Abstract
To design new strategies of antiviral therapy for chronic hepatitis B, we have evaluated the antiviral activity of the combination of amdoxovir (DAPD), emtricitabine [(−)FTC], and clevudine (l-FMAU) in the duck hepatitis B virus (DHBV) model. Using their triphosphate (TP) derivatives in a cell-free system expressing a wild-type active DHBV reverse transcriptase (RT), the three dual combinations exhibited a greater additive inhibitory effect on viral minus-strand DNA synthesis than the single drugs, according to the Bliss independence model. Both dual combinations with DAPD TP were the most efficient while the triple combination increased the inhibitory effect on the DHBV RT activity in comparison with the dual association, however, without additive effect. Postinoculation treatment of experimentally infected primary duck hepatocytes showed that dual and triple combinations potently inhibited viral DNA synthesis during treatment but did not inhibit the reinitiation of viral DNA synthesis after treatment cessation. Preinoculation treatment with the same combinations exhibited antiviral effects on intracellular viral DNA replication, but it was unable to prevent the initial covalently closed circular DNA (cccDNA) formation. Short-term in vivo treatment in acutely infected ducklings showed that the dual combinations were more-potent inhibitors of virus production than the single treatments, with the l-FMAU and FTC combination being the most potent. A longer administration of l-FMAU and FTC for 4 weeks efficiently suppressed viremia and viral replication. However, no viral clearance from the liver was observed, suggesting that the enhanced antiviral effect of this combination was not sufficient for cccDNA suppression and HBV eradication from infected cells.
Despite the existence of efficient vaccines, hepatitis B virus (HBV) infection remains a major public health problem worldwide with 400 million chronic carriers, who are exposed to a risk of developing liver cirrhosis and hepatocellular carcinoma (24). To date, alpha interferon and lamivudine (2′,3′-dideoxy-3′-thiacytidine, or 3TC) are the only approved drugs for chronic HBV infection. Alpha interferon therapy is only partially effective and is frequently limited by adverse effects (16). 3TC, a cytidine analog, is a very efficient inhibitor of HBV replication (23). Although 3TC efficiently inhibits HBV replication, the slow kinetics of viral elimination during 3TC therapy (33) and the spontaneous viral genome variability lead to the emergence of drug-resistant mutants which carry mutations affecting the reverse transcriptase (RT) domain (30, 32, 31, 36). Approximately 50% of treated patients develop viral resistance after 3 years of treatment with 3TC (26). Resistance to nucleoside analogs is associated with substitutions in the nucleic acid sequence of the polymerase gene causing changes in the amino acid sequence of the HBV RT, notably in the YMDD motif within the catalytic site. The most common polymerase variant is the rtL180M-plus-M204V change (according to the recent genotype-independent nomenclature for HBV drug-resistant variants) (39) that associates a mutation in the catalytic site (rtM204V) with a compensatory mutation in the B domain of the RT (rtL180M) which provides a higher replication capacity to the catalytic site variant (2, 4, 31, 34, 35).
Consequently, the anti-HBV activity of new analogs, such as PMEA (also called adefovir) and entecavir (also called BMS200-475) is under evaluation in experimental models and in clinical trials (12, 28, 40). The potent antiviral activity of clevudine (l-FMAU) and emtricitabine (FTC) has been previously demonstrated in in vitro and in vivo experimental studies (1, 9, 13, 20, 37). Furthermore, the duck model of HBV (DHBV) infection represents a suitable system for the study of in vitro and in vivo activity of anti-HBV agents as well as their toxicity. It provides relevant tools for detailed molecular studies of hepadnavirus replication and its inhibition (3, 11, 35, 37). Moreover, a previous study of the antiviral activity of five nucleoside an-alogues, 2R-cis-4-(2,6-diamino-9H-purin-9-yl)-1,3-dioxolane-2-methanol (DAPD, amdoxovir), 2R-cis-2-amino-1,9-dihydro-9-(2-[hydroxymethyl]-1,3-dioxolan-4-yl)-6H-purin-6-one, 4-amino-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxothiolan-5-yl]2(1H)-pyrimidinone (2R-cis) [(−)FTC], 3TC, and 1-(2-fluoro-5-methyl-β-l-arabinosyl) uracil (l-FMAU) showed that they inhibit HBV replication and exhibit different mechanisms of action (37). DAPD, a dioxolane nucleoside analog inhibits the priming and the elongation of viral minus-strand DNA in vitro (37). FTC, which is closely related to 3TC, inhibits the elongation of the viral minus-strand DNA, whereas l-FMAU, a thymidine analog, mainly inhibits DNA-dependent viral positive-strand DNA synthesis. While DAPD is also active against the rtL180M plus M204V mutant polymerase, FTC and l-FMAU showed minimal activity against 3TC-resistant mutants (5, 37).
In order to better control viral replication and delay the emergence of virus-resistant mutants, it is critical to develop new antiviral strategies based on combinations (42) that may also prevent the selection of cross-resistant mutants, as was shown with human immunodeficiency virus (6, 38, 41). Few studies focused on examination of the antiviral effect of the combination of polymerase inhibitors for the therapy of chronic hepadnavirus infection and used different experimental models (7, 8, 19). As DAPD, FTC, and l-FMAU are potent antiviral compounds that inhibit different steps of the HBV replication cycle, they represent potential candidates for such combinations. Therefore, we examined the inhibitory effect of DAPD, FTC, and l-FMAU associated with dual or triple combinations in comparison with single-drug administration in the DHBV model. We studied in vitro the effect of the combinations on reverse transcription by using the cell-free expression system of the DHBV polymerase and on DHBV genome replication by using experimentally infected primary duck hepatocytes. In addition, we analyzed in vivo the antiviral effect of the combination of l-FMAU and FTC in experimentally infected ducklings.
MATERIALS AND METHODS
Antiviral drugs.
DAPD, (−)FTC, and l-FMAU and their triphosphate (TP) forms, used for the cell-free assay, were provided by Triangle Pharmaceuticals, Inc. (Durham, N.C.).
Evaluation of inhibitory activity of compounds on DHBV RT activity.
Expression of the wild-type polymerase and the L489M-plus-M512V DHBV double mutant (corresponding to the rtL180M-plus-M204V double mutant in HBV) and analysis of viral minus-strand DNA chain extension, i.e., reverse transcription, were performed as previously described in detail (35, 37, 43). As this work focused on evaluation of the effect of drug combinations on viral minus-strand elongation, modifications were made to the reaction mixtures. They included dATP and dGTP (100 μM each) and 0.165 μM (each) [α-32P]dCTP and [α-32P]dTTP (3,000 Ci/mmol) for combinations of (−)FTC TP and l-FMAU TP, dGTP and dTTP (100 μM each), and 0.165 μM (each) [α-32P]dCTP and [α-32P]dATP (3,000 Ci/mmol) for combinations of (−)FTC TP and DAPD TP, and dGTP and dCTP (100 μM each) and 0.165 μM (each) [α-32P]dATP and [α-32P]dTTP (3,000 Ci/mmol) for combinations of DAPD TP and l-FMAU TP. The study of the combination of the 3 nucleoside analogues required dGTP (100 μM) and 0.165 μM (each) [α-32P]dATP, [α-32P]dCTP, and [α-32P]dTTP (3,000 Ci/mmol each).
Primary fetal duck hepatocyte cultures: evaluation of the anti-DHBV activity and cytotoxicity of the combinations.
