Abstract
An increasing number of clinical isolations of rapidly growing mycobacteria (RGM) at the National Taiwan University Hospital were noted from 1992 to 2001. Broth microdilution MICs of 15 antimicrobial agents were determined for 200 clinical isolates of RGM, including the Mycobacterium fortuitum group (69 isolates), M. chelonae (39 isolates), and M. abscessus (92 isolates). Our results showed that the resistance rates of these isolates to the currently available agents were remarkably high. Amikacin was active against nearly all RGM isolates. Clarithromycin was usually active against M. abscessus (79% susceptibility) and the M. fortuitum group (65% susceptibility). The majority of M. fortuitum group isolates were susceptible to ciprofloxacin (62%) and imipenem (61%). The susceptibilities to other conventional anti-RGM agents of these isolates were poor but differed markedly by species. The newer fluoroquinolones (levofloxacin, moxifloxacin, and gatifloxacin) and meropenem showed better in vitro activities against the M. fortuitum group isolates than against the other two species of RGM. Linezolid had fairly good activity against these RGM isolates, particularly against M. chelonae isolates (82% susceptible). Telithromycin had poor activity against these RGM isolates (the MICs at which 50% of the isolates tested are inhibited [MIC50s] were 32 to 64 μg/ml, and the MIC90s were >64 μg/ml).
Rapidly growing mycobacteria (RGM) are ubiquitous in nature (1, 10). The Mycobacterium fortuitum group (M. fortuitum, M. perigrinum, M. fortuitum third biovariant complex [sorbitol-negative and -positive], M. porcinium, and M. mageritense), Mycobacterium chelonae, and Mycobacterium abscessus are the species of RGM most often associated with human diseases (1, 10, 11, 14, 15, 21, 29). These organisms cause a variety of disseminated or localized diseases, particularly pulmonary infections as well as primary skin and soft tissue infections (1, 7, 21, 22). Pseudo-outbreaks of infection due to these organisms caused by contaminated medical equipment have been reported (30, 31). These organisms are resistant to the conventional antituberculous agents, and their susceptibilities to other antimycobacterial agents varied with different members of this group of mycobacteria (4-6, 17, 18, 22-25, 27, 32). Some investigators have reported that in vitro susceptibilities to several of these agents correlated with clinical response to therapy (18, 20). Furthermore, some recent in vitro susceptibility studies have demonstrated activity of newly developed antimicrobials (linezolid, telithromycin, tigecycline, and newer fluoroquinolones) against these organisms (3, 8, 26, 28, 32).
With the increasing clinical importance of RGM responsible for clinical infections at the National Taiwan University Hospital (NTUH) and with an effort to identify potentially useful agents to treat these infections (9, 14, 30), we investigated the in vitro susceptibilities of recent RGM isolates to 15 antimicrobial agents, including those developed recently.
MATERIALS AND METHODS
Bacterial isolates.
From January 1997 to June 2002, a total of 200 nonduplicate isolates (only one isolate per patient) of RGM, including 69 isolates of the M. fortuitum group, 92 of M. abscessus, and 39 of M. chelonae, that were recovered from various clinical samples were collected for the study (Table 1). These organisms were stored in Mueller-Hinton broth (BBL Microbiology Systems, Cockeysville, Md.) containing 15% glycerol at −70°C. Prior to testing, the isolates were subcultured, checked for purity, and reidentified to the species level by conventional growth and biochemical tests, including morphology of microcolonies, nitrate reduction, arylsulfatase reactions (3 days), and tolerance to 5% NaCl (10).
TABLE 1.
Sources of 200 isolates of RGM recovered from patients treated at NTUH from January 1997 to June 2002
| Source of specimens | No. (%) of indicated mycobacterial species
|
||
|---|---|---|---|
| M. fortuitum group (n = 69) | M. chelonae (n = 39) | M. abscessus (n = 92) | |
| Respiratory secretions (sputum, bronchial washing, pleural effusion) | 53 (77) | 34 (87) | 72 (78) |
| Pus (wound) | 14 (20) | 4 (10) | 13 (14) |
| Blood | 0 (0) | 1 (3) | 1 (1) |
| Lymph node | 1 (2) | 0 (0) | 4 (4) |
| Urine | 1 (2) | 0 (0) | 1 (1) |
| Synovial fluid | 0 (0) | 0 (0) | 1 (1) |
Antimicrobial susceptibility testing.
