Abstract
The stability of cefepime during simulated continuous infusion was determined with a motorized portable infusion pump worn over a period of 24 to 36 h. Susceptibility testing on cefepime solutions over time indicates that the degradation products do not exhibit antibacterial activity. Cefepime stability at 24 h following continuous infusion was 94.3% ± 1.0%, which supports the use of continuous infusion.
Continuous infusion (CI) is an efficient means of administering beta-lactams to maintain drug concentrations higher than the MIC throughout the dosing interval. Results from a number of clinical trials shows similar efficacy at a reduced dose (10, 12, 15). Thus, CI has a pharmacoeconomic advantage over intermittent dosing by achieving the same effect with a lower daily dose of drug (2, 6).
In order to support CI of a drug, it is necessary to establish that the desired drug is stable throughout the administration period. Previous studies indicate that cefepime is stable for 24 h at room temperature (4); however, cefepime is not stable for 24 h at body temperature (13). The temperature and stability of cefepime administered with motorized portable infusion pumps are presently unknown. The purpose of this study is to determine if cefepime can be administered via CI with a motorized portable infusion pump and to define optimal conditions for storage prior to administration. In addition, the major breakdown products of cefepime will be identified and their antibacterial activity will be characterized.
Methods and experimental design.
The high-performance liquid chromatography procedures utilized were based on a modification of a previously published cefepime assay (3). In brief, an analytical reversed-phase C18 column (250 by 4.6 mm; J&W Scientific) with 4.6-mm diameter particles was used to separate the various compounds found in the admixed drug solution. The column was connected onto a Hitachi L-6200 Intelligent pump, and the elutants were monitored at the cefepime absorption UV peak at 260 nm (9). The mobile phase consisted of 8% (vol/vol) acetonitrile in 20 mM ammonium acetate, adjusted to pH 4.9. The samples were kept at 4°C with a temperature-controlled water jacket. Sample concentrations were analyzed in duplicate. None of the degradation products interfered with the cefepime peak. The standard curve had a linear range from 25 to 250 μg/ml, with a correlation coefficient that was > 0.999. The intraday coefficients of variation measured in triplicate ranged from 0.05 to 1.15%, and interday during the 23 analytical days ranged from 1.33 to 3.48%. The assay lower limit of detection was 25 μg/ml, with a coefficient of variation of 0.54%.
CI cefepime was simulated by using a portable infusion pump (Microject 30; Sorenson Medical, West Jordan, Utah). The pump and the admixed solution (MediBag, ethylene vinyl acetate, 250 ml; Sorenson Medical) were placed inside individual pouches of a light protective bag worn on a belt. The drug solution was pumped through a cassette (Microject cassette and filter; Sorenson Medical) and into the vein. In this study, the drug was pumped into a waste bag (same type as drug reservoir), which was also worn on the same belt. During slumber the belt containing the pump and infusion bag was placed on a nightstand adjacent to the bed. The dose chosen for this study (100 mg/kg of body weight/24 h (maximum: 6 g/24 h) was derived from prior work evaluating CI ceftazidime (14), which exhibits pharmacokinetics similar to those of cefepime (1, 7, 8). Cefepime solutions were made with sterile Maxipime (lot no. 1A42352; expiration date, November 2003), containing arginine (as a buffer) in a concentration of 725 mg of cefepime/g to control the pH of the solution. Cefepime was admixed volumetrically to a final volume of 250 ml with sterile D5W (sterile dextrose, 5% in water). In order to simulate an actual home treatment situation, drug reservoirs containing cefepime were stored for 1 or 2 weeks in a freezer (−20°C) as well as in a refrigerator (4°C) (performed in duplicate) before CI. To mimic the patient situation, three individuals wore the pump and bag containing cefepime for 24 to 36 h. A total of 10 bags (four stored under refrigerated or frozen conditions and six freshly made solutions) were tested. Seven 2-ml aliquots were extracted from the administration bag at the following times: 0, 1, 2, 4, 8, 22, 24, and 36 h. These samples were frozen at −70°C until assayed. All samples were assayed within 3 months of collection. The measured stability after 24 h during simulated CI was used to determine clinical stability (defined as >90% cefepime remaining). Temperature changes in the drug reservoir during the administration were measured every 30 min with an electronic temperature detector (TempTrace; Dickson) that was placed adjacent to the drug reservoir.