Cell cultures and inoculations were performed as previously described (3, 11). Preinoculation and postinoculation treatment protocols used single, dual, or triple combinations. DAPD, FTC, and l-FMAU have been tested at low (1, 0.5, and 0.1 μM, respectively) and high (2, 1, and 0.5 μM, respectively) concentrations that were determined according to previous results (37). In the preinoculation protocol, the addition of an inoculum containing 30 DHBV genome equivalents per cell for 2 h at 37°C was performed 2 days after cell plating. Treatment started 1 day before inoculation for 7 consecutive days. For each experiment, cell cultures were performed in duplicate. At the end of treatment (day 8), one set of hepatocyte cultures was harvested and cells were lysed for the extraction of intracellular viral DNA, whereas the second set was maintained for 5 more days after cessation of the treatment (day 13). In the postinoculation protocol, cells were inoculated before plating with the same inoculum as described above. Hepatocytes were maintained for 9 to 13 days postinoculation. The addition of nucleoside analogs started 3 days postinoculation for 6 consecutive days. For each experiment, cell cultures were performed in duplicate. At the end of treatment (day 9), one culture set was harvested, cells were lysed, and intracellular viral DNA was extracted, whereas the second culture set was maintained for 4 additional days (day 13).
Cellular cytotoxicity was analyzed daily by light microscope examination. Briefly, cells were seeded in 24-well tissue culture plates and cultured in medium containing increasing concentrations of the different drugs for 7 consecutive days, with daily change of medium. Cell viability was estimated by neutral red assay as described by Hantz et al. (15). After incubation in medium containing 5 mg of neutral red dye (Sigma, L’Isle d’Abeau, France)/100 ml for 3 h at 37°C, the cells were fixed in a 4% formaldehyde-1% calcium chloride solution and finally lysed in an acetic acid-ethanol mixture as described earlier (37).
Experimental inoculations of ducklings.
Ducklings were maintained under normal daylight, fed with a standard commercial diet and water ad libitum, in accordance with the guidelines for animal care at the facilities of the Ecole Nationale Vétérinaire of Lyon, France. Five-day-old Pekin ducklings were inoculated intravenously with a DHBV-positive serum containing 4.3 × 108 viral genome equivalents (vge), following a protocol previously described (1, 11, 25, 43). Ducklings received nucleoside analogs (FTC, l-FMAU, or DAPD) in mono-, bi-, or tritherapy by daily intraperitoneal administration for 3 days post-viral inoculation for a duration of 7 days (short-term study, studies 1 and 2) or 28 days (long-term study, study 3) according to the protocols described in Results. All three studies were performed consecutively. Viremia, animal weight, and lactic acid levels (Lactate PAP; Biomerieux, Marcy l'Etoile, France) were monitored throughout the study period. Animals were monitored for 2 additional weeks after drug withdrawal for short-term studies. Serum samples were collected every other day for DHBV DNA quantification. In the long-term study, ducklings received nucleoside analogs (FTC plus l-FMAU) by daily intraperitoneal administration for 3 days postinoculation for a duration of 28 days at doses of 100 and 40 mg/kg of body weight/day, respectively. Blood samples were collected 6 days per week during the first 3 weeks, twice per week during the next 2 weeks, every day after treatment cessation for 2 weeks, and every week until the end of the study (day 223) for analysis of viral markers and drug tolerance. All animals alive at the end of the treatment (day 7 in short-term study 2) and at the end of follow-up (in short-term study 1 and long-term study 3) were euthanized by lethal injection of pentobarbital (Doléthal; Vétoquinol, Lure, France). Liver samples were snap-frozen in liquid nitrogen and subsequently stored at −80°C for viral DNA analyses.
Viral DNA extraction and radiolabeled detection.
Primary duck hepatocytes were lysed at day 8 or 9 and day 13 for intracellular viral DNA analysis, depending on the experimental protocol. Cells were rinsed with phosphate-buffered saline and stored at −80°C for DNA isolation. Total viral DNA was extracted and then subjected to agarose gel electrophoresis and Southern blotting as described earlier (17, 25). Samples containing 0.5 μg of cellular DNA were analyzed after electrophoresis through 1.2% agarose gels, transferred by Southern blotting to nylon membranes (Byodine B membrane; Merk Eurolab), and hybridized with an α-32P-labeled full-length DHBV genomic DNA probe as already described (44). Autoradiography of the Southern blot was quantitated by ImageQuant after PhosphorImager scanning (Amersham Pharmacia Biotech) as previously described (37).
For the in vivo study, viremia was assessed throughout the protocol period by a semiquantitative detection of DHBV DNA by a specific dot blot hybridization assay. Fifty microliters of serum was spotted directly onto nylon filters (Byodine B membrane; Merk Eurolab) with the Hybri-Dot Manifold apparatus (Life Technologies, Cergy Pontoise, France). After denaturation (0.2 M NaOH, 1 M NaCl), neutralization (0.5 M Tris-HCl [pH 8] with 1 M NaCl followed by 2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]), and fixation (80°C for 2 h), filters were hybridized with a full-length DHBV genomic DNA probe and analyzed by the ImageQuant software. (25).
Isolation of DHBV DNA and quantification of viral load by real-time PCR.
Isolation of viral DNA for quantitative amplification was extracted from 50 μl of serum by using the High Pure viral nucleic acid kit according to the manufacturer's instructions (Roche Diagnostics, Meylan, France). Elution was carried out in 40 μl of elution buffer. Quantitative analysis of viral load was carried out by real-time PCR (Roche Diagnostics). Primers were designed with Oligo 5 software (MedProbe, Olso, Norway) for conserved nucleic acid sequences within the DHBV POL gene. Amplification of a 159-bp product was detected by using hybridization probes designed by TibMolBiol (Berlin, Germany). Amplifications were performed in a 20-μl reaction mixture containing 4 μl of extracted DNA from serum (50 μl) or liver samples (0.2 g), Fast Start Taq DNA polymerase, reaction buffer dNTP mix (Roche Molecular Biochemicals), 3 mM MgCl2, 0.5 mM concentrations of forward primer PA2 (nucleotides [nt] 1569 to 1588, 5′ CTG ACG GAC AAC GGG TCT AC 3′) and reverse primer PAS1 (nt 1728 to 1710, 5′ GGG TGG CAG AGG AGG AAG TA 3′), and 0.2 and 0.4 μM concentrations, respectively, of 3′-labeled fluorescein probe DHBV FL (nt 1633 to 1658, 5′ CCT CCA TCT CTT CAC TAC TGC CCT CG X 3′) and 5′-labeled Red 640 DHBV LC (nt 1661 to 1685, 5′ Red 640 TCC GAA ATC TCT CGT CGC TTT AAC Gp 3′). The EcoRI site was referred to as nt 1. Amplification occurred after denaturation at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 10 s, fluorescence acquisition at 58°C for 3 s, annealing for 10 s at 61°C, and extension at 72°C for 15 s. The sensitivity limit of this real-time PCR has been evaluated at 100 copies of DHBV DNA per ml of serum after extraction of serial dilutions of cloned DHBV genome in DHBV-negative serum. For quantification of intrahepatic viral DNA, viral DNA was extracted at the time of animal autopsy according to a procedure described elsewhere (25). After homogenization in 10 mM Tris-HCl (pH 7.5) and 10 mM EDTA, the liver samples were processed for isolation of total viral DNA. After proteinase K digestion, phenol-chloroform extraction was followed by ethanol precipitation. GAPDH amplification was used for normalization of liver samples. Sequence primers were chosen from Clustal alignment of chicken cDNA (CHKGAPDH-K0145) with forward primer S2 (nt 387 to 419, 5′ AAG GGT GGG GTG CTA AGC GTG 3′) and reverse primer AS2 (nt 505 to 485, 5′ GCC AGG CAG TTG GTG GTG C 3′), producing a 118-bp fragment amplification after denaturation at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 s, annealing for 10 s at 55°C, and extension at 72°C for 6 s.
Statistical analysis and modeling of antiviral effects.
The use of several drugs in a same trial requires a detailed definition of the different observed effects: (i) additive (effect expected when two drugs are associated = product of the fractional effect of each drug), (ii) synergistic (observed effect in combination > additive effect), or (iii) antagonistic (effect of combination < additive effect). The Bliss independence model was used in this study to determine the effect of tested combinations. This approach defines the theoretical additive effect (Exy or Exyz) of 2 or 3 drugs (x, y, and z) used as Exy = Ex × Ey or Exyz = Ex × Ey × Ez, with E corresponding to the percentage of active viral replication. The observed experimental effect E is compared to the theoretical additive effect Exy: if E = Exy, the combination is additive; if E > Exy, the combination is synergistic; if E < Exy, the combination is antagonistic (14). Mann-Whitney tests were used to compare the inhibitory activities of drug combinations on viremia and intrahepatic viral DNA replication in experimentally infected ducklings. Differences were considered statistically significant when P values were below 0.05.