The following antimicrobial agents were provided by their manufacturers for use in this study: trimethoprim-sulfamethoxazole, doxycycline, and tobramycin (Sigma Chemical Co., St. Louis, Mo.), amikacin and gatifloxacin (Bristol-Meyer Squibb, Princeton, N.J.), cefoxitin and imipenem (Merck Sharp & Dohme, Rahway, N.J.), meropenem (Sumitomo Pharmaceuticals, Osaka, Japan), ciprofloxacin and moxifloxacin (Farbenfabriken Bayer GmbH, Leverkrusen, Germany), levofloxacin (Daiichi Pharmaceuticals, Tokyo, Japan), clarithromycin (Abbott Laboratories Pharmaceutical Products Division, North Chicago, Ill.), azithromycin (Pfizer Inc., New York, N.Y.), telithromycin (Aventis Pharma, Romainville, France), and linezolid (Pharmacia, Kalamazoo, Mich.).
MICs of these agents for the 200 RGM isolates were determined by the broth microdilution method and interpreted according to the tentative guidelines established by the National Committee for Clinical Laboratory Standards (NCCLS) (12). The isolates were grown on Trypticase soy agar plates supplemented with 5% sheep blood (BBL Microbiology Systems) at 30°C in ambient air for 72 h. Bacterial suspensions were prepared by sweeping the confluent portion of growth on the agar plate and adjusted to a final inoculum (5 × 105 CFU/ml) in cation-supplemented Mueller-Hinton broth (Difco, Detroit, Mich.) with 0.02% Tween 80 (Difco).
The medium used was cation-supplemented Mueller-Hinton broth without Tween 80. The final organism concentration was 1 × 104 to 5 × 104 CFU per well. Serial double dilutions of antimicrobial agents were prepared, and their concentrations in the wells followed the NCCLS recommendation (12). The concentrations tested for agents whose test concentrations were not provided by the NCCLS (i.e., azithromycin, levofloxacin, moxifloxacin, gatifloxacin, telithromycin, linezolid, and meropenem) ranged from 0.03 to 64 μg/ml (12). The inoculated trays were incubated at 30°C in ambient air and read after 72 h (12, 32).
The MIC was defined as the lowest concentration of the drug that inhibited visible growth. Otherwise, for trimethoprim-sulfamethoxazole, the endpoint was the well with approximately 80% inhibition of growth compared to the control well. Staphylococcus aureus ATCC 29213 and M. peregrinum ATCC 700686 were included as control strains (12).
Susceptibility categories of antimicrobial agents tested for these RGM isolates, except for telithromycin, were determined according to the breakpoints recommended by the NCCLS (M24-T2 and M100-S12) and those proposed by Wallace et al. (1, 12, 13, 26).
Sequencing of the 16S rRNA gene.
Parts (100 isolates) of these isolates were further confirmed by sequencing of their 16S rRNA gene (1,464 bp) (19). These isolates included 30 isolates of M. fortuitum group, 40 isolates of M. abscessus (including isolates for which the tobramycin MICs were 1 to 4 μg/ml and/or the cefoxitin MICs were >64 μg/ml), and 30 isolates of M. chelonae (including isolates for which the tobramycin MICs were 8 to 16 μg/ml and/or the cefoxitin MICs were 16 to 32 μg/ml). PCR amplifications of the nearly complete 16S rRNA gene were performed, and two primers (primers 8FPL and 1492) were used according to the previous description (19).
RESULTS
Isolation of RGM.
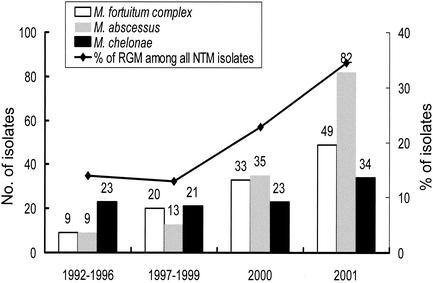
The number of clinical specimens sent for mycobacterial cultures at the NTUH was 6,447 in 1996 and increased to 18,433 in 2001. The rate of positive cultures for mycobacteria was 6.0% in 1996 and around 8 to 9% in 1999-2001. The number of isolates of the three RGM species increased during the past 10 years: a fourfold increase occurred from the period 1992-1996 (41 isolates) to the year 2001 (165 isolates) at the NTUH. The proportion of RGM isolations of all nontuberculous mycobacteria (NTM) also increased from 13.9% in 1992-1996 to 22.8% in 2000 and to 34.5% in 2001 (Fig. 1). The majority (about 60%) of these RGM isolates tested were from respiratory specimens as colonizers or as the causes respiratory infections, while the rest were associated with skin, soft-tissue, and disseminated infection (data not shown).
