Abstract
Invasive aspergillosis (IA) is the most common life-threatening invasive mold infection worldwide. The principal therapy for IA is amphotericin B, despite its known toxicity and immunosuppressive side effects. Studies in animal models of IA suggest a role for T lymphocytes in the pathology of the disease, although the precise role for Aspergillus-specific T cells remains undefined. The isolation and characterization of T lymphocytes in animal models of IA are hampered by the rapid outgrowth of the fungus in cultures derived from infected organs. In the present study, we tested the abilities of the antifungal drugs caspofungin acetate and voriconazole to inhibit fungal growth in vitro as a means of maintaining cultures of T cells from Aspergillus-infected mice. We demonstrate that while both antifungal drugs are inhibitory, only voriconazole completely inhibited fungal growth, allowing long-term maintenance of T-cell cultures. In addition, voriconazole had no inhibitory effect on the activation and maturation of dendritic cells or the proliferation of T lymphocytes. Thus, voriconazole appears to be a promising agent for use in in vitro studies of Aspergillus-specific T lymphocytes in animal models of IA.
Aspergillus fumigatus causes severe invasive disease in immunocompromised patients (15). Invasive aspergillosis (IA) occurs as a result of immunosuppressive treatments administered following allogeneic stem cell and organ transplantation or treatment of acute leukemia, but it rarely affects immunocompetent patients. Studies of IA in animal models have demonstrated a role for T-lymphocyte responses and associated cytokines in the control of A. fumigatus infections. Murine models of IA have shown that Th1-type responses, characterized by the production of interleukin-12 (IL-12) and gamma interferon, are important in the protection against lethal infection, while a predominance of Th2-type cytokines (e.g., IL-4 and IL-10) correlates with an inability to clear the fungus and eventual death (4-6, 19). While T cells are thought to be important players in establishing the cytokine milieu and determining the outcome of disease, their precise role in the protection against Aspergillus infections remains poorly characterized. In an effort to characterize these cells, we isolated T cells from Aspergillus-infected organs but were unable to maintain these cells in culture due to the rapid outgrowth of the fungus.
Antifungal drugs provide an effective means of preventing Aspergillus germination in vitro and thus may circumvent the problem of fungal outgrowth in cell cultures. However, the widely used antifungal amphotericin B is known to have myriad suppressive effects on cells of the immune system, including T cells and antigen-presenting cells, and would thus be undesirable for use in T-cell cultures (9, 13). The effects of other antifungal drugs on T-cell function has not been widely explored in vitro or in vivo.
In this study, we examined the effects of two new antifungal agents, caspofungin acetate and voriconazole, on fungal outgrowth, T-cell proliferation, and dendritic cell (DC) activation. Voriconazole is a broad-spectrum triazole antifungal that inhibits the sterol biosynthesis pathway, which is critical for the production of the fungal cell wall and sustained growth. Voriconazole is active against many Aspergillus species both in vitro and in vivo (17, 22) and has been shown to be at least as effective as amphotericin B and to result in fewer severe side effects in patients (10). Caspofungin is an echinocandin that inhibits fungal cell wall synthesis and has been shown to be effective in the treatment of disseminated aspergillosis in animal models (1, 12).
We show that voriconazole was effective in inhibiting fungal growth in cultures of cells derived from infected organs, while caspofungin was less effective. In addition, voriconazole did not inhibit T-cell proliferation following mitogenic or antigen-specific stimulation, nor did it affect the activation and maturation of DCs. Thus, voriconazole effectively inhibits Aspergillus germination in culture without measurable effects on T-cell proliferation or antigen-presenting cell activation. In vitro suppression of fungal growth by voriconazole will allow the propagation and maintenance of T cells from Aspergillus-infected organs, greatly enabling studies of T-cell responses during IA.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6 mice (age, 6 to 8 weeks) were purchased from The Jackson Laboratory (Bar Harbor, Maine) and were maintained under specific-pathogen-free conditions before and during the experiments. L9.6 transgenic mice, which express a T-cell response specific for Listeria monocytogenes-derived epitope p60217-225, were generated as described previously (16).