RESULTS
Inhibitory effect of dual and triple combinations on DHBV RT activity.
We have evaluated the effect of the dual and triple combinations of the TP forms of DAPD (DAPD TP), FTC (FTC TP), and l-FMAU (l-FMAU TP) on the wild-type RT and the L489M-plus-M512V mutant expressed in a cell-free system. Combinations were evaluated by using different concentrations of each nucleoside analog, which were determined in a previous assay (37).
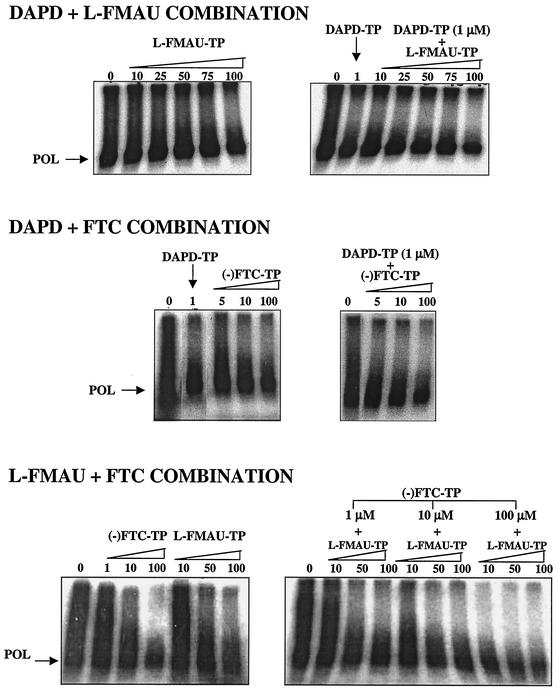
As reported in Table 1 (corresponding to 4 assays), a study of viral minus-strand DNA elongation showed that all combinations inhibited the RT activity more effectively than single drugs. Inhibition was dose dependent for all dual combinations. DAPD TP-l-FMAU TP and DAPD TP-FTC TP combinations had an additive inhibitory effect for almost all the tested concentrations, according to the Bliss independence model. In contrast, the observed effect of the dual combination FTC TP-l-FMAU TP was greater than the effect of the single drugs, but only two of nine drug ratios tested showed an additive effect (Table 1; Fig. 1). Furthermore, the triple combination, which was evaluated with three radiolabeled nucleotides and compared to the three dual combinations tested under similar conditions, effectively inhibited viral minus-strand DNA synthesis (Table 1). Most of the triple combinations exhibited a greater inhibition than that observed with single drugs and the dual combinations, but the triple combination showed no additivity according to the Bliss independence model (Table 1).
TABLE 1.
In vitro inhibitory activity of dual and triple combinations of DAPD TP, FTC TP, and l-FMAU TP on the RT activity of the wild-type DHBV polymerasea
| Combination and concn (μM) | 2 radiolabeled nucleosides
|
3 radiolabeled nucleosides
|
||||
|---|---|---|---|---|---|---|
| Experimental inhibition (%) | Theoretical additivity | Bliss independence | Experimental inhibition (%) | Theoretical additivity | Bliss independence | |
| DAPD-l-FMAU | ||||||
| 1, 10 | 29 ± 5 | 35 ± 11 | >Single | |||
| 1, 25 | 33 ± 4 | 36 ± 16 | >Single/ADD | 38 ± 7 | 51 ± 7 | >Single |
| 1, 50 | 34 ± 4 | 39 ± 13 | >Single/ADD | 50 ± 11 | 54 ± 9 | >Single/ADD |
| 1, 100 | 43 ± 6 | 45 ± 13 | >Single/ADD | 51 ± 16 | 60 ± 10 | >Single |
| DAPD-FTC | ||||||
| 1, 1 | 42 ± 12 | 45 ± 11 | >Single/ADD | |||
| 1, 5 | 57 ± 6 | 56 ± 8 | ADD | |||
| 1, 10 | 56 ± 13 | 45 ± 16 | SYN | 44 ± 5 | 56 ± 5 | >Single |
| 1, 50 | 46 ± 10 | 65 ± 9 | >Single | |||
| 1, 100 | 66 ± 9 | 79 ± 10 | >Single | 50 ± 12 | 68 ± 7 | >Single |
| FTC-l-FMAU | ||||||
| 1, 10 | 12 ± 10 | 24 ± 6 | >l-FMAU Single | |||
| 1, 50 | 34 ± 18 | 39 ± 1 | >Single/ADD | |||
| 1, 100 | 44 ± 8 | 49 ± 4 | >Single/ADD | |||
| 10, 10 | 37 ± 4 | 45 ± 0 | >Single | |||
| 10, 25 | 33 ± 14 | 44 ± 5 | >Single | |||
| 10, 50 | 43 ± 8 | 56 ± 4 | >Single | 36 ± 4 | 47 ± 6 | >Single |
| 10, 100 | 53 ± 4 | 63 ± 6 | >Single | 51 ± 13 | 54 ± 8 | >Single/ADD |
| 50, 25 | 40 ± 7 | 55 ± 11 | >l-FMAU Single | |||
| 50, 50 | 49 ± 8 | 57 ± 9 | >Single | |||
| 50, 100 | 49 ± 9 | 63 ± 11 | >Single | |||
| 100, 10 | 65 ± 5 | 74 ± 2 | >Single | |||
| 100, 25 | 44 ± 8 | 59 ± 7 | >l-FMAU Single | |||
| 100, 50 | 72 ± 3 | 79 ± 4 | >Single | 50 ± 4 | 61 ± 6 | >Single |
| 100, 100 | 71 ± 2 | 82 ± 4 | >Single | 66 ± 4 | 66 ± 8 | ADD |
| DAPD-FTC-l-FMAU | ||||||
| 1, 10, 25 | 48 ± 11 | 65 ± 7 | >Double | |||
| 1, 10, 50 | 47 ± 8 | 67 ± 7 | >Double | |||
| 1, 10, 100 | 58 ± 5 | 71 ± 6 | >Double | |||
| 1, 50, 25 | 48 ± 4 | 72 ± 8 | >Double | |||
| 1, 50, 50 | 51 ± 8 | 74 ± 9 | >Double | |||
| 1, 50, 100 | 56 ± 4 | 77 ± 9 | >Double | |||
| 1, 100, 25 | 45 ± 6 | 75 ± 6 | <FTC Single | |||
| 1, 100, 50 | 50 ± 7 | 76 ± 6 | >Double | |||
| 1, 100, 100 | 61 ± 13 | 79 ± 7 | <l-FMAU + FTC | |||
Results are expressed as percentages of inhibition of viral minus-strand DNA elongation and correspond to the mean values of 4 experiments. Abbreviations: ADD, additivity; SYN, synergy; >Single or double, effect superior to single or double drug combination.
FIG. 1.
Qualitative analysis of the inhibitory activity of dual and triple combinations with DAPD TP, FTC TP and l-FMAU TP on wild-type RT activity. The result of one typical experiment for one of the tested combinations with two radiolabeled deoxynucleoside TPs is presented. Autoradiograms of electrophoresis show the inhibition of viral minus-strand DNA synthesis by a single drug or a dual combination. Drug concentrations are indicated at the tops of the autoradiograms.
As a greater effect of the dual combination with DAPD TP on the wild-type DHBV polymerase activity was observed, it was important to determine whether the addition of FTC TP or l-FMAU TP to DAPD TP (in dual or triple combination) could enhance the inhibitory activity of these compounds on the L489M-plus-M512V mutant polymerase which was resistant to the inhibitory effect of FTC TP and l-FMAU TP but sensitive to the action of DAPD TP (37). The results showed that the inhibitory activity of DAPD TP on the mutant DHBV polymerase was not significantly increased by the addition of FTC TP or l-FMAU TP (data not shown).