FIG. 1.
Trends of isolation of M. fortuitum complex, M. chelonae, and M. abscessus and proportions of RGM among all NTM at the NTUH from 1992 to 2001.
The 16S rRNA gene analysis.
Species identification for the 100 RGM isolates by the 16S rRNA gene analysis was identical to that determined by the conventional methods.
Antimicrobial susceptibility testing results.
Table 2 shows the MIC ranges, the MIC at which 50% of the isolates tested are inhibited (MIC50), MIC90, and the categories of susceptibility of 15 antimicrobial agents against the 200 isolates of RGM. Nearly all of the M. fortuitum group, M. chelonae, and M. abscessus isolates were susceptible to amikacin. Amikacin also had better in vitro activity against the M. fortuitum group than tobramycin. Nonetheless, amikacin and tobramycin had similar in vitro activity against M. chelonae and M. abscessus. Nearly all M. chelonae and M. abscessus isolates were resistant to trimethoprim-sulfamethoxazole. More than 60% of M. fortuitum group isolates were susceptible to ciprofloxacin, clarithromycin, and imipenem, and about four-fifths of the M. abscessus isolates were susceptible to clarithromycin. Activities of cefoxitin, doxycycline, and tobramycin against the three RGM isolates were poor.
TABLE 2.
In vitro susceptiblities of 200 isolates of the M. fortuitum group, M. chelonae, and M. abscessus to 15 antimicrobial agents using the broth microdilution method
| Bacterium (no. of isolates tested) and antimicrobial agent | MIC (μg/ml)
|
No. (%) of isolates
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | Susceptible | Intermediate | Resistant | |
| M. fortuitum group (69) | ||||||
| Amikacin | 1-16 | 1 | 8 | 69 (100) | 0 (0) | 0 (0) |
| Tobramycina | 1-32 | 8 | 16 | 6 (9) | 31 (45) | 32 (46) |
| Cefoxitin | 8->256 | 32 | 64 | 13 (19) | 55 (80) | 1 (1) |
| Ciprofloxacin | 0.25-16 | 0.25 | 8 | 43 (62) | 3 (4) | 23 (33) |
| Moxifloxacinc | 0.06-16 | 0.06 | 16 | 46 (67) | 6 (9) | 17 (25) |
| Gatifloxacinc | 0.06-16 | 0.12 | 8 | 48 (70) | 7 (10) | 14 (20) |
| Levofloxacinc | 0.06-64 | 0.25 | 32 | 44 (64) | 2 (3) | 23 (33) |
| Clarithromycin | 0.12->64 | 2 | 8 | 45 (65) | 10 (15) | 14 (20) |
| Azithromycinc | 1-64 | 8 | 64 | 16 (24) | 16 (24) | 37 (52) |
| Telithromycinc | 0.06->64 | 64 | >64 | —f | — | — |
| Doxycycline | 0.25->32 | 16 | >32 | 9 (13) | 13 (19) | 47 (68) |
| Imipenem | 1-32 | 2 | 8 | 42 (61) | 22 (32) | 5 (7) |
| Meropenemc | 1->64 | 4 | 32 | 35 (51) | 6 (9) | 28 (41) |
| TMP-SMZd | 1->64 | 64 | >64 | 34 (49) | — | 35 (51) |
| Linezolide | 0.12-64 | 8 | 32 | 47 (68) | 5 (7) | 17 (25) |
| M. chelonae (39) | ||||||
| Amikacin | 2-16 | 8 | 16 | 39 (100) | 0 (0) | 0 (0) |
| Tobramycin | 1-16 | 8 | 16 | 12 (31) | 17 (44) | 10 (26) |
| Cefoxitin | 16->256 | 32 | 256 | 2 (5) | 25 (64) | 12 (31) |
| Ciprofloxacin | 2->16 | 16 | >16 | 0 (0) | 1 (3) | 38 (97) |