Microorganisms, culture conditions, and infection.
A. fumigatus strain 293 is a clinical isolate of Aspergillus from lung tissue and was generously provided by Michael Anderson (University of Manchester, Manchester, United Kingdom). The fungus was grown on Sabouraud dextrose agar slants (Becton Dickinson) for 7 to 10 days at 37°C. Conidia were harvested by inverting the slant over a 50-ml conical tube and gently tapping the side of the slant to dislodge the conidia. A total of 25 ml of sterile phosphate-buffered saline (PBS) plus 0.025% Tween 20 was added to the conidia, and the suspension was filtered twice through a 40-μm-pores-size filter to remove hyphal fragments and conidial aggregates. The conidia were enumerated with a hemocytometer. A. fumigatus hyphae were grown by inoculating minimal medium with 5 × 106 conidia/ml and incubating the cultures with shaking for 2 to 3 days at 37°C. Hyphae were harvested by filtering the cultures through Whatman no. 54 filters and washing the hyphae extensively with sterile PBS. The suspension was sonicated to generate hyphal fragments, and the relative concentration of hyphae was assessed by the chitin assay. The preparation used in this study contained 165 μg of glucosamine per ml.
Mice were infected via the lateral tail vein with 5 × 105 or 1 × 106 conidia in 0.2 ml of PBS-0.025% Tween 20. Control animals were given PBS-0.025% Tween 20. Infected animals were killed on day 4 postinfection, and their spleens were harvested.
Chitin assay.
Chitin contents were measured as described previously (18). The chitin contents of the samples were measured on the basis of a glucosamine standard curve and are expressed as micrograms of glucosamine per milliliter.
Antifungal agents.
Caspofungin acetate was provided by the pharmacy at Memorial Sloan-Kettering Cancer Center (New York, N.Y.). Voriconazole (UK-109496) was kindly provided by Pfizer Ltd. (Sandwich, United Kingdom).
Inhibition of fungal growth.
Splenocytes from infected and control C57BL/6 mice (n = 3 mice/group) were harvested 4 days after intravenous infection with 0.5 × 106 or 1 × 106 A. fumigatus conidia. Cells were plated at 5 × 105/well in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics. Antifungal drugs were added to the wells at final concentrations ranging from 0.5 to 10 μg/ml, as indicated where appropriate. Replicates of 12 wells were plated for each drug concentration. The cultures were maintained at 37°C for 7 days and each day were visually monitored for fungal growth.
DC activation.
Bone marrow was isolated from the femurs and tibias of adult C57BL/6 mice, filtered through nylon mesh, and depleted of red blood cells by hypotonic lysis with 0.17 M NH4Cl. The cells were resuspended at 1 × 106 to 2 × 106/ml of RPMI 1640 plus 2% granulocyte-macrophage colony-stimulating (GM-CSF) and were cultured for 4 to 5 days at 37°C. On day 2, nonadherent cells were removed by gentle pipetting and fresh RPMI-2% GM-CSF was added to the wells. Immature DCs were harvested on day 4 or 5 of culture and were stimulated with increasing dilutions of A. fumigatus hyphal fragments or with lipopolysaccharide (LPS; 10 ng/ml) or CpG DNA (1 μM) for 24 h at 37°C in the presence or absence of voriconazole. These concentrations were determined to be optimal in dose-response experiments. The supernatants were removed and stored at −80°C for cytokine analysis. DCs were stained for expression of surface molecules by using the following antibodies: anti-CD11c (HL3), anti-CD80 (16-10A1), anti-CD86 (GL1), and anti-major histocompatibility complex (anti-MHC) class II (I-A/I-E; M5/114.15.2) (PharMingen). The DCs were then analyzed by flow cytometry. Cytokine levels in DC culture supernatants were measured by an enzyme-linked immunosorbent assay according to the instructions of the manufacturer (PharMingen).
T-cell proliferation assays.
