Abstract
Microbes, some of which may be viable, have been found in ice cores drilled at Vostok Station at depths down to ≈3,600 m, close to the surface of the huge subglacial Lake Vostok. Two types of ice have been found. The upper 3,500 m comprises glacial ice containing traces of nutrients of aeolian origin including sulfuric acid, nitric acid, methanosulfonic acid (MSA), formic acid, sea salts, and mineral grains. Ice below ≈3,500 m comprises refrozen water from Lake Vostok, accreted to the bottom of the glacial ice. Nutrients in the accretion ice include salts and dissolved organic carbon. There is great interest in searching for living microbes and especially for new species in deepest Antarctic ice. I propose a habitat consisting of interconnected liquid veins along three-grain boundaries in ice in which psychrophilic bacteria can move and obtain energy and carbon from ions in solution. In the accretion ice, with an age of a few 104 years and a temperature a few degrees below freezing, the carbon and energy sources in the veins can maintain significant numbers of cells per cubic centimeter that are metabolizing but not multiplying. In the 4 × 105-year-old colder glacial ice, at least 1 cell per cm3 in acid veins can be maintained. With fluorescence microscopy tuned to detect NADH in live organisms, motile bacteria could be detected by direct scanning of the veins in ice samples.
It seems to be a fundamental law that, wherever microbial life can survive, it will be found to exist (1). Microbes are amazingly hardy; viable specimens of a spore-forming bacillus and of an extremely halophilic bacterium have been found in an inclusion in a 250-million-year-old salt crystal.† A bacterial spore has been revived, cultured, and identified from 40-million-year-old amber (2). Microbial life has been found at depths down to several kilometers in the earth's crust (3), and viable bacterial populations have been discovered at Pacific Ocean sites to depths of >500 m in sediments (4). Bacteria can grow and reproduce at temperatures ≤0°C in high-altitude cloud droplets (5). Microbial communities survive on wind-deposited sediment particles within liquid water inclusions in permanently ice-covered Antarctic lakes (6). Before Antarctica developed its permanent ice cap some 14 million years ago (7), microbes may well have existed in its continental crust, and their descendants may live in subglacial rock crevices, lakes, and sediments and perhaps even in the glacial ice itself. Far less is known about psychrophilic (cold-loving) bacteria than about thermophiles, and it is not even certain yet whether life on earth originated in a hot or cold environment (8). It seems worthwhile to devise an in situ search for live microorganisms in ancient polar ice. In this paper, I propose a habitat that will sustain a small population of psychrophilic bacteria in deep Antarctic ice in the absence of sunlight or oxygen, at pressures up to 400 bars (1 bar = 100 kPa), at temperatures well below 0°C, and in strongly acidic or saline solutions.
Evidence for Microbial Life in Deep Antarctic Glacial Ice.
Consider first airborne sources of microbes blown onto snow and compacted into the polar ice. Working with filtered (0.2 μm), melted ice from depths down to 2,750 m in the ice core from Vostok Station (east Antarctica), Abyzov et al. (9) studied the concentration and morphology of microbes stained with a fluorescent dye. Concentrations as a function of depth (and thus of age) ranged from ≈103 to ≈104 cells per cm3 and correlated with dust concentration, which suggests that they were deposited in the snow preferentially during glacial periods when the flux of dust and the wind speed were greatest. In addition, the authors used consumption of a 14C-labeled protein hydrolysate as a crude measure of cell viability. Superimposed on a general decline of radiocarbon uptake as a function of depth of origin of the sample (from 0.0044 μg per liter per h at 1,665 m to 0.0002 μg per liter per h at 2,750 m), they found a very low uptake in cells from depths corresponding to cold periods.
Willerslev et al. (10), using PCR amplification of fragments of the eukaryotic 18S rRNA gene, identified a diversity of fungi, plants, algae, and protists extracted from 2,000- and 4,000-year-old ice-core samples from North Greenland. They did not test them for viability.