| Moxifloxacinc | 1-32 | 8 | 8 | 9 (23) | 8 (21) | 22 (56) |
| Gatifloxacinc | 1-16 | 8 | 16 | 8 (21) | 9 (23) | 22 (56) |
| Levofloxacinc | 8->64 | 32 | 64 | 0 (0) | 0 (0) | 39 (100) |
| Clarithromycin | 0.06->64 | 4 | >64 | 19 (49) | 1 (3) | 19 (49) |
| Azithromycinc | 0.25->64 | >64 | >64 | 7 (18) | 5 (12) | 27 (70) |
| Telithromycinc | 0.12->64 | 64 | >64 | — | — | — |
| Doxycycline | 32->32 | >32 | >32 | 0 (0) | 0 (0) | 39 (100) |
| Imipenemb | 2-64 | 16 | 64 | 7 (18) | 12 (31) | 20 (51) |
| Meropenemc | 4-64 | 64 | >64 | 1 (3) | 2 (5) | 36 (92) |
| TMP-SMZd | 64->64 | >64 | >64 | 0 (0) | — | 39 (100) |
| Linezolide | 1-32 | 8 | 16 | 32 (82) | 5 (13) | 2 (5) |
| M. abscessus (92) | ||||||
| Amikacin | 1->128 | 8 | 8 | 88 (96) | 0 (0) | 4 (4) |
| Tobramycina | 1->32 | 8 | 16 | 25 (27) | 39 (42) | 28 (30) |
| Cefoxitin | 8-256 | 32 | 64 | 3 (3) | 85 (92) | 4 (4) |
| Ciprofloxacin | 0.25->16 | 8 | 16 | 3 (3) | 2 (2) | 87 (95) |
| Moxifloxacinc | 0.06-32 | 8 | 16 | 7 (8) | 10 (11) | 75 (82) |
| Gatifloxacinc | 0.06-32 | 8 | 16 | 6 (7) | 16 (17) | 70 (76) |
| Levofloxacinc | 0.5->64 | 16 | 64 | 2 (2) | 2 (2) | 88 (96) |
| Clarithromycin | 0.06->64 | 1 | 8 | 73 (79) | 9 (10) | 10 (11) |
| Azithromycinc | 0.25->64 | 2 | 32 | 48 (52) | 0 (0) | 44 (48) |
| Telithromycinc | 0.5->64 | 32 | >64 | — | — | — |
| Doxycycline | 4->32 | >32 | >32 | 0 (0) | 7 (8) | 85 (92) |
| Imipenemb | 1->64 | 8 | 16 | 11 (12) | 64 (70) | 17 (18) |
| Meropenemc | 8->64 | 32 | 64 | 0 (0) | 1 (1) | 9 (99) |
| TMP-SMZd | 2->64 | >64 | >64 | 1 (1) | — | 91 (99) |
| Linezolide | 1-32 | 16 | 32 | 29 (32) | 24 (26) | 39 (42) |
Tobramycin results were recommended to be reported only for M. chelonae isolates.
Imipenem results were not recommended to be reported for M. chelonae and M. abscessus isolates.
The breakpoints of these antimicrobial agents for RGM have not yet been addressed by the NCCLS (11). The NCCLS breakpoints for Staphylococcus species were used (12).
TMP-SMZ, trimethoprim-sulfamethoxazole.
The breakpoints of linezolid for RGM proposed by Wallace et al. (18) were used. These breakpoints of linezolid have not yet been addressed by the NCCLS (11).
—, no breakpoints are available.
The newer fluoroquinolones (levofloxacin, moxifloxacin, and gatifloxacin) showed slightly better in vitro activities against M. fortuitum group isolates than against the other two species of RGM. Meropenem had two- to fourfold less activity against these RGM isolates than imipenem. Azithromycin was two- to fourfold less active than clarithromycin against the three RGM species. The in vitro activities of telithromycin against these RGM isolates were very poor (MIC50s, 32 to 64 μg/ml; MIC90s, >64 μg/ml). Telithromycin at a concentration of 1 μg/ml inhibited the M. fortuitum group (4%), M. chelonae (4%), and M. abscessus (15%).