Antiviral activity of DAPD, FTC, and l-FMAU administered alone or in combination after DHBV inoculation of primary duck hepatocytes.
Primary duck hepatocytes received dual or triple combinations 3 days after inoculation for 7 consecutive days. Two concentrations, defined as efficient in a previous study (37), were tested for each compound. Cultures were maintained for 4 days after treatment cessation to analyze the influence of compound combinations on the reinitiation of viral DNA synthesis.
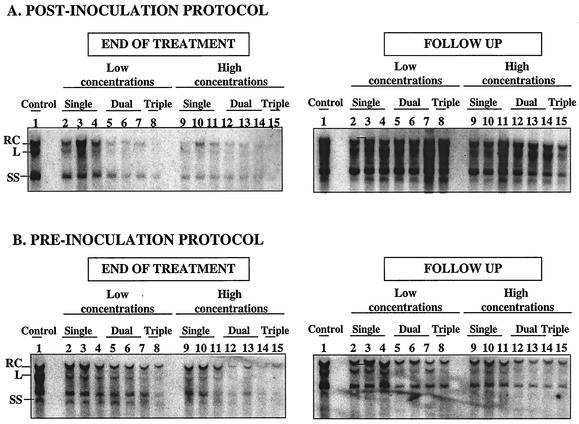
Analysis of intracellular viral DNA at the end of treatment showed that the inhibition of viral DNA synthesis by combinations of nucleoside analogs was dose dependent and more efficient than the observed inhibition with single drugs (Table 2, results corresponding to 3 assays). Combinations with low drug concentrations efficiently inhibited the synthesis of total viral DNA. The experimental effect of combinations was equivalent or greater than the theoretical additive effect according to the Bliss independence model (Table 2; Fig. 2A). Combinations with high drug concentrations potently inhibited total viral DNA synthesis. However, the observed effect was not additive for dual combinations according to the Bliss independence model (Table 2), whereas a synergistic effect was observed with the triple combination. In our experimental conditions, none of the combinations completely inhibit viral DNA synthesis.
TABLE 2.
Inhibition of total viral DNA synthesis at the end of treatment with dual and triple combinations in the postinoculation and preventive protocolsa
| Treatment | Low concnb results
|
High concnc results
|
||||
|---|---|---|---|---|---|---|
| Experimental result | Bliss independence
|
Experimental result | Bliss independence
|
|||
| Value | Additivity | Value | Additivity | |||
| Postinoculation | ||||||
| DAPD | 33 ± 3 | 62 ± 22 | ||||
| FTC | 23 ± 32 | 81 ± 26 | ||||
| l-FMAU | 32 ± 46 | 67 ± 34 | ||||
| DAPD-l-FMAU | 66 ± 2 | 54 ± 33 | SYN | 73 ± 38 | 84 ± 20 | >Single |
| DAPD-FTC | 62 ± 30 | 48 ± 24 | SYN | 76 ± 18 | 90 ± 14 | >Single |
| FTC-l-FMAU | 70 ± 12 | 40 ± 57 | SYN | 82 ± 26 | 89 ± 15 | >Single |
| DAPD-FTC-l-FMAU | 76 ± 2 | 59 ± 40 | SYN | 97 ± 5 | 94 ± 8 | SYN |
| Preinoculation | ||||||
| DAPD | 42 ± 33 | 42 ± 0 | ||||
| FTC | 45 ± 3 | 64 ± 9 | ||||
| l-FMAU | 48 ± 13 | 68 ± 16 | ||||
| DAPD-l-FMAU | 60 ± 8 | 68 ± 24 | >Single | 78 ± 4 | 81 ± 9 | ADD |
| DAPD-FTC | 58 ± 7 | 69 ± 16 | >Single | 83 ± 4 | 79 ± 5 | ADD |
| FTC-l-FMAU | 56 ± 5 | 71 ± 5 | >Single | 80 ± 26 | 87 ± 15 | ADD |
| DAPD-FTC-l-FMAU | 67 ± 14 | 83 ± 12 | >Double | 91 ± 2 | 94 ± 2 | ADD |
Results are expressed as percentages of inhibition of viral replication and correspond to the mean values of 3 independent experiments. Abbreviations: ADD, additivity; SYN, synergy; = or >Single, effect equivalent or superior to single drugs.
Low concentrations of DAPD, FTC, and l-FMAU are, respectively, 1, 0.5, and 0.1 μM.
High concentrations of DAPD, FTC, and l-FMAU are, respectively, 2, 1, and 0.5 μM.
FIG. 2.
Inhibitory effect of dual and triple combinations on intracellular DHBV DNA synthesis during drug administration and after treatment cessation in a postinoculation (A) or preinoculation (B) administration. Autoradiograms of Southern blot hybridizations of intracellular viral DNA are shown. The low concentrations of DAPD, FTC, and l-FMAU are 1, 0.5, and 0.1 μM, respectively; the high concentrations of DAPD, FTC, and l-FMAU are 2, 1, and 0.5 μM, respectively. Replication intermediates are represented as single strand (SS), linear (L), and relaxed circular (RC). Lanes: 1, no treatment; 2 and 9, DAPD; 3 and 10, FTC; 4 and 11, l-FMAU; 5 and 12, DAPD and l-FMAU; 6 and 13, DAPD and FTC; 7 and 14, FTC and l-FMAU; 8 and 15, DAPD, FTC, and l-FMAU.
After cessation of treatment, the observed effect of combinations was equivalent to that of the single drugs (Fig. 2A). In these experimental conditions, no delay in viral DNA synthesis rebound was observed after drug withdrawal.
Antiviral activity of DAPD, l-FMAU, and FTC alone or in combination administered prior to virus inoculation of primary duck hepatocytes.
To determine whether combinations are able to inhibit the initiation of infection, and especially the initial formation of covalently closed circular DNA (cccDNA), drugs were administered for 7 consecutive days beginning 1 day before inoculation. Cell cultures were maintained for 5 days after the cessation of treatment.
Intracellular DNA analysis at the end of treatment showed that compound combinations at low concentrations inhibited total DNA synthesis, with equivalent to greater effects than single drugs (Table 2, with results corresponding to 3 assays; Fig. 2B). However, at the end of treatment, only combinations with high concentrations had an additive inhibitory effect on the total DNA synthesis.
Interestingly, analysis of intracellular viral DNA showed that some combinations could delay the reinitiation of viral DNA synthesis (Fig. 2B). At low concentrations, dual and triple combinations seemed to have an equivalent to greater inhibitory effect on total DNA synthesis than single drugs. A synergistic effect of the dual combination of DAPD and FTC and the triple combination was observed at the end of follow-up. Moreover, at high concentrations, all compound combinations maintained a significant inhibitory effect on total viral DNA synthesis after drug withdrawal. This effect was synergistic according to the Bliss independence model (Fig. 2B) but was insufficiently potent to prevent the initial cccDNA formation.
Analysis of cellular toxicity.
An analysis of cell toxicity was performed in infected primary duck hepatocytes treated with 2.5, 5, 10, and 20 μM concentrations of each drug. Analysis based on the neutral red assay showed no toxicity for the concentrations used for the determination of the antiviral effect of the drugs and their combinations. A significant cellular toxicity was observed only with higher drug concentrations: 22 to 27% cell death was observed with concentrations of 10 and 20 μM (data not shown). These results suggest that the decrease of viral DNA synthesis observed in primary duck hepatocytes was not due to a deleterious effect of drug combinations on cellular viability.
Short-term administration of dual and triple drug combinations transiently inhibits viral replication in vivo in experimentally infected ducklings.
We used experimentally infected ducklings to determine whether a short-term administration of a combination of these nucleoside analogs may exhibit an enhanced antiviral effect. We first performed a dose-finding study (study 1) with FTC, l-FMAU, and DAPD administered as single drugs at two different doses and as dual combinations at the lower dose for each drug. We then compared the three dual therapies with the triple combination (study 2).