Additional experiments were performed to provide information about the clinical stability of cefepime (1 gm/50 ml) at various relevant temperatures (4, 21, 37, and 55°C) (performed in duplicate). The duration for each experiment was dependent upon the temperature for which they were stored: 4°C for 2 weeks, 21°C for 1 week, 37°C for 2 days, and 55°C for 8 h. Seven samples were obtained at evenly spaced intervals for each experiment. Additional samples (twice daily) were obtained from the bags stored at 37°C for a period of 6 days in order to more completely investigate the rate of degradation of cefepime over the entire concentration range. Since the degradation rate is pH dependent, pH was measured for each sample (pH meter type 320; Corning). All samples were frozen at −70°C until assayed by high-performance liquid chromatography. Putative cefepime degradation products were determined with a Micromass Quattro Ultima triple-quadrupole tandem mass spectrometer (Micromass, Beverly, Mass.) running in positive ion mode. By following the appearance of new mass ions in solutions containing freshly prepared cefepime and 5, 20, and 45% degraded solutions and a completely degraded cefepime solution, the major breakdown products were identified.
MICs were determined for a freshly prepared solution, and apparent MICs were determined for five solutions of cefepime containing 87 to 12% active cefepime and various concentrations of cefepime degradation products. All tests were performed in quadruplicate. The MICs were determined by using macrodilution with a fixed inoculum of 106 CFU of a reference strain of Pseudomonas aeruginosa (ATCC 27853)/ml and Mueller-Hinton broth adjusted with calcium and magnesium according to instructions by the National Committee for Clinical Laboratory Standards. Samples containing cefepime and various concentrations of degradation products were diluted with various amounts of broth resulting in the standard twofold dilutions and precise intermediate dilutions between the standard twofold dilutions (i.e., 6, 10, and 12 μg/ml for solutions containing 45 to 100% cefepime; 6 and 12 μg/ml for a solution containing 30% cefepime; and 12, 20, 24, and 28 μg/ml for a solution containing 12% cefepime).
Stability and temperature in infusion bags during simulated CI.
The percentage of cefepime remaining after 24 and 30 h of CI was 94.3% ± 1.0% and 92.2% ± 3.5%, respectively. Cefepime solutions administered over 24 h were significantly above the stability limit of 90% (P < 0.0001). The temperature in the drug reservoirs showed modest variation during the 24 h, with 87.5% of all the average temperatures falling in the range of 20 to 25°C (Fig. 2). One limitation is that stability was not tested at the extreme ambient temperature that might be expected during summertime.
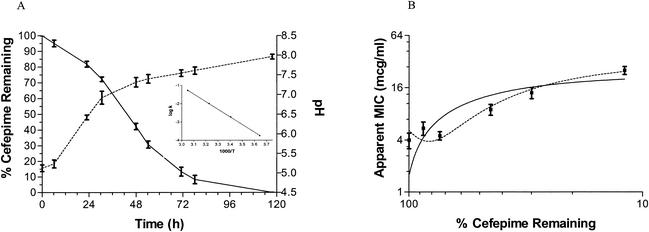
FIG. 2.
(A) Degradation of cefepime at 37°C. Observed degradation curve (solid line) and the measured pH during the cefepime degradation (dashed line). When the pH increases as a result of cefepime breakdown, cefepime degradation deviates from predicted first-order kinetics. Inset shows the Arrhenius plot of the degradation of cefepime (k) at four different temperatures ranging from 1°C to 55°C. Temperatures on the x axis are measured in kelvins. A log linear relationship exists between the degradation of cefepime and temperature (r2 > 0.99), with a slope of −4.16 × 103 and intercept of 11.4. The activation energy was calculated to be 81.4 kJ mol−1 K−1. (B) Antibacterial activity during cefepime degradation. The apparent MIC increases proportionally with the percentage of cefepime remaining (first-order polynomial, r2 > 0.73; second-order polynomial, r2 > 0.90), which indicates that the degradation products do not exhibit significant antibacterial activity.
Stability during various storage conditions.
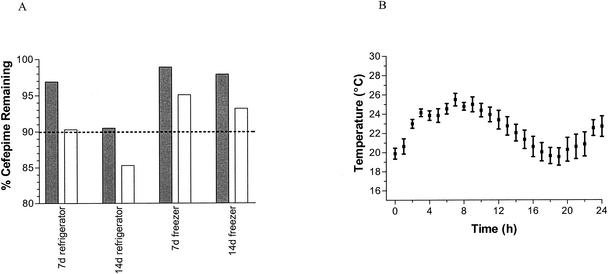
The results from all storage experiments are summarized in Fig. 1. The cefepime solution was stable after storage and simulated CI in all experiments, with the exception of the one following 2 weeks' storage in a refrigerator. Cefepime stored at 4°C for 2 weeks was found to have 90.5% parent drug, and after the same solution was worn to simulate CI for 24-h CI, the percentage of cefepime remaining was 85.3%.