Utilizing the linezolid breakpoints suggested by Wallace et al. (26), 68% of M. fortuitum group and 82% of M. chelonae isolates were within the susceptible ranges (MICs, ≤8 μg/ml), and more than 50% of isolates of M. abscessus were within susceptible or intermediate (moderately susceptible) (MICs, ≤16 μg/ml) range.
DISCUSSION
Many previous studies showed that the susceptibility of RGM varied widely with different species of this group, with isolates from different geographical areas, and with time (4-6, 17, 18, 22-25, 32). Members of the RGM also exhibit their own susceptibility profiles to conventional antimycobacterial agents (17, 21).
In the present study, in addition to the emerging nature of diseases caused by RGM and the increasing number of clinical isolations of RGM at the NTUH, our results clearly demonstrated that the resistance rates of these isolates to conventional and currently available anti-RGM agents were remarkably high. Amikacin was active against nearly all RGM isolates. Clarithromycin was usually active against the majority of the M. fortuitum group and M. abscessus. Similarly, ciprofloxacin and imipenem exhibited good activity (>60% susceptibility) against the M. fortuitum group only. The susceptibilities of RGM isolates to other agents were poor but differed markedly by species. Accordingly, for empirical therapy of infections caused by RGM (when the species was not identified and susceptibility results were not available) at our hospital, multidrug regimens containing amikacin were usually recommended.
Our data showed a poor activity of telithromycin against the M. fortuitum group and M. abscessus (MIC90s for the two species were both >64 μg/ml), a finding also previously reported (8). In contrast to the previous finding that 80% of M. chelonae isolates were inhibited by telithromycin at a concentration of 1 μg/ml (8), our results showed only 15% of M. chelonae isolates were inhibited by 1 μg of telithromycin per ml. Although successful treatment of disseminated M. chelonae infection with linezolid (the MIC for the isolate was 4 μg/ml) has been reported (2), a previous study showed high MIC90 for the M. fortuitum group (16 μg/ml), M. chelonae (16 μg/ml), and M. abscessus (64 μg/ml). These were all similar to our findings (26). Wallace et al. have proposed broth MIC breakpoints for RGM species (namely: susceptible, MICs of ≤8 μg/ml; moderately susceptible, MICs of 16 μg/ml; and resistant, MICs of ≥32 μg/ml) and further suggested the excellent potential of linezolid for therapy of infections caused by RGM (26). This suggestion should be assessed in the context of in vivo trials in the future.
Previous study demonstrated that gatifloxacin was generally fourfold more active than ciprofloxacin against the M. fortuitum group and M. chelonae (3). Our results did not support this finding (3). The newer fluoroquinolones tested in this study had activity only slightly superior to that of ciprofloxacin and only against the M. fortuitum group isolates. Moreover, meropenem had poorer potency against these RGM than imipenem. Accordingly, the role of the newly developed agents, such as telithromycin, meropenem, and newer fluoroquinolones, in the treatment of diseases due to RGM was limited.
Clinical isolates of RGM are identified to species and subgroup levels by using standard methods, antimicrobial susceptibility patterns, high-performance liquid chromatography, PCR restriction enzyme analysis of the 65-kDa hsp gene, and 16S rRNA gene (>1,400 bp) sequencing (1, 3, 10, 16, 29). There are numerous studies which show that susceptibility or resistance of RGM to a drug correlates with the species or taxon to which the organisms belong (1, 5, 18, 23). Previous studies showed that for all isolates of M. chelonae, cefoxitin MICs are >128 μg/ml, and for more than 90%, tobramycin MICs are ≤4 μg/ml (18, 23). For isolates of M. abscessus, cefoxitin MICs are 16 to 64 μg/ml and tobramycin MICs are ≥8 μg/ml (18, 23). Interestingly, in the present study, cefoxitin MICs were ≤64 μg/ml for 27 (68%) of the 39 isolates of M. chelonae, and tobramycin MICs were ≥8 μg/ml for 27 (69%) of the M. chelonae isolates. Isolates of M. abscessus for which tobramycin MICs were ≤4 μg/ml accounted for 27%. In this study, isolates of these two species were clearly identified by using conventional methods as well as 16S rRNA sequencing. Obviously, some of our data conflict with what is conventionally accepted (1, 5, 18, 23). This may indicate that resistance patterns among RGM isolates vary geographically.