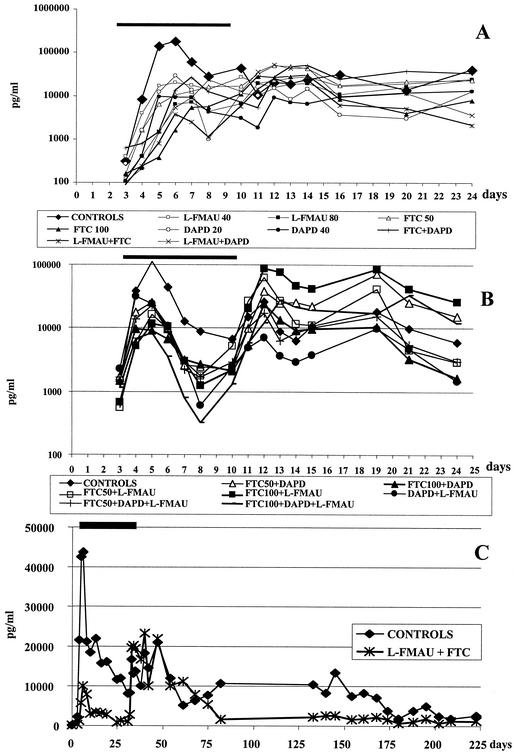
The results of the first dose-finding study (study 1) showed that all single and dual treatments inhibited DHBV replication but to different extents (Fig. 3A; Table 3). However, after drug withdrawal, a relapse of viral replication occurred in all groups. Comparison between untreated controls and monotherapies or dual therapies showed that the more-potent protocol was the administration of FTC at 100 mg/kg as well as the combination of FTC (50 mg/kg) and l-FMAU (40 mg/kg) (P < 0.05). Moreover, dual combination of DAPD (20 mg/kg) with FTC (50 mg/kg) or l-FMAU (40 mg/kg) exhibited a greater antiviral effect than DAPD, FTC, or l-FMAU monotherapies at the same dosage (P < 0.05). Using a quantitative real-time PCR assay for the detection of DHBV DNA, the results showed that, among the 3 dual combinations, l-FMAU plus FTC inhibited viral replication more effectively and significantly decreased virus production as calculated by the area under the curve (AUC) of viremia for each duck during drug administration (Table 3).
FIG. 3.
Dual and triple combination therapy decreased viremia in chronically infected ducks. Experimental protocols of the two short-term studies (studies 1 [A] and 2 [B]) and the long-term study (study 3 [C]) are described in detail in Materials and Methods and in Tables 3 and 4. Viremia (in picograms/milliliter) was monitored by DHBV DNA quantification by using a dot blot assay and/or real-time PCR. The mean viremia levels for each group from studies 1, 2, and 3 are plotted on the graphs shown in panels A, B, and C, respectively; the first two are displayed on a log y axis. The black bar indicates the antiviral treatment period. The limit of detection of DHBV DNA was 200 pg/ml for dot blot analysis and 100 copies/ml for the real-time PCR assay. In studies 1 and 3, all ducks were sacrificed at the end of study while in study 2 only half of the ducks were sacrificed at the end of treatment.
TABLE 3.
Short-term administration of double and triple combinations (DAPD, FTC, and l-FMAU) in experimentally infected ducklings
| Group | No. of ducks | Dosea (mg/kg) | DMSOb (μl/g) | Viremiac (day 10) | Viral productiond (AUC) | Intrahepatice viral DNA (day 10) |
|---|---|---|---|---|---|---|
| Study 1 | ||||||
| Controls | 6 | 0 or 8 | 4.10 ± 0.81 | 5.41 ± 0.7 | ||
| l-FMAU 40 | 6 | 40 | 1 | 3.90 ± 0.76 | 4.80 ± 0.39 | |
| l-FMAU 80 | 7 | 80 | 2 | 4.08 ± 0.78 | 4.92 ± 0.44 | |
| FTC 50 | 7 | 50 | 1 | 4.11 ± 0.4 | 4.69 ± 0.25 | |
| FTC 100 | 7 | 100 | 2 | 3.18 ± 1.47 | 3.14 ± 1.01 | |
| DAPD 20 | 6 | 20 | 4 | 3.81 ± 0.64 | 4.92 ± 0.75 | |
| DAPD 40 | 6 | 40 | 8 | 3.25 ± 0.57 | 3.91 ± 1.08 | |
| FTC + DAPD | 6 | 50 + 20 | 5 | 3.39 ± 0.93 | 3.80 ± 1.24 | |
| FTC + l-FMAU | 7 | 50 + 40 | 5 | 3.09 ± 1.15 | 3.56 ± 0.98 | |
| DAPD + l-FMAU | 7 | 20 + 40 | 5 | 3.33 ± 1.12 | 4.59 ± 0.89 | |
| Study 2 | ||||||
| Controls | 10 | 4 | 3.80 ± 0.50 | 5.10 ± 0.41 | 617 ± 519 | |
| FTC 50 + DAPD | 9 | 50 + 20 | 4 | 3.45 ± 0.61 | 4.54 ± 0.5 | 150 ± 57 |
| FTC 100 + DAPD | 10 | 100 + 20 | 4 | 3.32 ± 0.43 | 4.31 ± 0.49 | 204 ± 112 |
| FTC 50 + l-FMAU | 9 | 50 + 40 | 4 | 3.37 ± 0.85 | 4.37 ± 0.73 | 280 ± 194 |
| FTC 100 + l-FMAU | 10 | 100 + 40 | 4 | 3.12 ± 0.50 | 4.10 ± 0.54 | 214 ± 33 |
| DAPD + l-FMAU | 7 | 20 + 40 | 4 | 3.41 ± 0.62 | 4.45 ± 0.61 | 340 ± 217 |
| FTC 50 + l-FMAU + DAPD | 10 | 50 + 40 + 20 | 4 | 3.49 ± 0.51 | 4.52 ± 0.44 | 324 ± 174 |
| FTC 100 + l-FMAU + DAPD | 9 | 100 + 40 + 20 | 4 | 3.08 ± 0.78 | 4.02 ± 0.54 | 285 ± 202 |
In study 1, two doses for each drug were tested (40 or 80 mg/kg for l-FMAU, 50 or 100 mg/kg for FTC, and 20 or 40 mg/kg for DAPD) while only the lowest doses were chosen for combination. In study 2, dual and triple combinations were composed of DAPD at 20 mg/kg, l-FMAU at 40 mg/kg, and FTC at 50 or 100 mg/kg.
DAPD, FTC, and l-FMAU to be administered daily to Pekin ducklings by intraperitoneal injection were dissolved in dimethyl sulfoxide (DMSO) (expressed as microliters of DMSO per gram of duck).
Viremia at day 10 (treatment cessation) was determined by real-time PCR (values reported in log 10 picograms per milliliter). Results are means ± standard deviations.
Estimation of viral production was determined by the area under the curve (AUC) of viremia during the 7 days of treatment (AUC reported in log10 values). Results are means ± standard deviations.
In study 2, half of the ducklings were sacrificed at the end of treatment (day 10) in order to quantitate the amount of intrahepatic viral DNA (copies per vge of liver cell DNA). Results are means ± standard deviations.
In the second short-term study (study 2), the impacts of the three dual and the triple combination protocols were analyzed by using FTC at 50 or 100 mg/kg, l-FMAU at 40 mg/kg, and DAPD at 20 mg/kg (Fig. 3B; Table 3). The results showed that the triple combination and the dual combinations of FTC plus l-FMAU and FTC plus DAPD, with the high dose of FTC, inhibited viral production by more than 85% compared with the control duck groups (P < 0.05). However, the suppression of viremia in treated animals was not long lasting and a relapse of viral replication occured in all animal groups after drug withdrawal (Fig. 3B).
