Abstract
Four clinical U.S. glycopeptide intermediate resistant Staphylococcus aureus (GISA) isolates were resistant to Triton X-100-induced autolysis. Similar resistance was demonstrated in an isolate obtained after a single passage of a susceptible clinical isolate in low-level vancomycin. Strains with the vancomycin-induced Triton X-100 resistance phenotype produced active murein hydrolases but were resistant to lysis by murein hydrolases.
A common characteristic among glycopeptide intermediate resistant Staphylococcus aureus (GISA) isolates is a slightly thickened cell wall (10, 14, 22), although the explanation for this phenomenon is unknown. One hypothesized mechanism is a decrease in activity of murein hydrolases (MH) or autolysins that play physiologic roles in cell separation, penicillin-induced lysis, and ongoing peptidoglycan remodeling (1, 13, 17).
Lysis induced by incubation in Triton X-100 has been used to evaluate autolytic activity (15, 25). We and others observed that resistance to Triton X-100-induced lysis occurs in laboratory-derived GISA mutants obtained by selection of glycopeptide-susceptible clinical isolates in glycopeptide-containing media (3, 19, 24).
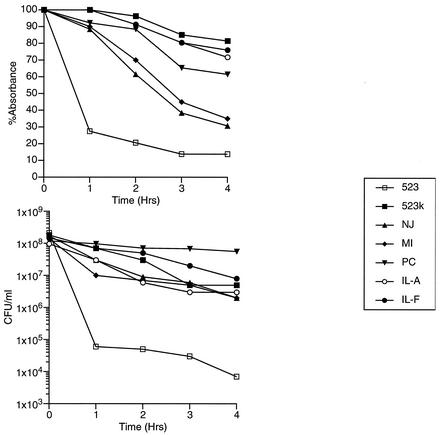
This phenomenon has been studied less well among clinical GISA isolates. It was reported that a clinical GISA isolate, IL-F, was resistant to Triton X-100-induced lysis (2), as was IL-A, an earlier blood isolate obtained from the same patient. IL-A and IL-F were both obtained after vancomycin therapy had been initiated. IL-A was susceptible to vancomycin but contained a bacterial subpopulation that could grow on media containing 4 μg of that antimicrobial per ml (2). Subsequently, clinical GISA isolates from Michigan, Port Chester, N.Y., and New Jersey were studied (6-9, 21, 23), and all were found to be relatively resistant to Triton X-100-mediated lysis compared with a vancomycin-susceptible and -naïve control S. aureus strain, 523 (10) (Fig. 1); another clinical GISA isolate, Mu50, from Japan did not have this phenotype.
FIG. 1.
Triton X-100 autolysis assay of clinical GISA isolates. GISA isolates IL-F, MI, PC, and NJ are clinical isolates from Illinois, Michigan, Port Chester, N.Y., and New Jersey, respectively (6-9, 21, 23). Cultures were grown to mid-logarithmic growth phase and suspended in buffered 0.05% Triton X-100. At 1-h intervals, an aliquot was collected for absorbance determinations at 600 nm and for determination of viable cell count (CFU/ml). Absorbances are represented as percentages of the absorbance at 600 nm relative to that at time zero for each sample.
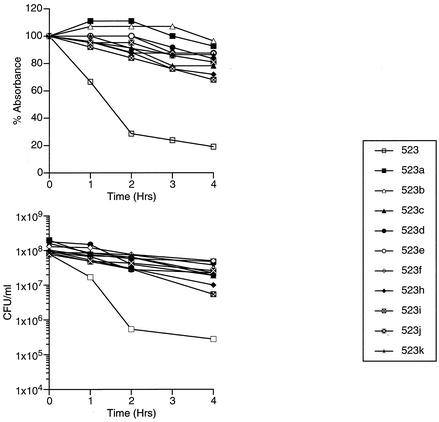
Previously characterized isolates (523a to 523k) (10), obtained by step-wise incubation of isolate 523 in medium containing vancomycin, provided an opportunity to study the time course of acquisition of Triton X-100 resistance and compare it with the acquisition of vancomycin resistance. This model is relevant, since laboratory-derived GISA isolate 523k has many properties accorded to clinical GISA isolates (2, 10). We found that the first isolate, 523a, was already resistant to Triton X-100-induced autolysis compared with the parent strain 523 (Fig. 2), although the MIC of vancomycin was 3 to 4 μg/ml (susceptible). The subsequent isolates (523b to 523k) were also resistant to Triton X-100-induced autolysis.
FIG. 2.