In conclusion, the presence of high variations in susceptibility by and within species of RGM to the conventional and currently available anti-RGM antimicrobials confirms the need for accurate identification of these isolates to the species level. Moreover, susceptibility of any isolate to antimicrobial agents should be tested individually. This is particularly true for clinically important isolates. Our results can offer clinicians choices for empirical treatment when RGM infection is suspected and the in vitro susceptibility is not available.
REFERENCES
- 1.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15:716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown-Elliott, B. A., R. J. Wallace, Jr., R. Blinkhorn, C. J. Crist, and L. B. Mann. 2001. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin. Infect. Dis. 33:1433-1434. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Elliott, B. A., R. J. Wallace, Jr., C. J. Crist, L. B. Mann, and R. W. Wilson. 2002. Comparison of in vitro activities of gatifloxacin and ciprofloxacin against four taxa of rapidly growing mycobacteria. Antimicrob. Agents Chemother. 46:3283-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown-Elliott, B. A., R. J. Wallace, Jr., G. O. Onyi, V. De Rosas, and R. J. Wallace III. 1992. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium chelonae-like organisms. Antimicrob. Agents Chemother. 36:180-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casal, M., and F. Rodriguez. 1983. In vitro susceptibility of Mycobacterium fortuitum and Mycobacterium chelonae to sisomicin, gentamicin, and tobramycin. Ann. Microbiol. 134B:451-454. [PubMed] [Google Scholar]
- 6.Dalovisio, J. R., and G. A. Pankey. 1978. In vitro susceptibility of Mycobacterium fortuitum and Mycobacterium chelonae to amikacin. J. Infect. Dis. 137:318-321. [DOI] [PubMed] [Google Scholar]
- 7.Debrunner, M., M. Salfinger, O. Brandli, and A. von Gravenitz. 1992. Epidemiology and clinical significance of nontuberculous mycobacteria in patients negative for human immunodeficiency virus in Switzerland. Clin. Infect. Dis. 15:330-345. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Roblas, R., J. Esteban, F. Cabria, J. C. Lopez, M. S. Jimenez, and F. Soriano. 2000. In vitro susceptibilities of rapidly growing mycobacteria to telithromycin (HMR 3647) and seven other antimicrobials. Antimicrob. Agents Chemother. 44:181-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsueh, P. R., L. J. Teng, P. C. Yang, Y. C. Chen, S. W. Ho, and K. T. Luh. 1998. Recurrent catheter-related infection caused by a single clone of Mycobacterium chelonae with two clonal morphotypes. J. Clin. Microbiol. 36:1422-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metchock, B. G., F. S. Nolte, and R. J. Wallace, Jr. 1999. Mycobacterium, p. 399-437. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 11.Meyers, H., B. A. Brown-Elliott, D. Moore, J. Curry, C. Truong, Y. Zhang, and R. J. Wallace, Jr. 2002. An outbreak of Mycobacterium chelonae infection following liposuction. Clin. Infect. Dis. 34:1500-1507. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2002. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes. Tentative standards—second edition. M24-T2. National Committee for Clinical Laboratory Standards, Wayne, Pa. [PubMed]
- 13.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing: twelfth informational supplement. M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Shih, J. Y., P. R. Hsueh, L. N. Lee, H. C. Wang, P. C. Yang, S. H. Kuo, and K. T. Luh. 1997. Nontuberculous mycobacteria isolates: clinical significance and disease spectrum. J. Formos. Med. Assoc. 96:621-627. [PubMed] [Google Scholar]