The possibility of finding subglacial sources of microbes is now of great international interest. In 1974–1975, airborne radar mapping (11) resulted in the discovery of a huge subglacial lake under nearly 4 km of ice near Vostok Station. Recently, seismic sounding and radar mapping plus radar altimetry (12) showed that the lake water is ≈650 m deep at the deepest end and has a density consistent with that of fresh water. Workshops in St. Petersburg, Russia (13), Washington, DC‡, and Cambridge, England§, were devoted to discussions of prospects for drilling into the lake. At the St. Petersburg workshop, Petit (14) reported that the bottom ≈100 m of a 3,623-m-deep Vostok ice core that reached a depth ≈120 m above the lake consists of refrozen lake water, called “accretion ice,” whose crystals range in size from ≈0.1 to 1 m. The accretion ice is believed to extend another 100 m from the bottom of the core to the lake surface. Ionic composition measurements and dc electrical conductivity measurements (see Table 1) have since shown that the accretion ice seems not to be acidic, presumably reflecting the composition of the lake water. At the Cambridge workshop, Priscu et al. (14) reported detection in melted accretion ice of 3 × 103 to 4 × 104 bacterial cells per ml, which were not culturable and did not incorporate radiocarbon during 52 h in air at 1 bar. Karl et al. (15) also reported evidence for microbial life in their sample of melted accretion ice: the presence of dissolved organic carbon (7 μM), including ≈100 pg/liter of lipopolysaccharides, and sluggish uptake of 14CO2 into biomolecules. A small fraction of the microbes in the accretion ice is viable but probably not capable of reproducing. In contrast to the atmospherically deposited microbial life found at depths down to 2,750 m in the Vostok ice core (9), life in Lake Vostok might have emerged from sediments, from cracks in bedrock, or even from thermal vents and might have migrated upward into the accretion ice. Without direct measurements, this hypothesis is only speculation. No one has yet observed living, motile organisms in situ in unmelted glacial freshwater ice.
Table 1.
Major ions in Vostok glacial ice at a depth of 3,300 m and in accretion ice at ≈3,600 m
| Ion or compound | Glacial ice (4 × 105
years)
|
Accretion ice (filtered)
|
||
|---|---|---|---|---|
| ng/g | Bulk molarity, μM | ng/g | Bulk molarity, μM | |
| SO42− | 122 | 1.24 | 38 | 0.39 |
| NaCl in solid grains | 58.2 | 1 | — | — |
| NO3− | 18.6 | 0.3 | 10 | 0.164 |
| Na+ | — | — | 29 | 1.26 |
| Cl− | 5 | 0.14 | 37.6 | 1.06 |
| CH3SO3− | 7.99 | 0.084 | — | — |
| HCOO− | 0.95 | 0.02 | — | — |
| CH3COO− | 0.77 | 0.013 | — | — |
| Mg2+ | — | — | 7.6 | 0.32 |
| Ca2+ | — | — | 9.7 | 0.24 |
| Acidity in bulk | — | ≈2 | — | ≈0 |
| DOC (ref. 15) | ? | ? | ? | ≈7 |
| DOC (ref. 14) | — | — | ∼500 | ? |
DOC, dissolved organic carbon of unknown composition.
Liquid Veins in Vostok Ice.
Fig. 1 shows a habitat that I argue can provide microbes in polar ice with the three ingredients essential for life: water, energy, and carbon. The high dc electrical conductivity of cold polar ice had long provided indirect evidence for aqueous acid solutions concentrated in veins (16). Aqueous veins at the linear junctions of three ice crystals in temperate glaciers are now known to form a continuous network, and laboratory experiments have clarified their geometry (17). Using a scanning electron microscope equipped with a cold stage and an energy-dispersive x-ray microanalyzer, Wolff and coworkers (18, 19) showed that sulfur was concentrated in veins and was undetectable in the bulk of the ice. They estimated that, in a region where a vein roughly 1 μm2 in cross section intersected the surface of an ice sample, the concentration of sulfuric acid was about 2.5 M. From the mean crystal size of their sample, they estimated that the melt water concentration of acid was ≈7 μM and thus that most, and perhaps all, of the acid was concentrated in veins. They later showed that hydrochloric acid can also concentrate in veins (20). Recently, using a micrometer-size laser beam, Fukazawa et al. (21) inferred from Raman spectra that aqueous solutions of sulfuric and nitric acid are concentrated in veins in Antarctic ice as HSO4− and NO3− ions. The evidence is thus strong that all three of the major mineral acids do collect in veins.
