Abstract
This study investigated the oral bioavailability and efficacy of BILS 45 BS, a selective herpes simplex virus (HSV) helicase-primase inhibitor, against acyclovir (ACV)-resistant (ACVr) infections mediated by the HSV type 1 (HSV-1) dlsptk and PAAr5 mutant strains. In vitro, the compound was more potent than ACV against wild-type clinical and laboratory HSV-1 strains and ACVr HSV isolates, as determined by a standard plaque reduction assay, with a mean 50% effective concentration of about 0.15 μM. The oral bioavailability of BILS 45 BS in hairless mice was 49%, with a peak concentration in plasma of 31.5 μM after administration of a single dose of 25 mg/kg. Following cutaneous infection of nude mice, both the HSV-1 dlsptk and PAAr5 mutant strains induced significant, reproducible, and persistent cutaneous lesions that lasted for more than 2 weeks. Oral treatment with ACV (100 or 125 mg/kg/day, three times a day by gavage) did not affect either mutant-induced infection. In contrast, BILS 45 BS at an oral dose of 100 mg/kg/day almost completely abolished cutaneous lesions mediated by both ACVr HSV-1 mutants. The 50% effective doses of BILS 45 BS were 56.7 and 61 mg/kg/day against dlsptk- and PAAr5-induced infections, respectively. Taken together, our results demonstrate very effective oral therapy of experimental ACVr HSV-1 infections in nude mice and support the potential use of HSV helicase-primase inhibitors for the treatment of nucleoside-resistant HSV disease in humans.
Nucleoside analogs such as acyclovir (ACV) and penciclovir and prodrugs thereof have been approved as drugs of choice for the treatment of herpes simplex virus (HSV) infections (1, 11). While nucleoside-based therapeutics are quite effective for the treatment of primary and recurrent mucocutaneous infections, present medications are not effective for the treatment of nucleoside-resistant herpesvirus infections in immunocompromised individuals (4, 5, 29). Moreover, nucleoside-based antiviral therapeutics have limited effects on the establishment of latent HSV infections (6, 17, 31). Therefore, significant improvements in therapy may be achievable with distinct inhibitors with improved efficacy and pharmacokinetic (PK) properties and a new mechanism of action. We have reported previously on the discovery of specific non-nucleoside-based inhibitors of the HSV type 1 (HSV-1) helicase-primase (10). This enzyme is composed of the virus-encoded UL5, UL8, and UL52 gene products, which are all essential for HSV DNA replication and growth (3, 7, 9, 19, 23). The most optimized aminothiazolyl-phenyl compounds exhibited potent antiviral activity against a series of HSV strains analyzed in vitro, including HSV-1 and HSV-2 strains that are ACVr. One of the inhibitors, BILS 179 BS, showed antiviral activity in murine models of wild-type HSV disease after oral administration (10), but its activity against ACVr HSV disease had not been evaluated. In the present report, we provide a detailed examination of the PK and pharmacodynamic properties of a helicase-primase inhibitor, BILS 45 BS, in a mouse model of ACVr HSV infection. PK studies were done with hairless mice, where BILS 45 BS demonstrated in vivo efficacy against wild-type HSV-1 infection with a 50% effective dose (ED50) of 56 mg/kg. The athymic nude mouse model was chosen for the present study because ACVr HSV-1 fails to induce significant disease in normal mice (2, 14-16). In addition, since ACVr HSV infections cause significant disease mainly in the immunocompromised patient population, information obtained with these immunodeficient animals may have clinical relevance (14-16). Our results show that BILS 45 BS, an analog structurally related to BILS 179 BS, exhibited excellent oral efficacy against ACVr HSV-1 infections in nude mice, highlighting the potential of this novel class of antiherpetic agents for the treatment of ACVr HSV disease in humans.
MATERIALS AND METHODS
Cell culture and viruses.
All of the cell culture reagents and media used in this study were obtained from Gibco BRL (Burlington, Ontario, Canada). Cells were from the American Type Culture Collection (Manassas, Va.). Vero (African green monkey kidney) cells were grown in Dulbecco's modified Eagle's medium supplemented with 8% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin sulfate per ml, and 100 μg of kanamycin sulfate per ml. Baby hamster kidney (BHK) 21/C13 (ATCC CCL10) cells were grown in α-MEM instead of Dulbecco's modified Eagle's medium. All cells were grown at 37°C in an atmosphere of 5% CO2. The laboratory strains KOS, PAAr5, and dlsptk represents wild-type and DNA polymerase- and thymidine kinase-negative mutant HSV-1 strains, respectively, as described previously (6, 14, 18). The clinical isolates 294, 615.8, and 615.9 represent wild-type and DNA polymerase- and thymidine kinase-negative mutant HSV-1 strains, respectively, as described previously (30). Briefly, virus stocks were routinely grown in Vero cells and virus titers were determined by a standard plaque assay on confluent Vero cells.