At the end of treatment, examination of intrahepatic viral DNA showed that the inhibition of viral production (>1 log, P < 0.05) by the FTC (100 mg/kg) plus l-FMAU combination with or without DAPD was associated with a moderate inhibition of intrahepatic DHBV replication (>65.3 and >53.8% for dual and triple therapy, respectively). These results are due to wide variations in liver DHBV DNA levels of two animals from the triple combination group with FTC at 100 mg/kg and two others from the group treated with 50 mg of FTC/kg plus l-FMAU which exhibited DHBV DNA levels equivalent to controls (Table 3).
As combination of FTC and l-FMAU exhibited the greatest antiviral effect, the antiviral effect of this combination was studied during a longer administration period.
A 4-week combination of FTC and l-FMAU transiently inhibits viral replication in vivo in experimentally infected ducklings.
The combination of FTC and l-FMAU was determined to be potent in short-term protocols. Therefore, we examined the antiviral activity of this combination in a 4-week administration period (long-term study, study 3). Acutely infected ducks received a combination of 100 mg of FTC/kg and 40 mg of l-FMAU/kg for 4 weeks and were monitored for 192 days. As illustrated in Fig. 3C, the combination of l-FMAU plus FTC decreased the peak level of viremia by >80% (P < 0.05) during the week following DHBV inoculation as well as during the rest of the treatment. The suppression of viremia was maintained during the entire treatment period. After drug withdrawal, mean viremia levels of treated ducks remained lower than those of controls (Fig. 3C; Table 4). At the end of follow-up (day 223), serum DHBV DNA was undetectable by dot blot analysis in 3 ducks (1 control [no. 326] and 2 treated [no. 337 and 340] ducks). This was confirmed by the real-time PCR quantification which showed viral DNA detection of <0.1 pg/ml in the serum and <0.09 vge in the liver. Moreover, a four- to fivefold reduction of the mean total DHBV DNA in the livers of treated ducks was observed in comparison with that of the control group (Table 4).
TABLE 4.
Four-week administration of a combination of FTC (100 mg/kg) and l-FMAU (40 mg/kg) in experimentally infected ducklings
| Group and duck no. | Viremia (pg/ml)a
|
Viral productionb (AUC) (day 3-31) | Intrahepaticc viral DNA (day 223) | ||
|---|---|---|---|---|---|
| Day 10 | Day 31 | Day 223 | |||
| Controls | |||||
| 321 | 4.7 | 4.5 | 3.3 | 5.2 | 339 |
| 322 | 4.3 | 3.4 | 4.9 | ||
| 323 | 4.2 | 4.2 | 3.1 | 5.0 | 80 |
| 324 | 4.2 | 4.1 | 3.7 | 4.8 | 1,894 |
| 325 | 4.2 | 3.1 | 3.5 | 4.9 | 1,693 |
| 326 | 4.2 | 2.7 | 0.1 | 4.8 | 0.14 |
| 327 | 3.7 | 1.1 | 4.5 | ||
| 328 | 4.1 | 3.5 | 3.7 | 4.7 | 345 |
| 329 | 4.1 | 3.1 | 4.5 | ||
| 330 | 4.2 | 3.6 | 3.2 | 4.8 | 196 |
| Mean | 4.2 ± 0.3 | 3.4 ± 1 | 2.9 ± 1.3 | 4.8 ± 0.2 | 649.5 ± 793.5 |
| l-FMAU + FTC | |||||
| 331 | 2.1 | 0 | 2.7 | ||
| 332 | 3.7 | 2.9 | 3.2 | 4.3 | 127 |
| 333 | 1.6 | ||||
| 334 | 3.6 | 2.9 | 4.7 | ||
| 335 | 3.5 | 3.4 | 3.4 | 4.4 | 160 |
| 336 | 2.9 | 0.3 | 3.1 | ||
| 337 | 3.1 | 1.7 | 0.1 | 4 | 0.86 |
| 338 | 3.6 | 3.4 | 4.2 | ||
| 339 | 3.4 | 3.2 | 3.2 | 4 | 425 |
| 340 | 3.8 | 2.7 | 0.1 | 4.1 | 0.18 |
| Mean | 3.1 ± 0.8 | 2.3 ± 1.3 | 2 ± 1.9 | 3.7 ± 0.7 | 142 ± 173.7 |
Viremia was determined after 7 (day 10) and 28 days of treatment (day 31, treatment cessation) and at the end of follow-up (day 223) by real-time PCR as described in Materials and Methods (log10 values are reported).
Estimation of viral production was determined by calculation of the area under the curve (AUC) during the 28 days of treatment (from day 3 to 31) and reported as log10 AUC values.
Quantitation of intrahepatic viral DNA in copies per vge of liver cell DNA by real-time PCR was performed on the remaining ducklings that were sacrificed at the end of the study (day 223).
DISCUSSION
Little information is available on the combination of polymerase inhibitors for the therapy of chronic hepadnavirus infection. Previous studies examined the antiviral effect of the combination of 3TC with penciclovir and/or PMEA in DHBV-infected primary duck hepatocytes (7, 8), while Korba et al. examined the antiviral benefit of combination therapy with 3TC and famciclovir in the 2.2.15 cells transfected with HBV (18) and in woodchuck hepatitis virus-infected woodchucks (19). Our results show that dual and triple combinations of purine and pyrimidine analogs exhibit a potent inhibitory effect on DHBV replication both in vitro and in vivo. The originality of our study relied on the association of compounds that inhibit different steps of viral DHBV DNA synthesis, i.e., priming of reverse transcription, viral minus-strand DNA elongation, and viral positive-strand DNA synthesis, and on their evaluation in a complete set of experimental systems with a viral RT assay, a real-time PCR, a primary hepatocyte culture system, and in vivo studies in infected ducklings.
The study with a cell-free system for the expression of an enzymatically active DHBV polymerase showed that the dual or triple association of the three nucleoside analog TPs, i.e., DAPD TP, FTC TP, and l-FMAU TP has a greater effect on the wild-type RT activity than single drugs that may be additive under some experimental conditions. However, the combination of DAPD TP with FTC and/or l-FMAU did not enhance the sensitivity of the mutant polymerase to the FTC or l-FMAU (37). Previous studies showed the importance of the conformation of the RT catalytic site for its enzymatic activity and susceptibility to nucleoside analogs (2, 10). One could have hypothesized that the incorporation of an active compound, such as DAPD, could modify the interaction of the replication complex, including the catalytic site of the RT, the RNA template, and the substrate, to promote the antiviral activity of FTC and/or l-FMAU against this mutant polymerase. However, our experimental results showed that this was not the case.
A detailed investigation of the effect of these combinations on intracellular viral DNA synthesis in experimentally infected primary duck hepatocytes provided additional information on the efficiency of these combinations. In postinoculation protocols, dual and triple combinations with low drug concentrations had an additive-to-synergistic inhibitory effect on viral DNA replication at the end of drug administration. Noteworthy, combinations administered at the highest concentrations did not exhibit an additive or synergistic effect except for the triple combination. This could be due to a maximum inhibitory effect of the single molecules that could not be exceeded. Nevertheless, the additive-to-synergistic effect that was observed at low concentrations during drug administration was not maintained after treatment cessation, suggesting that these combinations were not able to eradicate the DHBV genome from infected cells. Interestingly, the dual and triple combinations administered in preinoculation treatment delayed the relapse of viral DNA synthesis after treatment cessation, most likely by decreasing the amplification of viral cccDNA during administration. This lasting antiviral effect may be due to a relatively long half-life of the active metabolites of these compounds. Moreover, the persistence of viral cccDNA at low levels indicated that these combinations of nucleoside analogs were not sufficient to inhibit the initial formation of cccDNA and also to eradicate the pool of already established viral cccDNA, as this was already shown with single drugs such as 3TC or PMEA (11, 29, 30).