Splenocytes from infected (n = 3) or uninfected (n = 3) C57BL/6 or L9.6 transgenic mice were plated at 2 × 105 to 3 × 105 cells/well in 96-well flat-bottom plates (Costar). The cells were stimulated with immobilized anti-CD3 antibody (10 μg/ml), phorbol myristate acetate (PMA; 50 ng/ml), ionomycin (500 ng/ml), specific peptide (L. monocytogenes p60217-225) at the indicated concentrations or live A. fumigatus hyphae for 4 to 5 days at 37°C. Replicates of five wells were plated for each stimulation condition. Voriconazole was added to the wells at 0.5 to 10 μg/ml for the duration of the assay except for the stimulation with live hyphae, in which voriconazole was added after the first 24 h of incubation. [3H]thymidine was added for the final 16 h of culture.
Immunization with live hyphae.
C57BL/6 mice were immunized intravenously (i.v.) (n = 2) or intraperitoneally (i.p.) (n = 2) with live A. fumigatus hyphal fragments diluted in PBS (1:4, which is equal to 41.3 μg of glucosamine per ml) and were boosted 2 weeks later in the same way. Control mice were given PBS alone. Four weeks after the mice were boosted, splenocytes were harvested for analysis in proliferation assays in the presence of 0.5 μg of voriconazole per ml, as described above.
RESULTS
Voriconazole prevents outgrowth of A. fumigatus in cell culture.
We have found that the isolation and propagation of T lymphocytes specific for A. fumigatus antigens are hampered by rapid outgrowth of the fungus in cultures of cells derived from infected organs. In an attempt to circumvent this problem, we tested the abilities of two antifungal agents, caspofungin acetate and voriconazole, to prevent fungal outgrowth in cultures of spleen cells from mice infected i.v. with A. fumigatus conidia. Splenocytes from mice infected with a sublethal dose (0.5× 106 to 1 × 106) of A. fumigatus conidia 4 days earlier were cultured in the presence or absence of increasing concentrations of caspofungin or voriconazole for 7 days at 37°C and were monitored daily for fungal growth. In the absence of drug, A. fumigatus germination was seen in 100% of the wells within 2 to 3 days of culture, as indicated by appearance of extensively branched hyphae throughout the culture. The presence of caspofungin acetate partially inhibited fungal outgrowth in cultures of cells from mice infected with 5 × 105 conidia (growth was seen in 30 to 40% of the wells with drug and in 100% of the wells without drug) but had no inhibitory effect on fungal growth in cultures of cells derived from mice infected with 1 × 106 A. fumigatus conidia (growth was seen in 100% of the wells) (Table 1). The lowest dose of caspofungin tested (0.5 μg/ml) reduced fungal growth by 60% in cultures of cells from mice infected with 5 × 105 conidia, and increasing doses of caspofungin (1.0 to 10 μg/ml) had no additional effect. In broth microdilution assays, caspofungin was found to significantly inhibit hyphal growth at concentrations as low 0.04 μg/ml, but it did not completely inhibit germination at any concentration (data not shown). This finding is in agreement with those of previous studies (2, 3, 8), which reported similar morphological changes associated with inhibition of fungal growth by caspofungin. In contrast, addition of voriconazole to spleen cell cultures completely prevented the outgrowth of fungus, regardless of the dose of conidia used to infect the mice (Table 1). Voriconazole was effective at concentrations as low as 0.5 μg/ml and did not have to be replenished in the culture medium for continued inhibition of fungal growth. Cultures remained negative for fungal growth for the duration of the experiment (7 days). In broth microdilution analysis, voriconazole inhibited hyphal growth at 0.08 μg/ml and completely inhibited germination at 0.3 to 0.6 μg/ml (data not shown). Thus, voriconazole effectively inhibited the growth of Aspergillus in cultures of cells derived from Aspergillus-infected mice.
TABLE 1.