Figure 1.
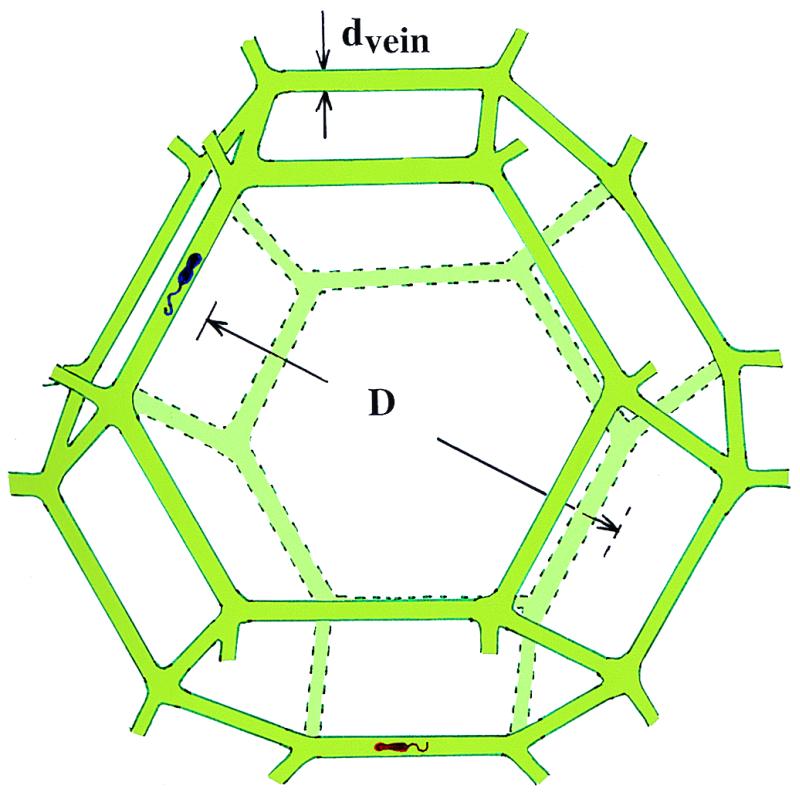
Microbial habitat consisting of solid ice grains (approximated by truncated semiregular octahedra) bounded by liquid veins (not to scale). Two microbes are depicted as living in the vein of diameter dvein surrounding a single grain of diameter D.
The explanation for how foreign ions migrate into veins has to do with the fact that micrometer-size droplets of acids and sea salts deposited as aerosols are essentially insoluble in ice crystals. Coarsening and recrystallization of deep ice, in response to the shear stress induced by the weight of the overlying ice, take place by migration of grain (crystal) boundaries, which sweep through and scavenge the droplets. A droplet first encounters a two-grain boundary and then diffuses into veins at the edges of the boundary. The driving force for concentrating in a vein rather than in a planar boundary is the greater decrease in the freezing point of a vein because of its curvature. The eutectic temperatures of acids, such as sulfuric acid (−73°C), hydrochloric acid (−88°C), nitric acid (−43°C), MSA (−75°C), and formic acid (−49°C), are low enough to ensure that, in thermodynamic equilibrium, they will exist as liquids in veins within solid pure ice. It seems unlikely, however, that solid salt grains intersected by a grain boundary will end up in aqueous solution in veins, and certainly mineral dust grains will not.