Antiviral compounds.
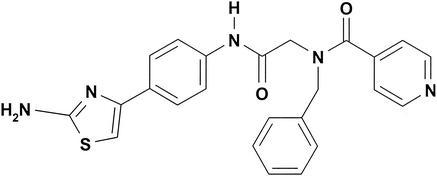
ACV was from Sigma (St. Louis, Mo.). BILS 45 BS was synthesized in house (Fig. 1) in three steps, starting with the coupling of 4′-aminoacetophenone and BOC-N-benzylglycine. Treatment of the resulting substituted acetophenone first with iodine and thiourea and then with isonicotinic acid provided BILS 45 BS. For oral administration, BILS 45 BS was dissolved in an acidified vehicle containing 0.033 N HCl, and ACV was given in water. Intravenous (i.v.) drug administration was achieved by infusing the compounds into the tail vein in a vehicle containing (vol/vol) 10% ethanol, 30% DMA, 20% glycerol, and 40% phosphate-buffered saline (PBS).
FIG. 1.
Molecular structure of BILS 45 BS.
Antiviral plaque reduction assay.
BHK 21/C13 cells were seeded in 12-well culture plates (Corning, Cambridge, Mass.) at a density of 20,000 cells per well in α-MEM medium containing 8% (vol/vol) fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin sulfate per ml, and 100 μg of kanamycin sulfate per ml, and incubated at 37°C with 5% CO2 to reach 90% confluency. In all cases, cell monolayers were infected with 50 PFU/well in α-MEM. After 1 h, the HSV-infected cells were incubated for 48 to 72 h. For studies of inhibition by ACV and BILS 45 BS, compounds were assayed in threefold serial dilutions. All compounds were dissolved in dimethyl sulfoxide and then diluted with cell culture medium to yield a 1% final concentration of dimethyl sulfoxide. All stock compound solutions were filter sterilized through 0.22-μm-pore-size Millex-GV filters (Millipore, Bedford, Mass.). Compound concentrations were routinely verified by high-performance liquid chromatography. After the postinfection incubation period, cells were fixed with 4% formaldehyde and stained with 2% crystal violet in 20% ethanol. HSV plaques were visualized and counted under a microscope. The results were expressed as percent inhibition of virus replication compared to control plaques obtained in the absence of inhibitor. The drug concentrations causing 50% inhibition of plaque formation (EC50s) were extrapolated from dose-response curves of the percent inhibition-versus-concentration data. The values reported are the average ± the SEM (standard error of the mean) from the indicated number of experiments. The cytotoxicity of BILS 45 BS for BHK cells was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay described elsewhere (14, 25). The concentration of BILS 45 BS resulting in 50% cytotoxicity in five independent experiments under the antiviral assay conditions was 19 ± 6 μM.
Therapeutic effects in vivo.
Athymic nude mice (female, nu/nu, CD1 from Charles River Canada, Quebec) at 5 to 6 weeks of age were used for all experiments. Animals were housed in microisolator cages inside semirigid isolators with sterile food, water, and bedding. All experiments with infected mice were done within class II-type safety cabinets (NuAire, Plymouth, Minn.), in accordance with protocols approved by the Canadian Council on Animal Care (Ottawa, Ontario). Animals were inoculated with ACVr HSV-1 dlsptk or PAAr5 mutants under halothane anesthesia by needle scarification and rubbing for 10 s with 10 μl of virus stock (107 PFU) on an area of about 1 cm2 on each side of the dorsal skin. ACV and BILS 45 BS were administered by oral gavage in a vehicle containing 0.033 N HCl three times per day (between 8:30 am and 4:30 pm) for 10 days starting 3 h postinoculation. Cutaneous lesions were scored as follows: 0, no lesions; 1, discrete vesicles; 2, two or more open lesions; 3, separate ulcerations; 4, zoster band formations. Under the experimental conditions used, topical lesions become visible within 2 to 3 days and peaks become visible within about 10 to 13 days. Although some degree of spontaneous regression occurs in animals infected with the PAAr5 mutant virus, topical lesions persist over the whole duration of the experiment. Only a few of the PAAr5-infected mice (<10%) developed systemic disease (disseminated infections, neurological and physical abnormality, and mortality) following the development of severe topical lesions. Therefore, systemic disease was not used for drug evaluation. The in vivo ACV resistance of both mutants was fully characterized in our previous report, which described partial or marginal effects of high doses of ACV applied either topically (5%) or orally in drinking water (5 mg/ml) (14).