Inhibitory effects of caspofungin and voriconazole on fungal growth
| Drug and expt (dose) | % Wells with visible fungal growth at drug concn (μg/ml) of:
|
||||
|---|---|---|---|---|---|
| 10 | 5 | 1 | 0.5 | 0 | |
| Caspofungin | |||||
| 1 (5 × 105) | 42 | 33.5 ± 6.4 | 33.5 ± 6.4 | 40 ± 2.8 | 100 |
| 2 (1 × 106) | 100 | 100 | 100 | 100 | 100 |
| 3 (1 × 106) | 100 | 100 | 100 | 100 | 100 |
| Vorizonazole | |||||
| 1 (5 × 105) | 0 | 0 | 0 | 0 | 100 |
| 2 (1 × 106) | 0 | 0 | 0 | 0 | 100 |
| 3 (1 × 106) | 0 | 0 | 0 | 0 | 100 |
A. fumigatus hyphae activate murine DCs, and activation is not inhibited in the presence of voriconazole.
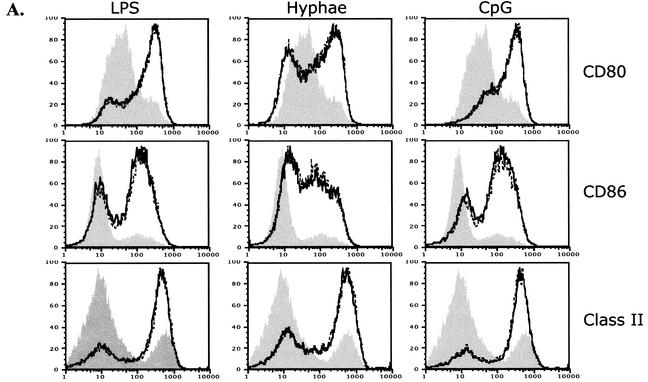
Activation of DCs following stimulation with microbial products such as LPS results in the upregulation of costimulatory molecules (CD80, CD86) and MHC class II on the surface of the cell and the production of cytokines such as IL-12 and tumor necrosis factor alpha. To determine whether A. fumigatus hyphae activate DCs, we tested the effect of exposure of bone marrow-derived DCs to live A. fumigatus hyphal fragments. We found that contact with the hyphae caused the activation and maturation of DCs, as reflected by the upregulation of MHC class II, CD80, and CD86 and the secretion of IL-12 (Fig. 1). This activation was dependent on direct contact with the hyphae, since exposure of DCs to supernatants from the solution of hyphal fragments had no activating effect (data not shown)
FIG. 1.
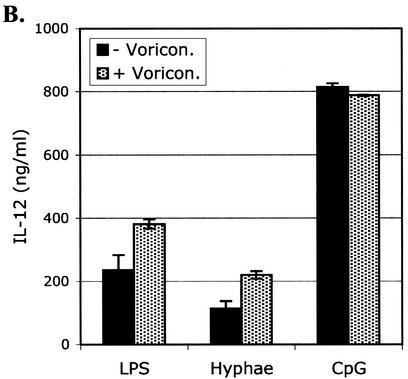
Activation of DCs by A. fumigatus hyphae, LPS, and CpG in the presence or absence of voriconazole. Bone marrow-derived DCs were cultured with A. fumigatus hyphae (1:1,000 dilution), LPS (10 ng/ml), or CpG (1 μM) in the absence or presence of voriconazole (0.5 μg/ml) for 24 h at 37°C. (A) Surface expression of CD80, CD86, and MHC class II on unstimulated DCs (filled histogram) or following activation with the indicated stimulus in the presence (dashed lines) or absence (solid lines) of voriconazole (0.5 μg/ml). (B) Production of IL-12 by unstimulated DCs (medium alone) or following activation with the indicated stimulus in the absence (−) or presence (+) of voriconazole (Voricon.; 0.5 μg/ml). The data shown are representative of greater than five independent experiments.
