence of yeasts in the feces. This emergence was much more frequent than any other side effect and it persisted for some time after the end of treatment. Microorganisms with DS to the treatment received were selected in both groups of volunteers in all the ecosystems studied. In the feces, the increase in yeast counts was very important but did not differ between groups, although previously the emergence of yeast had been reported after treatment with amoxiclav (14, 19, 26) but not telithromycin (9). This may be due to differences in the selection of volunteers or in stool conservation methods. Intestinal adverse effects were significantly more frequent in the amoxiclav group, but colonization by C. difficile and resistance to colonization by P. aeruginosa and S. aureus were not. The remnants of the ecological impact of antibiotic treatments over the weeks that followed the discontinuation of therapy that we observed were previously reported for intestinal (16, 20) and oral bacteria (11, 22). The method used was strict. The volunteers were monitored daily, all drug intake was monitored, and concentrations of drugs in plasma were measured. Volunteers had not taken antibiotics for 3 months and had fecal counts of enterobacteria greater than 104 CFU/g at inclusion. We chose to evaluate the impact of treatments by screening strains with DS to antibiotics on selective agar supplemented with antibiotics, first, because this method is more sensitive than testing individual isolates grown on nonselective agar for susceptibility (15) and, secondly, because it yields both qualitative and quantitative results. Concentrations in agar lower than the recommended breakpoints (6) were chosen to increase the sensitivity of the screening. To avoid any confusion, we described here bacteria that grew on the corresponding antibiotic-containing agar as DS to the given antibiotic (and not as resistant). We used the term resistant only for NGS coresistant to erythromycin, telithromycin, and amoxiclav, as tested by the disk diffusion technique.
Overall, our results demonstrated that the simultaneous emergence of microorganisms with DS to antibiotics in major human ecosystems after antibiotic treatments was much more frequent than any other drug-related side effect. Differences were observed between the effects of amoxiclav and telithromycin regimens, but they were essentially related to the spectrum of the antibiotic ingested and not as marked for their magnitude for the phenotype of DS of the bacteria selected.
Acknowledgments
This work was partly supported by a grant from Aventis Laboratories.
We thank C. Carbon and C. Safran for fruitful discussions, M. Dreyfus for English revision, and M. J. Julliard and S. Couriol for secretarial assistance.
REFERENCES
- 1.Balfour, J. A., and D. P. Figgitt. 2001. Telithromycin. Drugs 61:815-831. [DOI] [PubMed] [Google Scholar]
- 2.Balle, V. H., S. E. Stangerup, J. Sederberg-Olsen, J. Thomsen, and R. Vejlsgaard. 1990. Amoxicillin/clavulanate treatment in secretory otitis media. Bacteriological findings in the nasopharynx. Acta Otolaryngol. 110:274-278. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. G. 2002. Clinical practice. Antibiotic-associated diarrhea. N. Engl J. Med. 346:334-339. [DOI] [PubMed] [Google Scholar]
- 4.Brook, I., and A. E. Gober. 1998. Bacterial interference in the nasopharynx following antimicrobial therapy of acute otitis media. J. Antimicrob. Chemother. 41:489-492. [DOI] [PubMed] [Google Scholar]
- 5.Bryskier, A. 2000. Ketolides-telithromycin, an example of a new class of antibacterial agents. Clin. Microbiol. Infect. 6:661-669. [DOI] [PubMed] [Google Scholar]
- 6.Comité de l'Antibiogramme de la Société Française de Microbiologie. February2003, posting date. Communiqué du Comité de l'Antibiogramme de la Société Française de Microbiologie. [Online.] http://www.sfm.asso.fr.