To develop a quantitative model, consider ice at temperature T in which an aqueous solution of acids and other soluble impurities is entirely located in veins of uniform radius rvein, where rvein is the radius of an equivalent cylinder (not the radius of curvature of the vein interface, which is convex inwards). In accordance with the work of Frank (22), I model polycrystalline ice as consisting of grains of semiregular truncated octahedra of diameter (between square faces) D. The volume of a grain is D3/2, and it has 36 edges, each of length √2D/4, each shared with three other grains. The total fractional volume (f) in veins is
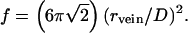 |
1 |
The molar concentration (Cvein) of an aqueous solution in veins is
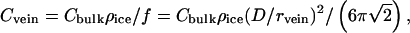 |
2 |
where Cbulk is measured in melt water and ρice = 0.915 g/cm3. Cvein is determined by the free-energy requirement that, in equilibrium, the two-phase system (i.e., pure ice + aqueous solution) be on the freezing line of its phase diagram. Thus, Cvein is a function of ice temperature that can be found by judicious use of tables that give the depression of the freezing point as a function of concentration for various solutions (23). Once Cvein is known, vein diameter is found from
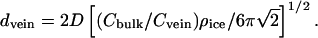 |
3 |
Ionic Composition.
Table 1 shows the data from which the molarity in veins and the vein diameter were calculated as a function of temperature of Vostok ice. The bulk concentrations of ions in Vostok ice at a depth of ≈3,300 m (corresponding to an age of ≈4 × 105 years) were taken from unpublished tables provided by M. Legrand (personal communication; see also ref. 24), and the concentrations for accretion ice were provided by Priscu et al. (14) and Karl et al. (15). The ionic activities of each of the ion species contribute additively to depression of the freezing point (23, 25). The balance of positive ions in the ancient ice is provided by H+ (26), whereas the composition of the accretion ice seems to be accounted for by salts and not to require H+ ions.
Composition and Size of Veins.
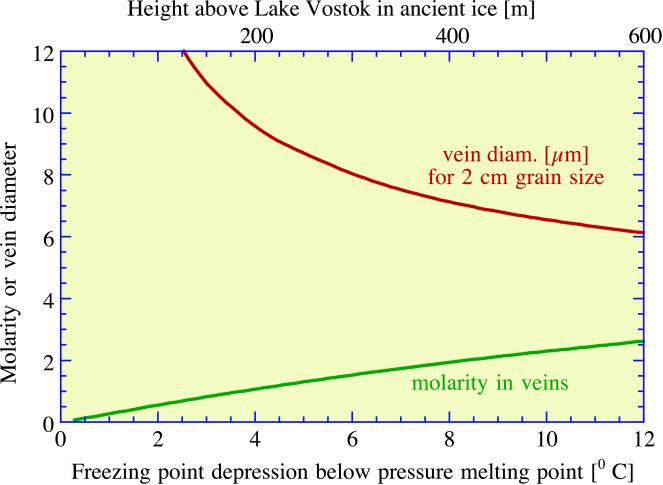
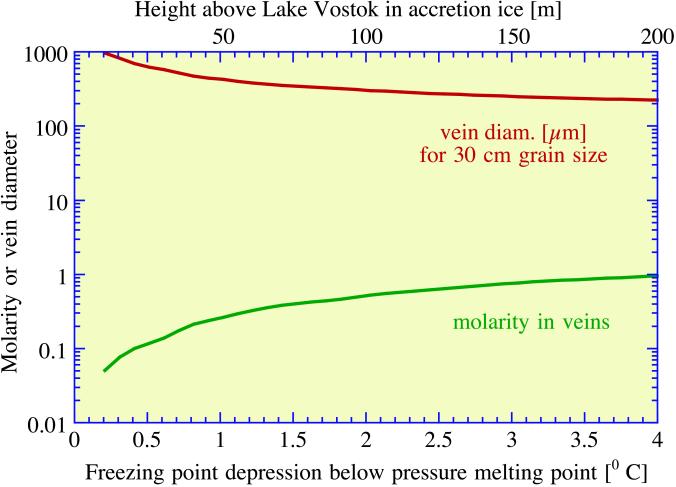
Fig. 2 shows results for ancient Vostok ice, and Fig. 3 shows results for accretion ice. The accretion ice extends from ≈3,538 m to the lake surface at ≈3,750 m, and the Vostok core retrieved ice down to a depth of 3,623 m. From 3,538 to 3,608 m, the ice contained numerous mud inclusions ≈1 mm in diameter, presumably incorporated from materials along the lake margin. Below 3,608 m, the ice is very clear and is thought to have accreted from freezing of lake water to the base of the ice sheet as it passed over the lake. The temperature gradient in the lowest few hundred meters of ice is taken to be 0.02°C/m. The abscissae in Figs. 2 and 3 give freezing point depression relative to the pressure melting point, found from the relation dT/dP = 0.0074°C/bar to be −2.8°C for pure ice just above the lake surface. The mean grain size of the glacial ice was taken to be D = 2 cm, and that of accretion ice was taken to be D = 30 cm (13). Because vein diameter scales linearly with D, it is easy to find dvein for other choices of D. The main difference between the two environments, other than differences in impurity concentrations and differences in grain size, is that ancient ice scavenges only acids, not salts, into veins, whereas accretion ice freezes from lake water in which all the ions are dissolved. Figs. 2 and 3 show that, at temperatures down to a few degrees below the pressure melting point, vein diameter is easily large enough to accommodate motile micrometer-size bacteria. With decreasing depth, the temperature decreases, reaching −56°C at the surface, and the vein diameter decreases to a few micrometers, with a concomitant increase in molarity of the acids.