FIG. 1.
Left panel, stability of cefepime solution under refrigerated and freezer storage conditions. Each set of paired columns represents one storage condition (shaded bar) and stability after storage and use as a 24-h simulated CI (unshaded bar). Ninety percent stability is indicated by the dashed line. Right panel, variability in temperature throughout the 24-h CI period.
The complete degradation of cefepime in D5W at 37°C demonstrates that the degradation follows first-order kinetics for the first 25% of the degradation (r2 > 0.97), while the remainder of the degradation deviates from first-order kinetics. An explanation of this is illustrated in Fig. 2, which shows that the amount of cefepime remaining varies in inverse proportion to the pH of the solution. This suggests that accumulation of alkaline degradation products may be responsible for increases to the pH and thereby for increases to the rate of cefepime degradation. The degradation of cefepime solutions was also associated with colorimetric changes, with the completely degraded solution providing a characteristic orange or brown. The stability results from all the experiments at four different temperatures were used to construct an Arrhenius plot seen in Fig. 2. Assuming first-order degradation, the temperature limit for clinical stability of cefepime when administered via CI is 29.1°C. In addition, the stability in the refrigerator was calculated to be 95.8% after 1 week and 91.8% after 2 weeks and in the freezer was calculated to be 99.6 and 99.3% at 1 and 2 weeks, respectively.
Determination of degradation products.
Mass spectrometry data indicated that degradation of cefepime includes cleavage of N-methylpyrrolidine (R2 side chain) and opening of the cephem (β-lactam ring). This was the expected breakdown of cefepime, since two related compounds, ceftazidime and cefpirome, have shown similar breakdown (5, 11). It also indicates that the ring opening occurs before the cleavage of N-methylpyrrolidine. This conclusion is taken from the relative amounts of degradation products seen in the mass scans after different amounts of cefepime have degraded. There were no observed degradation products where ring opening had occurred without N-methylpyrrolidine being cleaved first. A concern with the degradation products is their possible toxicological effects. Studies performed with ceftazidime demonstrate that it breaks down to form the known toxic compound pyridine (13). This is not a concern for cefepime, since pyridine is the R2 side chain only of ceftazidime; however, cefepime has N-methylpyrrolidine as a R2 side chain, and the toxicological effects of this compound or other breakdown products are presently unknown.
Correlation between cefepime stability and in vitro antibacterial activity.
The apparent MICs for cefepime increased linearly, with decreasing amounts of cefepime remaining in the solutions (r2 > 0.73; P < 0.0001). This observation suggests that the degradation products do not exhibit antibacterial activity, since the apparent MIC increases during degradation. One limitation is that the MIC measurements are not continuous values and that some concentration gaps were up to 50%; however, despite this limitation, it is clear that the solutions that had significant quantities of the degradation products exhibited reduced activity against the reference strain of P. aeruginosa.
Several studies have been conducted to evaluate the stability of cefepime under various conditions. The results of these studies indicate that cefepime is stable at room temperature for a minimum of 24 h (4, 16) (cefepime product monograph, Bristol-Myers Squibb, July 2000). These data indicate that cefepime should be stable when administered as a CI over 24 h. Our data support this conclusion demonstrating that the temperature in the drug reservoir remained below 30°C, resulting in clinically insignificant drug loss over a 24-h interval. In contrast, Viaene et al. recently published an evaluation of cefepime stability and concluded that cefepime is not sufficiently stable to support CI with portable infusion pumps (13). In their study, portable elastomeric pumps were used to evaluate the 24-h stability of cefepime. These pumps are utilized commonly in the outpatient setting due to their ease of use and are relatively inexpensive. However, they are typically placed under the clothing near the body. Thus, the temperature of the drug solution within the reservoir approximates normal body temperature. Since our study utilized a motorized portable infusion pump that was placed in an external pouch (separate from the drug reservoir) worn around the waist, the higher temperature could explain the greater degradation noted in their study (10% degradation over 13 h for 37°C and 10% degradation over 20.5 h at 25°C) than in our study (5.8% degradation over 24 h). The use of motorized portable infusion pumps and the bag system may be more suitable for CI regimens with drugs that exhibit temperature-dependent stability.
In summary, this study has shown that both the stability and the antibacterial activity of cefepime solutions support 24-h CI with a motorized portable infusion pump. Patients who will be outside in temperatures exceeding 29°C for any length of time should place a cold pouch adjacent to the drug reservoir to ensure stability of the cefepime administered. Cefepime solutions must be stored in a refrigerator, and if the supply is for more than 5 days, it should be kept in a freezer and allowed to thaw in a refrigerator 1 day before use.