- 7.Doucet-Populaire, F., P. Trieu-Cuot, A. Andremont, and P. Courvalin. 1992. Conjugal transfer of plasmid DNA from Enterococcus faecalis to Escherichia coli in digestive tracts of gnotobiotic mice. Antimicrob. Agents Chemother. 36:502-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doucet-Populaire, F., P. Trieu-Cuot, I. Dosbaa, A. Andremont, and P. Courvalin. 1991. Inducible transfer of conjugative transposon Tn1545 from Enterococcus faecalis to Listeria monocytogenes in the digestive tracts of gnotobiotic mice. Antimicrob. Agents Chemother. 35:185-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edlund, C., G. Alvan, L. Barkholt, F. Vacheron, and C. E. Nord. 2000. Pharmacokinetics and comparative effects of telithromycin (HMR 3647) and clarithromycin on the oropharyngeal and intestinal microflora. J. Antimicrob. Chemother. 46:741-749. [DOI] [PubMed] [Google Scholar]
- 10.Foulstone, M., and C. Reading. 1982. Assay of amoxicillin and clavulanic acid, the components of Augmentin, in biological fluids with high-performance liquid chromatography. Antimicrob. Agents Chemother. 22:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghaffar, F., L. S. Muniz, K. Katz, J. L. Smith, T. Shouse, P. Davis, and G. H. McCracken, Jr. 2002. Effects of large dosages of amoxicillin/clavulanate or azithromycin on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, nonpneumococcal alpha-hemolytic streptococci, and Staphylococcus aureus in children with acute otitis media. Clin. Infect. Dis. 34:1301-1309. [DOI] [PubMed] [Google Scholar]
- 12.Hofstra, W., G. W. Welling, and D. Van der Waaij. 1988. A comparative study of the effect of oral treatment with augmentin, amoxycillin and bacampicillin on the faecal flora in mice. Zentbl. Bakteriol. Mikrobiol. Hyg. A 269:78-85. [DOI] [PubMed] [Google Scholar]
- 13.Janoir, C., I. Podglajen, M. Kitzis, C. Poyart, and L. Gutmann. 1999. In vitro exchange of fluoroquinolone resistance determinants between Streptococcus pneumoniae and viridans streptococci and genomic organization of the parE-parC region in S. mitis. J. Infect. Dis. 180:555-558. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq, R. 2001. Overcoming antimicrobial resistance: profile of a new ketolide antibacterial, telithromycin. J. Antimicrob. Chemother. 48(Suppl. T1):9-23. [DOI] [PubMed] [Google Scholar]
- 15.Lester, S., M. Del Pilar Pla, F. Wang, I. Perez Schaeli, and T. O'Brien. 1990. The carriage of Escherichia coli resistant to antimicrobial agents by healthy children in Boston, Caracas, Venezuela, and in Qin Pu, China. N. Engl. J. Med. 323:285-289. [DOI] [PubMed] [Google Scholar]
- 16.Murray, B. E., E. R. Rensimer, and H. L. DuPont. 1982. Emergence of high-level trimethoprim resistance in fecal Escherichia coli during oral administration of trimethoprim or trimethoprim-sulfamethoxazole. N. Engl. J. Med. 306:130-135. [DOI] [PubMed] [Google Scholar]
- 17.Neu, H. C., A. P. Wilson, and R. N. Gruneberg. 1993. Amoxycillin/clavulanic acid: a review of its efficacy in over 38,500 patients from 1979 to 1992. J. Chemother. 5:67-93. [DOI] [PubMed] [Google Scholar]
- 18.Noble, W. C., Z. Virani, and R. G. Cree. 1992. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol. Lett. 72:195-198. [DOI] [PubMed] [Google Scholar]
- 19.Samonis, G., A. Gikas, P. Toloudis, S. Maraki, G. Vrentzos, Y. Tselentis, N. Tsaparas, and G. Bodey. 1994. Prospective study of the impact of broad-spectrum antibiotics on the yeast flora of the human gut. Eur. J. Clin. Microbiol. Infect. Dis. 13:665-667. [DOI] [PubMed] [Google Scholar]
- 20.Scanvic-Hameg, A., E. Chachaty, J. Rey, C. Pousson, M. Ozoux, E. Brunel, and A. Andremont. 2002. Impact of quinupristin/dalfopristin (RP 59500) on the faecal microflora in healthy volunteers. J. Antimicrob. Chemother. 49:135-139. [DOI] [PubMed] [Google Scholar]
- 21.Simes, R. 1986. An improved Bonferroni procedure for multiple tests of significance. Biometrika 73:751-754. [Google Scholar]
- 22.Smith, G. E., M. R. Fulford, R. W. Matthews, and D. C. Speller. 1989. Roxithromycin as a possible agent for prophylaxis of endocarditis: a study in normal volunteers. J. Antimicrob. Chemother. 23:417-425. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 24.Terpstra, S., G. T. Noordhoek, H. G. Voesten, B. Hendriks, and J. E. Degener. 1999. Rapid emergence of resistant coagulase-negative staphylococci on the skin after antibiotic prophylaxis. J. Hosp. Infect. 43:195-202. [DOI] [PubMed] [Google Scholar]
- 25.Varon, E., and L. Gutmann. 2000. Mechanisms and spread of fluoroquinolone resistance in Streptococcus pneumoniae. Res. Microbiol. 151:471-473. [DOI] [PubMed] [Google Scholar]
- 26.Vlaspolder, F., G. de Zeeuw, M. Rozenberg-Arska, P. Egyedi, and J. Verhoef. 1987. The influence of flucloxacillin and amoxicillin with clavulanic acid on the aerobic flora of the alimentary tract. Infection 15:241-244. [DOI] [PubMed] [Google Scholar]
- 27.Wu, S. W., H. de Lencastre, and A. Tomasz. 2001. Recruitment of the mecA gene homologue of Staphylococcus sciuri into a resistance determinant and expression of the resistant phenotype in Staphylococcus aureus. J. Bacteriol. 183:2417-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]