Figure 2.
Relationships among molarity of impurities in aqueous solution, vein diameter, and depression of freezing point below pressure melting point for the composition of impurities in glacial ice. Also shown is approximate height above Lake Vostok. For arbitrary grain size, vein diameter scales as grain size.
Figure 3.
Relationships among molarity, vein diameter, and depression of freezing point for the composition of impurities in accretion ice, with D taken to be 30 cm.
Energy Source.
For bacteria in veins, aqueous sulfuric acid and nitric acid are the main electron acceptors. MSA (HCH3SO3), formic acid (HCOOH), and acetic acid (CH3COOH) will also be swept into veins and will provide energy and carbon for biosynthesis. Dissolved organic carbon is present in Vostok ice (Table 1), but its composition and location are not known.
I have shown that segregation of acids into veins raises the concentrations of nutrients and of dissolved carbon as much as a million-fold, depending on ice temperature and crystal size, thus providing channels of aqueous solution in which microorganisms can live and extract energy. For those microbes that find themselves in veins, maintenance of life seems likely, given the demonstrated adaptability of life to extreme environments. Eqs. 4 and 5 provide examples of reactions that provide both energy and carbon: decomposition of MSA at a concentration of ≈0.1 M in ancient ice (Eq. 4) and formic acid at a lower composition together with sulfuric acid (Eq. 5). The substantial changes in standard-state Gibbs free energy, ΔG°, shown under each equation are essentially independent of temperature and concentration in the ranges of interest. Formic + nitric acid would also work. Formate is interesting, because some bacteria are known to sense and move toward formate under anaerobic conditions.
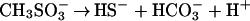 |
4 |
(ΔG° = −55 to −56 kcal/mol at −10 to −40°C)
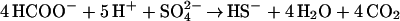 |
5 |
(ΔG° = −84.5 to −81 kcal/mol at −10 to −40°C)
Hyperacidophiles are known (27–29) that can exist at pH ≈0. A fungus, Acontium velatum, has been cultured in 2.5 M H2SO4 (30). Thiobacillus-like bacteria are able to grow (27, 28) both at very low pH and at temperatures below 0°C in a mine in Greenland at 83°N. To survive in veins in the deep ice, the microbes depicted in Fig. 1 must be able to withstand temperatures below 0°C, without oxygen, in the dark, and at pressures up to 400 bars. In addition, those in strongly acidic veins must consume energy to operate a strong proton pump or have a low proton membrane permeability to maintain their interior at nearly neutral pH. Those in accretion ice at subfreezing temperature will have to tolerate salt at 1 to 2 M.
Carbon Supply Limits Population Size.
The age of the glacial ice at ≈3,300 m in the Vostok core is ≈4 × 105 years. A freezing rate of several millimeters to several centimeters per year‡ for the accretion ice implies that it is much younger, reaching only a few 104 years at 100 m above Lake Vostok. Assuming that the source of energy and carbon is MSA and taking a bulk molarity of 0.084 μM for MSA in liquid veins, I use data from refs. 5 and 31 to estimate the bacterial population size that could be maintained for times of 4 × 105 years in glacial ice and for 104 years in accretion ice. I assume that microbes in veins in ice have a composition CH1.8O0.5N0.2, a cell volume of 0.084 μm3, and a dry mass of 30 fg (5).