- 15.Silcox, V. A., R. C. Good, and M. M. Floyd. 1981. Identification of clinically significant Mycobacterium fortuitum complex isolates. J. Clin. Microbiol. 14:686-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steingrube, V. A., J. L. Gibson, B. A. Brown, Y. Zhang, R. W. Wilson, M. Rajagopalan, and R. J. Wallace, Jr. 1995. PCR amplification and restriction endonuclease analysis of a 65-kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J. Clin. Microbiol. 33:149-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swenson, J. M., C. Thornsberry, and V. A. Silcox. 1982. Rapidly growing mycobacteria: testing of susceptibility to 34 antimicrobial agents by broth microdilution. Antimicrob. Agents Chemother. 22:186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swenson, J. M., R. J. Wallace, Jr., V. A. Silcox, and C. Thornsberry. 1985. Antimicrobial susceptibility testing of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob. Agents Chemother. 28:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turenne, C. Y., L. Tschetter, J. Wolfe, and A. Kabani. 2001. Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. J. Clin. Microbiol. 39:3637-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace, R. J., Jr., J. M. Swenson, V. A. Silcox, and M. G. Bullen. 1985. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonae on the basis of in vitro susceptibilities. J. Infect. Dis. 152:500-514. [DOI] [PubMed] [Google Scholar]
- 21.Wallace, R. J., Jr., J. Glassroth, D. E. Griffith, K. N. Olivier, J. L. Cook, and F. Gordin. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. American Thoracic Society statement. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed] [Google Scholar]
- 22.Wallace, R. J., Jr., B. A. Brown, G. O. Onyi. 1992. Skin, soft tissue, and bone infections due to Mycobacterium chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J. Infect. Dis. 166:405-412. [DOI] [PubMed] [Google Scholar]
- 23.Wallace, R. J., Jr., B. A. Brown, and G. Onyi. 1991. Susceptibilities of Mycobacterium fortuitum biovar fortuitum and the two subgroups of Mycobacterium chelonae to imipenem, cefmetazole, cefoxitin, and amoxicillin-clavulanic acid. Antimicrob. Agents Chemother. 35:773-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace, R. J., Jr., D. B. Jones, and K. Wiss. 1981. Sulfonamide activity against Mycobacterium fortuitum and Mycobacterium chelonae. Rev. Infect. Dis. 3:898-904. [DOI] [PubMed] [Google Scholar]
- 25.Wallace, R. J., Jr., G. Bedsole, G. Sumter, C. V. Sanders, L. C. Steele, B. A. Brown, J. Smith, and D. R. Graham. 1990. Activities of ciprofloxacin and ofloxacin against rapidly growing mycobacteria with demonstration of acquired resistance following single-drug therapy. Antimicrob. Agents Chemother. 35:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace, R. J., Jr., B. A. Brown-Elliott, S. C. Ward, C. J. Crist, L. B. Mann, and R. W. Wilson. 2001. Activities of linezolid against rapidly growing mycobacteria. Antimicrob. Agents Chemother. 45:764-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace, R. J., Jr., A. Meier, B. A. Brown, Y. Zhang, P. Sander, G. O. Onyi, and E. C. Bottger. 1996. Genetic basis for clarithromycin resistance among isolates Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob. Agents Chemother. 40:1676-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace, R. J., Jr., B. A. Brown-Elliott, C. J. Crist, L. B. Mann, and R. W. Wilson. 2002. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob. Agents Chemother. 46:3164-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace, R. J., Jr., B. A. Brown-Elliott, L. Hall, G. Roberts, R. W. Wilson, L. B. Mann, C. J. Crist, S. H. Chiu, R. Dunlap, M. J. Garcia, J. T. Bagwell, and K. C. Jost, Jr. 2002. Clinical and laboratory features of Mycobacterium mageritense. J. Clin. Microbiol. 40:2930-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, H. C., Y. S. Liaw, P. C. Yang, S. H. Kuo, and K. T. Luh. 1995. A pseudoepidemic of Mycobacterium chelonae infection caused by contamination of a fibreoptic bronchoscope suction channel. Eur. Respir. J. 8:1259-1262. [DOI] [PubMed] [Google Scholar]
- 31.Wilson, R. W., V. A. Steingrube, E. C. Bottger, B. Springer, B. A. Brown-Elliott, V. Vincent, K. C. Jost, Jr., Y. Zhang, M. J. Garcia, S. H. Chiu, G. O. Onyi, H. Rossmoore, D. R. Nash, and R. J. Wallace, Jr. 2001. Mycobacterium immunogenum sp. nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. Int. J. Syst. E vol. Microbiol. 51:1751-1764. [DOI] [PubMed] [Google Scholar]
- 32.Woods, G. L., J. S. Bergmann, F. G. Witebsky, G. A. Fahle, A. Wanger, B. Boulet, M. Planunt, B. A. Brown, and R. J. Wallace, Jr. 1999. Multisite reproducibility of results obtained by the broth microdilution method for susceptibility testing of Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum. J. Clin. Microbiol. 37:1676-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]