Since we are interested in using DCs as a means of stimulating T-cell responses in vitro, we tested whether the addition of voriconazole to the culture medium affects the activation of DCs. We stimulated DCs using various agents, including LPS, CpG DNA, and A. fumigatus hyphae, in the presence or absence of voriconazole. The presence of voriconazole at concentrations that inhibited the outgrowth of the fungus (0.5 μg/ml) did not affect the upregulation of MHC class II, CD80, or CD86 on the surfaces of the DCs (Fig. 1A) or the production of IL-12 (Fig. 1B). Production of tumor necrosis factor alpha and IL-6 by DCs was also unaffected in the presence of voriconazole (data not shown).
Voriconazole does not inhibit mitogen- or antigen-driven proliferation of T cells.
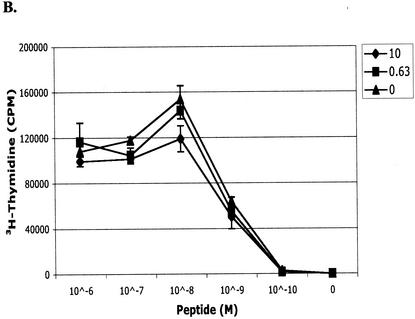
To assess whether the presence of voriconazole affects the proliferative function of T cells, we stimulated spleen cells from mice infected i.v. with 106 A. fumigatus conidia or uninfected mice in the presence or absence of voriconazole. In the absence of voriconazole, fungus grew in all wells that were seeded with cells from infected mice, precluding analysis of the assay. The presence of voriconazole at concentrations as high as 10 μg/ml did not affect T-cell proliferation in response to stimulation with either anti-CD3 or PMA-ionomycin (Fig. 2). Proliferative responses were similar when splenocytes were derived from infected or uninfected mice (data not shown). We next assessed whether the presence of voriconazole would affect antigen-specific proliferation of T cells by stimulating transgenic CD8+ T cells specific for an epitope from the L. monocytogenes p60 protein with the cognate peptide (p60217-225) in the presence or absence of drug. As shown in Fig. 2B, the levels of proliferation of the p60217-225-specific transgenic T cells in response to increasing dilutions of peptide were comparable in the presence and absence of voriconazole, indicating that proliferative signals delivered via the T-cell receptor were unaffected by the presence of the antifungal drug.
FIG. 2.
T-cell activation in the presence and absence of voriconazole. Splenocytes from infected C57BL/6 or L9.6 × Rag−/− transgenic mice were plated at 2 × 105 to 3 × 105/well in 96-well plates. (A) Splenocytes were stimulated with immobilized anti-CD3 antibody, PMA-ionomycin (P+I), or medium alone in the presence of the indicated concentrations of voriconazole for 5 days at 37°C. [3H]thymidine was added for the final 16 h of culture. Incubation in the absence of voriconazole resulted in the outgrowth of fungus in the wells, so these wells were not analyzed. (B) Splenocytes from L9.6 × Rag−/− mice were stimulated with the indicated concentration of peptide (L. monocytogenes p60217-225) in the presence (0.63 μg/ml [squares] or 10 μg/ml [circles]) or absence (diamonds) of voriconazole for 5 days at 37°C. [3H]thymidine was added for the final 16 h of culture. The data in panels A and B are representative of three independent experiments. (C) Splenocytes from mice immunized twice with live A. fumigatus hyphal fragments (i.v. or i.p.) were plated at 4 × 105 cells/well in 96-well plates. Splenocytes were stimulated with the indicated concentration of hyphal fragments (expressed as micrograms of glucosamine per milliliter) or were left unstimulated and cultured for 5 days. Voriconazole (0.5 μg/ml) was added after the first 24 h of incubation, and [3H]thymidine was added for the final 16 h.
Finally, we immunized mice with live A. fumigatus hyphal fragments in an attempt to generate Aspergillus-specific T-cell responses. After the mice were immunized twice (i.v. or i.p.) with live hyphae, we were able to detect dose-dependent antigen-specific proliferation in response to secondary stimulation with live hyphae in three of four animals (Fig. 2C). One of the mice immunized i.p. did not have a detectable proliferative response to hyphal stimulation (data not shown). The addition of voriconazole to the wells after the first 24 h of incubation allowed us to detect an antigen-specific response while inhibiting the outgrowth of fungus. Control wells incubated in the absence of voriconazole became positive for fungal growth by day 3, indicating that the mice were productively infected with Aspergillus. When we immunized mice with inactivated preparations of A. fumigatus hyphae, we were unable to detect proliferation in response to in vitro stimulation (data not shown).