Triton X-100 autolysis assay of vancomycin-susceptible strain 523 and its vancomycin-exposed derivatives, 523a to 523k. Cultures were grown to mid-logarithmic growth phase and suspended in buffered 0.05% Triton X-100. At 1-h intervals, an aliquot was collected for determinations of absorbance at 600 nm and viable cell count (CFU/ml). Absorbances are represented as percentages of the absorbance at 600 nm relative to that at time zero for each sample.
The atl gene, encoding the major bifunctional autolysin, was sequenced from strains IL-A and IL-F to assess whether the Triton X-100 resistance phenotype in these vancomycin-exposed strains was due to a change in the function of the atl gene product. For sequencing, a 5,890-bp PCR product containing the atl gene, two contiguous upstream open reading frames, and a portion of open reading frame 1 (18) was obtained with primers ATL-F (5′-GGTACCAAAAATTAAATGGTGATG-3′) and ATL-R (5′-GCACGTTGCGAATTGATTGAAGC-3′) and the Advantage2 PCR kit (Clontech Laboratories, Inc., Palo Alto, Calif.). Sequencing reactions were performed with fluorescent dye-labeled terminator chemistry with the use of primers designed against the sequence of strain N315 (16).
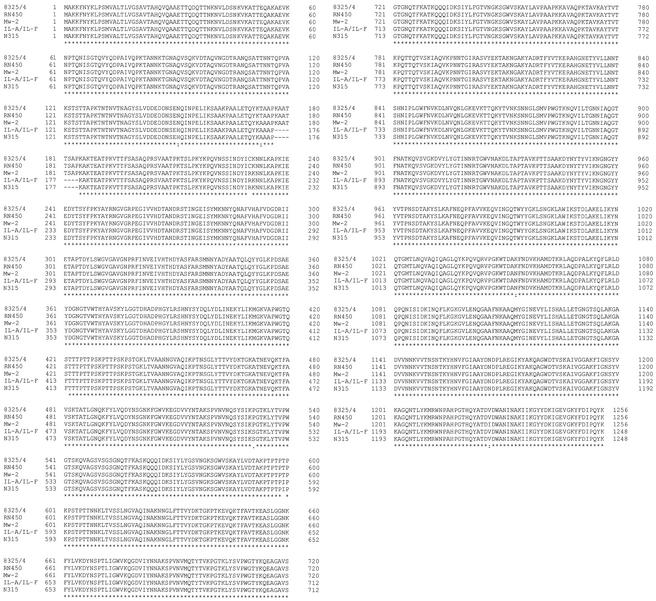
Sequence alignment (Fig. 3) and mutation detection were performed using public-domain software, namely, Blast (on the NCBI web site at www.ncbi.nlm.nih.gov/blast/Blast.cgi) and ClustalW version 1.8 (http://clustalw.genome.ad.jp). The sequence of the 5,890-bp fragment was identical between strains IL-A, IL-F (accession number AF537210), and vancomycin-susceptible methicillin-resistant S. aureus control isolate N315 (16). The amino acid sequence alignment of ATL from five strains of S. aureus is shown in Fig. 3. The identity of the atl gene sequence with that of N315 suggests that ATL is not responsible for vancomycin resistance in strains IL-A and IL-F.
FIG. 3.
Clustal W alignment of the Atl amino acid sequences of strains IL-A and IL-F (accession number AF537210), N315 (protein ID: BAB42150.1), RN450 (protein ID: BAA04185.1), MW2 (protein ID: AP004825.1), and 8325/4 (protein ID: AAA99982.1). Asterisks indicate positions with a single, fully conserved residue; colons indicate positions with polymorphism but with one of the following strong groups fully conserved: STA, NEQK, NHQK, NDEQ, QHRK, MILV, MILF, HY, FYW; periods indicate positions with one of the following weaker groups fully conserved: CSA, ATV, SAG, STNK, STPA, SGND, SNDEQK, NDEQHK, NEQHRK, FVLIM, HFY. Strong and weak groups are defined as a strong score of >0.5 and a weak score of ≤ 0.5, respectively, according to the Gonnet Pam250 matrix.