In a nutrient-poor soil, Morita (31) found that ≈3 × 10−5 g of carbon per g of biomass carbon per h at 15°C was needed to prevent microbial carbon loss during incubation. (This amount is about 1/1,000 of that typically consumed by biomass in laboratory cultures.) Then, for 4 × 105 years, the MSA in glacial ice would maintain a population per cubic centimeter of ice of approximately seven cells that are biologically functional but not multiplying. In 104-year-old accretion ice, more than 102 cells per cm3 would be found. Because the ice temperature is at least 20°C colder than that of the biomass studied by Morita, the lower metabolism rate in ice would permit an even larger population to survive.
In their study of bacteria collected from supercooled cloud droplets, Sattler et al. (5) measured an uptake rate at 0°C of ≈2 × 10−21 mol thymidine h−1 per cell, a rough measure of cell growth. Equating MSA to thymidine as a source of carbon, the thymidine uptake rate leads to estimates of 10 and 400 cells per cm3 for the sustainable populations of bacteria in ice of ages 4 × 105 and 104 years, respectively. These estimates are very similar to those based on Morita's data.
If the dissolved organic carbon listed in Table 1 can also be incorporated into cells, a still larger population could be maintained.
Searching for Living Bacteria in Veins.
By exploiting their confinement to liquid veins, one could easily search for bacteria in situ. In collecting an ice core from great depth where the ice is relatively warm, it would be desirable to preserve bacteria in the veins in an anaerobic and thermal environment similar to that to which they were acclimated. Because vein size in equilibrium depends on ice temperature, one would have to maintain the ice samples at a roughly constant temperature during shipping and study. In ancient ice with D = 2 cm, the length of vein that would have to be scanned for bacterial cells would be only ≈2 cm per cm3 of ice. It would be easy to scan 10 cm3 or more in a low-power microscope by using phase contrast or fluorescence microscopy. In principle, one could introduce a dye specific to certain molecules into liquid veins. Saito (32) has shown that 8-anilinonaphthalene 1-sulfonate will indicate the presence of cells; sulfofluorescein diacetate can be used to detect certain enzymes such as esterase; and ethidium bromide can identify nucleic acids. It would be easier, however, to avoid dyes by using 366-nm light to search for NADH, which marks the existence of a living cell by fluorescing at ≈440 nm (33).¶ With 103 to 106 NADH molecules per living cell, the signal from a single cell would be strong. In a dead cell, NADH is oxidized to NAD, which does not fluoresce.
In accretion ice, with D = 30 cm, veins would be very large in diameter, but the probability of finding a vein in a random sample would be very low. The best strategy would be to use a stereoscopic microscope to search for a portion of the core that contains a vein before preparing a sample for fluorescence microscopy. The risk of contamination by external organisms is far lower with the vein technique than with techniques that require melting and filtering of ice samples.
A Subglacial Lake at the South Pole.
In their analysis of airborne radar surveys of Antarctica, Siegert et al. (34) found evidence for at least 77 subglacial lakes, one of which has subsequently proved to be only a few kilometers from the South Pole Station (D. Blankenship and D. Morse, unpublished results). The idea of collecting water, ice, and sediments from the South Pole subglacial lake has both advantages and disadvantages compared with sampling Lake Vostok. Being only a few kilometers in size, the South Pole lake and the surrounding bedrock would be easy to characterize by radar mapping and seismic sounding, and being close to the infrastructure of the South Pole Station, researchers would have easy access to modern laboratory facilities for study of samples to be recovered by sterile drilling techniques. One could, for example, use a pressure-resistant waterproof fluorescent camera for assaying ice for living cells as a drill is being lowered toward the lake.
Greenland Glacial Ice.