DISCUSSION
The antifungal drugs voriconazole and caspofungin have both been shown to inhibit the germination of Aspergillus species in vitro (7, 21, 25) and to significantly increase survival rates in animal models of IA (1, 11, 12). In a guinea pig model, treatment of transiently immunosuppressed animals with voriconazole for 5 days after a lethal challenge with A. fumigatus provided 100% protection, while caspofungin was much less effective, achieving a maximum rate of protection of only 50 to 60% (11, 12). In our study, we showed that both drugs inhibited fungal outgrowth in cultures of cells derived from infected mice (Table 1) and inhibited the growth of A. fumigatus hyphae in pure cultures. However, voriconazole completely inhibited germination and growth in cultures of cells from infected mice, while caspofungin provided only partial growth inhibition. This is consistent with our observation that voriconazole, but not caspofungin, effectively inhibits germination of A. fumigatus when it is incubated directly with conidia (data not shown). Caspofungin has been reported to inhibit the growth of A. fumigatus isolates in vitro, with mean inhibitory concentrations as low as 0.06 μg/ml and with inhibitory concentrations ranging from 0.06 to >16 μg/ml (3, 7). However, while inhibition of fungal growth by caspofungin results in characteristic morphological changes, including short, extensively branched hyphal clusters (8, 14), it has not been reported to completely inhibit germination of conidia. Since the recovery and subsequent culture of cells from infected mice require the complete absence of fungal growth, we considered wells to be positive for fungus even if the growth was inhibited compared to the growth in the wells to which no drug was added. The concentration of caspofungin necessary to inhibit fungal outgrowth in vitro varies depending on the strain of fungus in question, and it is possible that higher concentrations would more effectively inhibit the growth of the 293 strain used in this study. However, we think that this is unlikely, since we did not observe increased inhibition with increasing concentrations of caspofungin. While it is not possible to directly compare the in vitro potencies of these drugs, voriconazole was a more reliable inhibitor of fungal outgrowth in this study.
In vitro studies of Aspergillus-specific T cells derived from infected animals require absolute inhibition of fungal outgrowth in cell culture. Without a reliable means of neutralizing fungus in cultures of tissue derived from infected mice, immunologic studies have depended on immunization with inactivated forms of the fungus. While immunization with inactive pathogens may elicit T-cell responses, studies that have used other disease models have shown that immune responses to dead pathogens are qualitatively different from those generated during infection with live organisms (16, 24).
Amphotericin B has been widely used for the treatment of invasive fungal infections in immunocompromised patients; however, it is also known to have various immunomodulatory effects. These effects of amphotericin B include inhibition of macrophage differentiation and function (20), B- and T-cell proliferation (20, 23), and CD8+-T-cell cytolytic activity and gamma interferon production (9, 13). Although liposomal formulations of amphotericin B have been shown to significantly reduce the toxicity and immunosuppressive effects of the drug in some models, suppression of antigen-specific T-cell proliferation and inhibition of protective immunity are still observed (9, 13). Voriconazole is an attractive alternative to amphotericin B, as it has been reported to be as effective or more effective than amphotericin B for the treatment of fungal infections and is significantly less toxic to patients (10). Voriconazole is also of use in animal models of Aspergillus infection, particularly those focused on the analysis of specific T-cell responses. We demonstrate here that voriconazole facilitates the culture of T cells from infected organs without compromising T-cell proliferation or DC activation. Thus, voriconazole appears to be a promising antifungal drug that will enable the isolation and characterization of Aspergillus-specific T cells in animal models of IA.
Acknowledgments
This work was supported by an award to E.G.P. from The Sandler Program for Asthma Research.