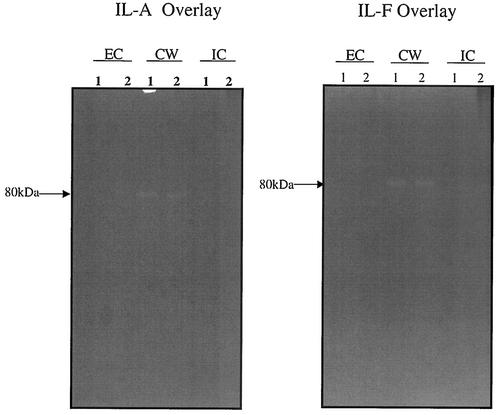
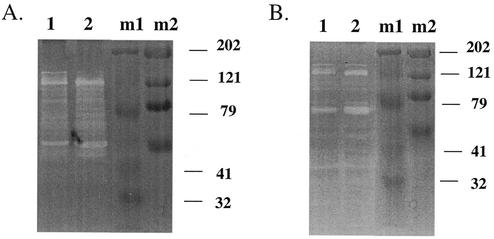
To determine whether decreased autolysis in vancomycin-exposed S. aureus isolates IL-A, IL-F, 523a to -k, and clinical GISA isolates was due to a changed physical property of vancomycin-exposed cell walls, heat-killed cells from strains 523, 523a, 523k, IL-A, and IL-F were incorporated as overlays into zymograms. Zymography was performed as described previously (20) with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis to resolve MHs. Proteins were obtained from the intracellular, cell wall, and extracellular fractions, as described previously (5). Overlays of strain 523 could be hydrolyzed by MHs from any fraction of any of the S. aureus test strains (data not shown). In contrast, overlays of the Triton X-100-resistant S. aureus strains 523a, 523k (data not shown), IL-A, and IL-F were resistant to MHs from all fractions from strains IL-A and IL-F (Fig. 4) and all other sources we tested (data not shown). The poor activity was not due to inactive enzymes, since the MHs from the Triton X-100-resistant S. aureus isolates were highly active (IL-F more so than IL-A) when evaluated on heat-killed cells of Micrococcus luteus or S. aureus strain 523 (Fig. 5). These data demonstrate that vancomycin-selected resistance to Triton X-100 in strains IL-A, IL-F, 523a, and 523k is correlated with resistance to MHs, presumably due to change(s) in the cell wall.
FIG. 4.
Zymograms containing overlays of heat-killed cells from strains IL-A and IL-F showing resistance to murein hydrolases extracted from the extracellular (EC), cell wall (CW), and intracellular (IC) fractions from strains IL-A and IL-F. Shown are murein hydrolases extracted from strains IL-A (lanes 1) and IL-F (lanes 2).
FIG. 5.
Intracellular murein hydrolases evaluated on zymograms containing overlays of heat-killed cells from M. luteus (A) or S. aureus strain 523 (B). Lanes: 1, MH from strain IL-A; 2, MH from strain IL-F; m1, Bio-Rad Kaleidoscope prestained standards; m2, Bio-Rad High Range prestained sodium dodecyl sulfate-polyacrylamide gel electrophoresis standard.
The data from isolates 523 to 523k prove that exposure of a vancomycin-naïve strain to low-level vancomycin in vitro was sufficient for selecting Triton X-100 resistance concurrently with decreasing susceptibility to vancomycin and that Triton resistance preceded the vancomycin-intermediate resistance phenotype. Moreover, we have now shown that four clinical GISA isolates and one vancomycin heteroresistant isolate (IL-A) all have decreased lytic capability compared with vancomycin-susceptible strains; therefore, the phenotype of Mu50 is an exception among the clinical GISA isolates we studied.
These data suggest that autolysis plays a role in vancomycin-mediated killing in S. aureus, and that a strain with decreased autolytic capacity could evade the lysis-inducing effect of vancomycin at an early stage. Thus, we propose a model whereby acquisition of intermediate vancomycin resistance requires at least two steps. The first involves acquisition of a decrease in autolytic activity mediated by resistance of cell walls to MHs. An isolate that could avoid lysis would have a prolonged survival time and an enhanced opportunity for a second-step mutation to lead to intermediate vancomycin resistance.
Resistance to autolysis was not likely due to a defect in the enzymatic activity of ATL in clinical isolates IL-A and IL-F. Although it might be hypothesized that decreased autolysis might explain the thickened cell walls described in GISA isolates, the cell wall of strain IL-F is 1.5-fold thicker than that of strain IL-A (2) without a further increase in resistance to autolytic activity. Understanding the increase in resistance to an exogenous MH, lysostaphin, as documented in GISA strain IL-F (2), may provide insight into the mechanism by which this occurs.
Several regulators of autolysin activity have been identified in S. aureus (4, 11-13). Although strains IL-A and IL-F were resistant to lysis, the MH activity was higher in strain IL-F than in strain IL-A. Since this increase was not explained by a change in the sequence of ATL, it will be interesting to learn if any of the regulators of autolysin activity were involved in that change.
Acknowledgments
We thank Fred Tenover (Centers for Disease Control and Prevention) for providing clinical GISA isolates from Michigan, Port Chester, N.Y., and New Jersey, and Keiichi Hiramatsu for providing isolate Mu50 from Japan.