The greater effects observed at the end of administration of these combinations could be due to a complex mechanism including a combination of different inhibitory activities on the viral RT as well as allosteric effects on enzymes of nucleotide metabolism leading to the depletion of competing intracellular deoxynucleoside TPs. In the case of the combination of FTC plus l-FMAU, the synergistic effect may be surprising because a previous study showed that the metabolism of l-FMAU to its active TP form depends on cellular thymidine and deoxycytidine kinases, which may predict a competition of both compounds (27). Nevertheless, our results show that this phenomenon did not prevent this combination from being synergistic under our experimental conditions. However, two recent publications reported that in the last step, l-deoxynucleoside analog diphosphates (such as l-FMAU) are phosphorylated by 3-phosphoglycerate kinase preferentially to the d-form deoxynucleosides or nucleoside analogs (21, 22). Such relevant results allowing the prediction of competition for activating enzymes should help to design further combination regimens.
Short-term in vivo treatment in acutely infected ducklings showed that single-drug administration significantly inhibited viremia, especially the high dose of FTC. The dual combinations more potently inhibited viral production than did single drugs at the same concentrations (P < 0.05), the more potent being the combination of l-FMAU and FTC. The addition of DAPD to this latter combination induced a greater inhibition of virus production in serum. However, none of these combinations allowed the clearance of viral infection or prevented the chronicity of viral infection. Furthermore, a longer administration of l-FMAU and FTC for 4 weeks allowed the suppression of viremia and viral replication. However, this was not sufficient to clear viral cccDNA and viral infection from the livers of infected animals, although viral eradication assessed by a sensitive PCR assay was observed in 2 of 5 treated ducks versus 1 of 7 untreated animals. These in vivo results together with those obtained in preinoculation protocols in primary hepatocyte cultures may indicate that a longer duration of combination therapy may allow the intrahepatic viral load to decrease progressively by delaying the infection of new cells as well as the amplification of cccDNA in already infected cells.
Taken together, these results showed that single-drug administration in the DHBV duckling model required higher drug concentrations than drugs in combinations to obtain a similar inhibitory effect; this is important when considering the risk of drug toxicity versus antiviral efficacy. The use of these combinations of compounds with different mechanisms of action and resistance profiles (such FTC and DAPD) together with an enhanced antiviral effect may be of particular interest to prevent or delay the emergence of drug-resistant mutants. However, while the combination of FTC and DAPD exhibited the most potent effect on DHBV RT activity, this combination was equivalent in action to the two other dual combinations on DHBV replication in primary duck hepatocyte cultures while the most potent was the triple combination. Nevertheless, in vivo this marked benefit of the triple combination was not observed, rather pointing out a better efficacy of the l-FMAU-FTC combination. These results may suggest that drug metabolism and the half-life of their active metabolites in the liver are key determinants in the establishment of an optimized therapy and therefore warrant further pharmacological and pharmacodynamical studies on the combination of l-FMAU and FTC. As our study also underlined the lack of clearance of viral cccDNA from infected hepatocytes, the association of nucleoside analogs with immunotherapeutic approaches may be considered in the future to eradicate residual infected hepatocytes (42).
Acknowledgments
This work was performed in part with the support of a grant from ARC (Association pour la Recherche sur le Cancer).
B.S. and P.M. contributed equally to this work.
REFERENCES
- 1.Aguesse-Germon, S., S. H. Liu, M. Chevallier, C. Pichoud, C. Jamard, C. Borel, C. K. Chu, C. Trépo, Y. C. Cheng, and F. Zoulim. 1998. Inhibitory effect of 2′-fluoro-5-methyl-β-l-arabinofuranosyl-uracil on duck hepatitis B virus replication. Antimicrob. Agents Chemother. 42:369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, M. I., M. Deslauriers, C. W. Andrews, G. A. Tipples, K. A. Walters, D. L. J. Tyrell, N. Brown, and L. D. Condreay. 1998. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology 27:1670-1677. [DOI] [PubMed] [Google Scholar]
- 3.Borel, C., O. Schorr, I. Durand, F. Zoulim, A. Kay, C. Trepo, and O. Hantz. 2001. Initial amplification of duck hepatitis B virus covalently closed circular DNA after in vitro infection of embryonic duck hepatocytes is increased by cell cycle progression. Hepatology 34:168-179. [DOI] [PubMed] [Google Scholar]
- 4.Chayama, K., Y. Suzuki, M. Kobayashi, M. Kobayashi, A. Tsubota, M. Hashimoto, Y. Miyamo, H. Koike, M. Kobayashi, I. Koida, Y. Arase, S. Saito, N. Murashima, K. Ikeda, and H. Kumada. 1998. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology 27:1711-1716. [DOI] [PubMed] [Google Scholar]
- 5.Chin, R., T. Shaw, J. Torresi, V. Sozzi, C. Trautwein, T. Bock, M. Manns, P. Furman, and S. Locarnini. 2001. In vitro susceptibilities of wild-type or drug-resistant hepatitis B virus to (−)-β-d-2,6-diaminopurine dioxolane and 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil. Antimicrob. Agents Chemother. 45:2495-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow, Y. K., M. S. Hirsch, D. P. Merrill, L. J. Bechtel, J. J. Eron, J. C. Kaplan, and R. T. D'Aquila. 1993. Use of evolutionary limitations of HIV-1 multidrug resistance to optimize therapy. Nature 361:650-654. [DOI] [PubMed] [Google Scholar]
- 7.Colledge, D., G. Civitico, S. Locarnini, and T. Shaw. 2000. In vitro antihepadnaviral activities of combinations of penciclovir, lamivudine, and adefovir. Antimicrob. Agents Chemother. 44:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colledge, D., S. Locarnini, and T. Shaw. 1997. Synergistic inhibition of hepadnaviral replication by lamivudine in combination with penciclovir in vitro. Hepatology 26:216-225. [DOI] [PubMed] [Google Scholar]
- 9.Cullen, J. M., S. L. Smith, M. G. Davis, S. E. Dunn, C. Botteron, A. Cecchi, D. Linsey, D. Linzey, L. Frick, M. T. Paff, A. Goulding, and K. Biron. 1997. In vivo antiviral activity and pharmacokinetics of (−)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob. Agents Chemother. 41:2076-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das, K., X. Xiong, H. Yang, C. E. Westland, C. S. Gibbs, S. G. Sarafianos, and E. Arnold. 2001. Molecular modeling and biochemical characterization reveal the mechanism of hepatitis B virus polymerase resistance to lamivudine (3TC) and emtricitabine (FTC). J. Virol. 75:4771-4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmas, J., O. Schorr, C. Jamard, C. Gibbs, C. Trepo, O. Hantz, and F. Zoulim. 2002. Inhibitory effect of adefovir on viral DNA synthesis and CCC DNA formation in duck hepatitis B virus-infected hepatocytes in vivo and in vitro. Antimicrob. Agents Chemother. 46:425-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Man, R. A., L. M. Wolters, F. Nevens, D. Chua, M. Sherman, C. L. Lai, A. Gadano, Y. Lee, F. Mazzotta, N. Thomas, and D. DeHertogh. 2001. Safety and efficacy of oral entecavir given for 28 days in patients with chronic hepatitis B virus infection. Hepatology 34:578-582. [DOI] [PubMed] [Google Scholar]