Acknowledgments
This study was supported in part by an educational grant from Elan Pharmaceuticals. The portable infusion bag and supplies were provided as a gift from Sorenson Medical.
REFERENCES
- 1.Arguedas, A. G., H. R. Stutman, M. Zaleska, C. A. Knupp, M. I. Marks, and E. Nussbaum. 1992. Cefepime pharmacokinetics and clinical response in patients with cystic fibrosis. Am. J. Dis. Child. 146:797-802. [DOI] [PubMed] [Google Scholar]
- 2.Craig, W. A. 2002. Pharmacodynamics of antimicrobials: general concepts and applications, p. 1-22. In C. H. Nightingale (ed.), Antimicrobial pharmacodynamics in theory and clinical practice. Marcel Dekker, Inc., New York, N.Y.
- 3.Elkhaili, H., L. Linger, H. Monteil, and F. Jehl. 1997. High-performance liquid chromatographic assay for cefepime in serum. J. Chromatogr. B 690:181-188. [DOI] [PubMed] [Google Scholar]
- 4.Fubara, J. O., and R. E. Notari. 1998. Influence of pH, temperature and buffers on cefepime degradation kinetics and stability predictions in aqueous solutions. J. Pharm. Sci. 87:1572-1576. [DOI] [PubMed] [Google Scholar]
- 5.Fubara, J. O., and R. E. Notari. 1997. A kinetic oxymoron: concentration-dependent first-order rate constants for hydrolysis of ceftazidime. J. Pharm. Sci. 87:53-57. [DOI] [PubMed] [Google Scholar]
- 6.Grant, E. M., J. Kuti, D. P. Nicolau, C. H. Nightingale, and R. Quintiliani. 2002. Clinical efficacy and pharmacoeconomics of a continuous-infusion piperacillin-tazobactam program in a large community teaching hospital. Pharmacotherapy 22:471-483. [DOI] [PubMed] [Google Scholar]
- 7.Hamelin, B. A., N. Moore, C. A. Knupp, M. Ruel, F. Vallee, and M. LeBel. 1993. Cefepime pharmacokinetics in cystic fibrosis. Pharmacotherapy 13:465-470. [PubMed] [Google Scholar]
- 8.Huls, C. E., R. A. Prince, D. K. Seilheimer, and J. A. Bosso. 1993. Pharmacokinetics of cefepime in cystic fibrosis patients. Antimicrob. Agents Chemother. 37:1414-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ip, M., C. Au, S. W. Cheung, C. Y. Chan, and A. F. Cheng. 1998. A rapid high-performance liquid chromatographic assay for cefepime, cefpirome and meropenem. J. Antimicrob. Chemother. 42:121-123. [PubMed] [Google Scholar]
- 10.MacGowan, A. P., and K. Bowker. 1998. Continuous infusion of beta-lactam antibiotics. Clin. Pharmacokinet. 35:391-402. [DOI] [PubMed] [Google Scholar]
- 11.Sugioka, T., T. Asano, Y. Chikaraishi, E. Suzuki, A. Sano, T. Kuriki, M. Shirotsuka, and K. Saito. 1990. Stability and degradation pattern of cefpirome (HR 810) in aqueous solution. Chem. Pharm. Bull. 38:1998-2002. [DOI] [PubMed] [Google Scholar]
- 12.Tessier, P. R., D. P. Nicolau, C. Onyeji, and C. H. Nightingale. 1999. Pharmacodynamics of intermittent- and continuous-infusion cefepime alone and in combination with once-daily tobramycin against Pseudomonas aeruginosa in an in vitro infection model. Chemotherapy 45:284-295. [DOI] [PubMed] [Google Scholar]
- 13.Viaene, E., H. Chanteux, H. Servais, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative stability studies of antipseudomonal β-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob. Agents Chemother. 46:2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinks, A. A., D. J. Touw, H. G. Heijerman, M. Danhof, G. P. de Leede, and W. Bakker. 1994. Pharmacokinetics of ceftazidime in adult cystic fibrosis patients during continuous infusion and ambulatory treatment at home. Ther. Drug Monit. 16:341-348. [DOI] [PubMed] [Google Scholar]
- 15.Vondracek, T. G. 1995. Beta-lactam antibiotics: is continuous infusion the preferred method of administration? Ann. Pharmacother. 29:415-423. [DOI] [PubMed] [Google Scholar]
- 16.Williamsen, J., D. Volles, P. Lynch, P. Rogers, and D. Haverstick. 1999. Stability of cefepime in peritoneal dialysis solution. Ann. Pharmacother. 33:906-909. [DOI] [PubMed] [Google Scholar]