S. Boyle-Vavra and R. S. Daum were supported by NIH R01 AI40481-01A1 to R.S.D. and R03 AI 44999-01 to S.B.-V. and by a grant from the Grant Healthcare Foundation (Lake Forest, IL).
REFERENCES
- 1.Blackman, S. A., T. J. Smith, and S. J. Foster. 1998. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology 144:73-82. [DOI] [PubMed] [Google Scholar]
- 2.Boyle-Vavra, S., R. B. Carey, and R. S. Daum. 2001. Development of vancomycin and lysostaphin resistance in a methicillin-resistant Staphylococcus aureus isolate. J. Antimicrob. Chemother. 48:617-625. [DOI] [PubMed] [Google Scholar]
- 3.Boyle-Vavra, S., S. Stenberg, and R. S. Daum. 1997. Decreased triton-induced lysis in two vancomycin resistant Staphylococcus aureus mutants derived in the laboratory from clinical susceptible isolate 523. 97th General Meeting American Society for Microbiology Abstract A-131, Miami, Fla.
- 4.Brunskill, E. W., and K. W. Bayles. 1996. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J. Bacteriol. 178:611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunskill, E. W., and K. W. Bayles. 1996. Identification of LytSR-regulated genes from Staphylococcus aureus. J. Bacteriol. 178:5810-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1997. Reduced susceptibility of Staphylococcus aureus to vancomycin—Japan, 1996. Morb. Mortal. Wkly. Rep. 46:624.. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1999. Staphylococcus aureus with reduced susceptibility to vancomycin—Illinois, 1999. Morb. Mortal. Wkly. Rep. 48:1165-1167. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1997. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb. Mortal. Wkly. Rep. 46:765-766. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1997. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, Sept. 1997. Morb. Mortal. Wkly. Rep. 46:813-815. [PubMed] [Google Scholar]
- 10.Daum, R. S., S. Gupta, R. Sabbagh, and W. M. Milewski. 1992. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomycin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J. Infect. Dis. 166:1066-1072. [DOI] [PubMed] [Google Scholar]
- 11.Fournier, B., and D. C. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 182:3955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto, D. F., E. W. Brunskill, and K. W. Bayles. 2000. Analysis of genetic elements controlling Staphylococcus aureus lrgAB expression: potential role of DNA topology in SarA regulation. J. Bacteriol. 182:4822-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groicher, K. H., B. A. Firek, D. F. Fujimoto, and K. W. Bayles. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 182:1794-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanaki, H., K. Kuwahara-Arai, S. Boyle-Vavra, R. S. Daum, H. Labischinski, and K. Hiramatsu. 1998. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 42:199-209. [DOI] [PubMed] [Google Scholar]
- 15.Holtje, J. V., and A. Tomasz. 1975. Lipoteichoic acid: a specific inhibitor of autolysin activity in pneumococcus. Proc. Natl. Acad. Sci. USA 72:1690-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroda, M., T. Ohta, Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1218-1219. [DOI] [PubMed] [Google Scholar]
- 17.Madiraju, M. V. V. S., D. P. Brunner, and B. J. Wilkinson. 1987. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 31:1727-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oshida, T., M. Sugai, H. Komatsuzawa, Y.-M. Hongh, H. Suginaka, and A. Tomasz. 1995. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L alanine amidase domain and an endo-β-N-acetylglucosaminidase domain: cloning, sequence analysis and characterization Proc. Natl. Acad. Sci. USA 92:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeltz, R. F., V. K. Singh, J. L. Schmidt, M. A. Batten, C. S. Baranyk, M. J. Nadakavukaren, R. K. Jayaswal, and B. J. Wilkinson. 2000. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob. Agents Chemother. 44:294-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qoronfleh, M. W., and B. J. Wilkinson. 1986. Effects of growth of methicillin-resistant and -susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob. Agents Chemother. 29:250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotun, S. S., V. McMath, D. J. Schoonmaker, P. S. Maupin, F. C. Tenover, B. C. Hill, and D. M. Ackman. 1999. Staphylococcus aureus with reduced susceptibility to vancomycin isolated from a patient with fatal bacteremia. Emerg. Infect. Dis. 5:147-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanyal, D., and D. Greenwood. 1993. An electron microscope study of glycopeptide antibiotic-resistant strains of Staphylococcus epidermidia. J. Med. Microbiol. 39:204-210. [DOI] [PubMed] [Google Scholar]
- 23.Sieradzki, K., R. B. Roberts, S. W. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517-523. [DOI] [PubMed] [Google Scholar]
- 24.Sieradzki, K., and A. Tomasz. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomasz, A., and S. Waks. 1975. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc. Natl. Acad. Sci. USA 72:4162-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]