- 13.Gish, R. G., N. W. Y. Leung, T. L. Wright, H. Trinh, W. Lang, H. A. Kessler, L. Fang, L. H. Wang, J. Delehanty, A. Rigney, E. Mondou, A. Snow, and F. Rousseau. 2002. Dose range study of pharmacokinetics, safety, and preliminary antiviral activity of emtricitabine in adults with hepatitis B virus infection. Antimicrob. Agents Chemother. 46:1734-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco, W. R., G. Bravo, and J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 15.Hantz, O., C. Borel, C. Trabaud, F. Zoulim, J. Dessolin, M. Camplo, P. Vlieghe, M. Bouygues, C. Trépo, and J. L. Kraus. 1997. Selective inhibition of the duck hepatitis B virus by a new class of tetraazamacrocycles. Antimicrob. Agents Chemother. 41:2579-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoofnagle, J. H., and A. M. Di Bisceglie. 1997. The treatment of chronic viral hepatitis. N. Engl. J. Med. 336:347-356. [DOI] [PubMed] [Google Scholar]
- 17.Jilbert, A. R., T. T. Wu, J. M. England, P. M. Hall, N. Z. Carp, A. P. O'Connell, and W. S. Mason. 1992. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J. Virol. 66:1377-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korba, B. E. 1996. In vitro evaluation of combination therapies against hepatitis B virus replication. Antivir. Res. 29:49-51. [DOI] [PubMed] [Google Scholar]
- 19.Korba, B. E., P. Cote, W. Hornbuckle, R. Schinazi, J. L. Gerin, and B. C. Tennant. 2000. Enhanced antiviral benefit of combination therapy with lamivudine and famciclovir against WHV replication in chronic WHV carrier woodchucks. Antivir. Res. 45:19-32. [DOI] [PubMed] [Google Scholar]
- 20.Korba, B. E., R. F. Schinazi, P. Cote, B. C. Tennant, and J. L. Gerin. 2000. Effect of oral administration of emtricitabine on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob. Agents Chemother. 44:1757-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan, P., Q. Fu, W. Lam, J. Y. Liou, G. Dutschman, and Y. C. Cheng. 2002. Phosphorylation of pyrimidine deoxynucleoside analog diphosphates: selective phosphorylation of l-nucleoside analog diphosphates by 3-phosphoglycerate kinase. J. Biol. Chem. 277:5453-5459. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan, P., J. Y. Liou, and Y. C. Cheng. 2002. Phosphorylation of pyrimidine l-deoxynucleoside analog diphosphates. Kinetics of phosphorylation and dephosphorylation of nucleoside analog diphosphates and triphosphates by 3-phosphoglycerate kinase. J. Biol. Chem. 277:31593-31600. [DOI] [PubMed] [Google Scholar]
- 23.Lai, C. L., R. W. Chine, N. W. Y. Leung, T. T. Chang, R. Guan, D. I. Tai, K. Y. Ng, P. C. Wu, J. C. Dent, J. Barber, S. L. Stephenson, and F. Gray. 1998. A one year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 339:61-68. [DOI] [PubMed] [Google Scholar]
- 24.Lee, W. M. 1997. Hepatitis B virus infection. N. Engl. J. Med. 337:1733-1745. [DOI] [PubMed] [Google Scholar]
- 25.Leguerhier, F., C. Pichoud, S. Guerret, M. Chevallier, C. Jamard, O. Hantz, X. Y. Li, S. H. Chen, I. King, C. Trepo, Y. C. Cheng, and F. Zoulim. 2000. Characterization of the antiviral effect of 2′,3′-dideoxy-2′, 3′-didehydro-β-l-5-fluorocytidine in the duck hepatitis B virus infection model. Antimicrob. Agents Chemother. 44:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung, N. W., C. L. Lai, T. T. Chang, R. Guan, C. M. Lee, K. Y. Ng, S. G. Lim, P. C. Wu, J. C. Dent, S. Edmundson, L. D. Condreay, and R. N. Chien. 2001. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 33:1527-1532. [DOI] [PubMed] [Google Scholar]
- 27.Liu, S.-H., K. L. Grove, and Y.-C. Cheng. 1998. Unique metabolism of a novel antiviral l-nucleoside analog, 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil: a substrate for both thymidine kinase and deoxycytidine kinase. Antimicrob. Agents Chemother. 42:833-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marion, P. L., F. H. Salazar, M. A. Winters, and R. J. Colonno. 2002. Potent efficacy of entecavir (BMS-200475) in a duck model of hepatitis B virus replication. Antimicrob. Agents Chemother. 46:82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason, W., J. Cullen, J. Saputelli, T. Wu, C. Liu, W. London, E. Lustbader, P. Schaffer, A. O'Connell, I. Fourel, C. Aldrich, and A. Jilbert. 1994. Characterization of the antiviral activity of 2′carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology 19:398-411. [PubMed] [Google Scholar]
- 30.Mason, W. S., J. Cullen, G. Moraleda, J. Saputelli, C. E. Aldrich, D. S. Miller, B. Tennant, L. Frick, D. Averett, L. D. Condreay, and A. R. Jilbert. 1998. Lamivudine therapy of WHV-infected woodchucks. Virology 245:18-32. [DOI] [PubMed] [Google Scholar]
- 31.Melegari, M., P. P. Scaglioni, and J. R. Wands. 1998. Hepatitis B virus mutants associated with 3TC and famciclovir administration are replication defective. Hepatology 27:628-633. [DOI] [PubMed] [Google Scholar]
- 32.Nafa, S., S. Ahmed, D. Tavan, C. Pichoud, F. Berby, L. Stuyver, M. Johnson, P. Merle, H. Abidi, C. Trepo, and F. Zoulim. 2000. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology 32:1078-1088. [DOI] [PubMed] [Google Scholar]
- 33.Nowak, M., S. Bonhoeffer, A. Hill, R. Boehme, H. Thomas, and H. McDade. 1996. Viral dynamics in hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 93:4398-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono, S. K., N. Kato, Y. Shiratori, J. Kato, T. Goto, R. F. Schinazi, F. J. Carrilho, and M. Omata. 2001. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J. Clin. Investig. 107:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seignères, B., S. Aguesse-Germon, C. Pichoud, I. Vuillermoz, C. Jamard, C. Trépo, and F. Zoulim. 2001. Duck hepatitis B virus polymerase gene mutants associated with resistance to lamivudine have a decreased replication capacity in vitro and in vivo. J. Hepatol. 34:114-122. [DOI] [PubMed] [Google Scholar]
- 36.Seignères, B., C. Pichoud, S. S. Ahmed, O. Hantz, C. Trepo, and F. Zoulim. 2000. Evolution of hepatitis B virus polymerase gene sequence during famciclovir therapy for chronic hepatitis B. J. Infect. Dis. 181:1221-1233. [DOI] [PubMed] [Google Scholar]
- 37.Seignères, B., C. Pichoud, P. Martin, P. Furman, C. Trépo, and F. Zoulim. 2002. Inhibitory activity of dioxolane purine analogs on wild-type lamivudine-resistant mutants of hepadnaviruses. Hepatology 36:710-722. [DOI] [PubMed] [Google Scholar]
- 38.Snyder, S., D. Z. D'Argenio, O. Weislow, J. A. Bilello, and G. L. Drusano. 2000. The triple combination indinavir-zidovudine-lamivudine is highly synergistic. Antimicrob. Agents Chemother. 44:1051-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuyver, L. J., S. A. Locarnini, A. Lok, D. D. Richman, W. F. Carman, J. L. Dienstag, R. F. Schinazi, and the HEP DART International Committee. 2001. Nomenclature for antiviral-resistant human hepatitis B virus mutations in the polymerase region. Hepatology 33:751-757. [DOI] [PubMed] [Google Scholar]
- 40.Tsiang, M., J. F. Rooney, J. J. Toole, and C. S. Gibbs. 1999. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology 29:1863-1869. [DOI] [PubMed] [Google Scholar]
- 41.Villahermosa, M. L., J. J. Martinez-Irujo, F. Cabodevilla, and E. Santiago. 1997. Synergistic inhibition of HIV-1 reverse transcriptase by combinations of chain-terminating nucleotides. Biochemistry 36:13223-13231. [DOI] [PubMed] [Google Scholar]
- 42.Zoulim, F. 2001. Evaluation of novel strategies to combat hepatitis B virus targetting wild-type and drug-resistant mutants in experimental models. Antivir. Chem. Chemother. 12(Suppl. 1):131-142. [PubMed] [Google Scholar]
- 43.Zoulim, F., E. Dannaoui, C. Borel, O. Hantz, T. S. Lin, S. H. Liu, C. Trépo, and Y. C. Cheng. 1996. 2′,3′-Dideoxy-β-l-5-fluorocytidine inhibits duck hepatitis B virus reverse transcription and suppresses viral DNA synthesis in hepatocytes, both in vitro and in vivo. Antimicrob. Agents Chemother. 40:448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zoulim, F., J. Saputelli, and C. Seeger. 1994. Woodchuck hepatitis virus X protein is required for viral replication in vivo. J. Virol. 68:2026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]