No subglacial lakes exist in Greenland. Two cores have been drilled to bedrock at Dome Summit, Greenland (35).‖ The grain size reaches 2–3 cm in the bottom 80 m, at depths below 2,950 m. The temperature and age near bedrock are ≈−9°C and ≈250,000 years, respectively. The impurities in Dome Summit ice are generally not acidic (unlike Vostok ice), and there is somewhat more formate and acetate than at Vostok. dc electrical measurements show that the conductivity (a measure of [H+] and of conducting veins) is much lower than that in Antarctic ice. It thus seems unlikely that there is a network of liquid veins hospitable to psychrophiles in these cores.
Concluding Comments.
The liquid vein habitat provides microbes with intimate access to water, energy, and carbon. By contrast, metabolism of microbes encased in solid ice must overcome a seemingly insuperable barrier: diffusion of nutrients through a solid is orders of magnitude slower than it is through a liquid. The advantages of scanning liquid veins in solid ice with fluorescence microscopy are that contamination would be ruled out, that living microbes could be distinguished from dead ones, and that a concentration of as low as 1 cell per cm3 could be detected in veins, despite a high background of dead matter not located in veins. We do not yet know of any species capable of tolerating all of the following conditions: no sunlight, no oxygen, pressure of ≈400 bars, temperature below 0°C, and a highly acidic or saline medium. It is thus likely that microbes living in liquid veins in ancient ice would be different from any yet known. Another interesting outcome of a search for living microbes in liquid veins in ice of known age would be to determine from the population density whether oligotrophic psychrophiles have developed an unusually high metabolic efficiency that permits long-term survival on very limited extracellular energy resources.
Acknowledgments
I am indebted to Sridhar Anandakrishnan, Don Blankenship, Frank Carsey, Michael Goldfeld, Michel Legrand, Donal Manahan, Rollie Myers, Ken Nealson, Norm Pace, Linda Powers, John Priscu, Roland Psenner, Takeshi Saito, Jose de la Torre, and Eric Wolff for discussions. This work was supported in part by National Science Foundation Grant PHY-9971390.
Abbreviation
- MSA
methanosulfonic acid
Footnotes
Vreeland, R. H., Vay, H., Bartell, J. H. & Rosenzweig, W. D. American Society for Microbiology, 99th General Meeting, June 2, 1999, Chicago.
Bell, R. & Karl, D., Proceedings of the Lake Vostok Workshop: A Curiosity or a Focus for Interdisciplinary Study?, Nov. 7 and 8, 1998, Washington, DC.
Ellis-Evans, C., Proceedings of the Workshop on the Exploration of Antarctic Subglacial Lakes, Sept. 25–27, 1999, Cambridge, England.
Powers, L. & Ellis, W., Jr., Defense Advanced Research Projects Agency. Conference on Biological Agent Detection and Identification, April 27–30, 1999, Washington, DC.
See ref. 35 for 47 papers devoted to research on the two deep ice cores drilled in central Greenland during 1989–1993 by the U.S. Greenland Ice Sheet Project 2 and the European Greenland Ice Core Program.
References
- 1.Gold T. Proc Natl Acad Sci USA. 1992;89:6045–6049. doi: 10.1073/pnas.89.13.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cano R J, Borucki M K. Science. 1995;268:1060–1064. doi: 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen K. Earth Sci Rev. 1993;34:243–260. [Google Scholar]
- 4.Parkes R J, Cragg B A, Bale S J, Getliff J M, Goodman K, Rochelle P A, Fry J C, Weightman A J, Harvey S M. Nature (London) 1994;371:410–413. [Google Scholar]
- 5.Sattler, B., Puxbaum, H. & Psenner, R. (2000) Geophys. Res. Lett., in press.