REFERENCES
- 1.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cenci, E., A. Mencacci, G. Del Sero, A. Bacci, C. Montagnoli, C. F. d'Ostiani, P. Mosci, M. Bachmann, F. Bistoni, M. Kopf, and L. Romani. 1999. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J. Infect. Dis. 180:1957-1968. [DOI] [PubMed] [Google Scholar]
- 5.Cenci, E., A. Mencacci, C. Fe d'Ostiani, G. Del Sero, P. Mosci, C. Montagnoli, A. Bacci, and L. Romani. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750-1760. [DOI] [PubMed] [Google Scholar]
- 6.Cenci, E., S. Perito, K. H. Enssle, P. Mosci, J. P. Latge, L. Romani, and F. Bistoni. 1997. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect. Immun. 65:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2000. Influence of human sera on the in vitro activity of the echinocandin caspofungin (MK-0991) against Aspergillus fumigatus. Antimicrob. Agents Chemother. 44:3302-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A. 2003. Evaluation of broth microdilution testing parameters and agar diffusion etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991). J. Clin. Microbiol. 41:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geginat, G., M. Kretschmar, S. Walter, D. Junker, H. Hof, and T. Nichterlein. 1999. Suppression of acquired immunity against Listeria monocytogenes by amphotericin B-mediated inhibition of CD8 T cell function. J. Infect. Dis. 180:1186-1194. [DOI] [PubMed] [Google Scholar]
- 10.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 11.Kirkpatrick, W. R., R. K. McAtee, A. W. Fothergill, M. G. Rinaldi, and T. F. Patterson. 2000. Efficacy of voriconazole in a guinea pig model of disseminated invasive aspergillosis. Antimicrob. Agents Chemother. 44:2865-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kretschmar, M., G. Geginat, T. Bertsch, S. Walter, H. Hof, and T. Nichterlein. 2001. Influence of liposomal amphotericin B on CD8 T-cell function. Antimicrob. Agents Chemother. 45:2383-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauvau, G., S. Vijh, P. Kong, T. Horng, K. Kerksiek, N. Serbina, R. A. Tuma, and E. G. Pamer. 2001. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science 294:1735-1739. [DOI] [PubMed] [Google Scholar]
- 17.Manavathu, E. K., J. L. Cutright, D. Loebenberg, and P. H. Chandrasekar. 2000. A comparative study of the in vitro susceptibilities of clinical and laboratory-selected resistant isolates of Aspergillus spp. to amphotericin B, itraconazole, voriconazole and posaconazole (SCH 56592). J. Antimicrob. Chemother. 46:229-234. [DOI] [PubMed] [Google Scholar]
- 18.Mehrad, B., R. M. Strieter, T. A. Moore, W. C. Tsai, S. A. Lira, and T. J. Standiford. 1999. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J. Immunol. 163:6086-6094. [PubMed] [Google Scholar]
- 19.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633-1640. [PubMed] [Google Scholar]
- 20.Mehta, R. T., K. Mehta, G. Lopez-Berestein, and R. L. Juliano. 1985. Effect of liposomal amphotericin B on murine macrophages and lymphocytes. Infect. Immun. 47:429-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meletiadis, J., J. F. Meis, J. W. Mouton, J. P. Donnelly, and P. E. Verweij. 2000. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 38:2949-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy, M., E. M. Bernard, T. Ishimaru, and D. Armstrong. 1997. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:696-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindler, J. J., R. P. Warren, S. D. Allen, and M. K. Jackson. 1993. Immunological effects of amphotericin B and liposomal amphotericin B on splenocytes from immune-normal and immune-compromised mice. Antimicrob. Agents Chemother. 37:2716-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Koenig, C. H., H. Finger, and H. Hof. 1982. Failure of killed Listeria monocytogenes vaccine to produce protective immunity. Nature 297:233-234. [DOI] [PubMed] [Google Scholar]
- 25.Vora, S., S. Chauhan, E. Brummer, and D. A. Stevens. 1998. Activity of voriconazole combined with neutrophils or monocytes against Aspergillus fumigatus: effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob. Agents Chemother. 42:2299-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]