- 6.Priscu J C, Fritsen C H, Adams E E, Giovannoni S J, Paerl H W, McKay C P, Doran P T, Gordon D A, Lanoil B D, Pinckney J L. Science. 1998;280:2095–2098. doi: 10.1126/science.280.5372.2095. [DOI] [PubMed] [Google Scholar]
- 7.Kennett J P. J Geophys Res. 1977;82:3843–3860. [Google Scholar]
- 8.Levy M, Miller S L. Proc Natl Acad Sci USA. 1998;95:7933–7938. doi: 10.1073/pnas.95.14.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abyzov S S, Mitskevich I N, Poglazova M N. Microbiology. 1998;67:451–458. [Google Scholar]
- 10.Willerslev E, Hansen A J, Christensen B, Steffensen J P. Proc Natl Acad Sci USA. 1999;96:8017–8021. doi: 10.1073/pnas.96.14.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robin G de Q, Robin G de Q, Drewry D J, Meldrum D T. Philos Trans R Soc London B. 1977;179:185–196. [Google Scholar]
- 12.Kapitsa A P, Ridley J K, Robin G de Q, Robin G de Q, Siegert M J, Zotikov I A. Nature (London) 1996;381:74–76. [Google Scholar]
- 13.Petit J R. In: Proceedings of the Lake Vostok Study: Scientific Objectives and Technological Requirements. Barkov N I, Lipenkov V Y, editors. St. Petersburg, Russia: Arct. Antarct. Res. Inst.; 1998. pp. 43–46. [Google Scholar]
- 14.Priscu J C, Adams E E, Lyons W B, Mogk D W, Voytek M A, Brown R L, McKay C P, Takacs C D, Welch K A, Kirshtein J D, et al. Science. 1999;286:2141–2144. doi: 10.1126/science.286.5447.2141. [DOI] [PubMed] [Google Scholar]
- 15.Karl D M, Bird D F, Björkman K, Houlihan T, Shackelford R, Tupas L. Science. 1999;286:2144–2147. doi: 10.1126/science.286.5447.2144. [DOI] [PubMed] [Google Scholar]
- 16.Wolff E W, Paren J G. J Geophys Res. 1984;89:9433–9438. [Google Scholar]
- 17.Mader H M. J Glaciol. 1992;38:333–347. [Google Scholar]
- 18.Wolff E W, Mulvaney R, Oates K. Ann Glaciol. 1988;11:194–197. [Google Scholar]
- 19.Mulvaney R, Wolff E W, Oates K. Nature (London) 1988;331:247–249. [Google Scholar]
- 20.Wolff E W, Mulvaney R, Oates K. Geophys Res Lett. 1989;16:487–490. [Google Scholar]
- 21.Fukazawa H, Sugiyama K, Mae S, Narita H, Hondoh T. Geophys Res Lett. 1998;25:2845–2848. [Google Scholar]
- 22.Frank F C. Nature (London) 1968;220:350–352. [Google Scholar]
- 23.Lide D R, editor. CRC Handbook of Chemistry and Physics. 78th Ed. Boca Raton, FL: CRC Press; 1997. pp. 8-56–8-78. [Google Scholar]
- 24.Legrand M, Mayewski P. Rev Geophys. 1997;35:219–243. [Google Scholar]
- 25.James A M, Lord M P. Index of Chemical and Physical Data. New York: Van Nostrand Reinhold; 1992. [Google Scholar]
- 26.Legrand M R, Lorius C, Barkov N I, Petrov V N. Atmos Environ. 1988;22:317–331. [Google Scholar]
- 27.Schleper C, Pühler G, Kühlmorgen B, Zillig W. Nature (London) 1995;375:741–742. doi: 10.1038/375741b0. [DOI] [PubMed] [Google Scholar]
- 28.Johnson D B. FEMS Microbiol Ecol. 1998;27:307–317. [Google Scholar]
- 29.Langdahl B R, Ingvorsen K. FEMS Microbiol Ecol. 1997;23:275–283. [Google Scholar]
- 30.Sletten O, Skinner C E. J Bacteriol. 1948;56:679–681. doi: 10.1128/jb.56.5.679-681.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morita R Y. Can J Microbiol. 1988;34:436–441. [Google Scholar]
- 32.Saito T. Proc SPIE Int Soc Opt Eng. 1999;3755:24–32. [Google Scholar]
- 33.Chance B, Thorell B. J Biol Chem. 1959;234:3044–3050. [PubMed] [Google Scholar]
- 34.Siegert M J, Dowdeswell J A, Gorman M R, McIntyre N F. Antarct Sci. 1996;8:281–286. [Google Scholar]
- 35.U.S. Greenland Ice Sheet Project 2 and the European Greenland Ice Core Program (1997) J. Geophys. Res.102.