Topical-lesion data are presented as the mean and SEM. Daily lesion scores and the areas under the curves (AUC) of lesion scores were compared for statistical significance by analysis of variance (ANOVA), followed by Student-Newman-Keuls multiple comparisons with SAS software (SAS Institute, Cary, N.C.). A P value of <0.05 was considered statistically significant.
Study of bioavailability after oral administration.
Oral administration of BILS 45 BS was achieved by gavage in a vehicle containing 0.033 N HCl. i.v. drug administration was achieved by infusing the compounds into the tail vein in a vehicle containing (vol/vol) 10% ethanol, 30% DMA, 20% glycerol, and 40% phosphate-buffered saline. Blood samples were collected at designated time points via tail snipping. Plasma was obtained by centrifugation and stored at −20°C until analyzed. Aliquots of plasma (25 to 100 μl) were adjusted to a final volume of 250 μl with 10% bovine serum albumin (BSA) in 100 mM NaCl, alkalized with 50 μl of 1.5 N sodium hydroxide solution, and extracted twice with 3 ml of diethyl ether-hexane (80:20). The samples were vortexed for 30 s, and the solvents were separated by centrifugation at 1,400 × g for 10 min at 4°C. Each solvent extract was then transferred to a 3.5-ml polypropylene tube and evaporated to dryness under a nitrogen gas stream. The dried extracts were reconstituted with 100 μl of 50% acetonitrile in milli-Q water. Compounds used for standard curves were prepared in 10% BSA daily and stored in a methanol solution in a refrigerator until analyzed (up to 6 months). Plasma extracts were analyzed with a high-performance liquid chromatography system (Waters Limited, Mississauga, Ontario, Canada). The system consists of a 600E controller and a 625 LC pump, a (WISP) 715 sample processor set at 10°C to minimize evaporation of samples, and a 996 diode array detector with Millennium 2010 version 2.10 system management. Seventy-five microliters of the reconstituted sample extracts was injected onto a Symmetry C8 column (3.0 by 150 mm; Waters Limited) at 40°C. The mobile phase contained acetonitrile and Milli-Q water. A gradient (curve 9) of 40 to 100% acetonitrile in 10 min was used. The flow rate was set at 0.5 ml min−1. BILS 45 BS was detected at a wavelength of 298 nm. The correlation coefficient of standard curves was 0.99967 ± 0.00016 over a concentration range of 0.02 to 50 μM (n = 5).
All PK parameters were determined with the noncompartmental analysis methods provided by the TopFit version 2.0 data analysis system. Cmax values represent the highest observed drug concentrations in plasma, and the time required to achieve Cmax is Tmax. The AUC was calculated with the trapezoidal rule from time zero to the last nonzero data point and extrapolated to infinity. The elimination half-life was determined from the slope of the regression line that best fit the terminal portion of the log-linear concentration-time curve. The mean residence time, the total clearance, and the apparent volume of distribution at steady-state were estimated by the standard noncompartmental method.
RESULTS
In vitro antiviral activity.
The effects of inhibitors on the replication of wild-type and ACVr isolates of HSV-1 were determined with a standard plaque reduction assay. Both laboratory and clinical isolates were evaluated. Confluent BHK cells were infected with a known amount of virus, and the EC50s of the test compounds were determined. Table 1 shows the effects of BILS 45 BS and ACV on HSV-1 growth. The mean EC50s of BILS 45 BS ranged from 0.13 to 0.25 μM. In this assay, the helicase-primase inhibitor was about five times more potent than ACV against wild-type HSV strains and >20 times more potent than ACV against ACVr isolates. The EC50 of BILS 45 BS was significantly lower than the concentration that resulted in 50% cytotoxicity under identical conditions, with a selectivity index of about 100. In conclusion, BILS 45 BS inhibited wild-type and ACVr strains of HSV equally well whether they were derived from laboratory strains or isolated from infected patients in the clinic.
TABLE 1.
Antiviral activities of BILS 45 BS and ACV in a plaque reduction assay against wild-type and ACVr strains of HSV-1 in cell culture
| HSV-1 strain | EC50 (μM)a
|
|
|---|---|---|
| BILS 45 BS | ACV | |
| Laboratory isolates | ||
| KOS (WT)b | 0.13 ± 0.02 (8) | 0.97 ± 0.21 (8) |
| dlsptk (ACVr) | 0.14 ± 0.02 (6) | 24.7 ± 2.3 (6) |
| PAAr5 (ACVr) | 0.15 ± 0.03 (6) | 7.8 ± 2.2 (6) |
| Clinical isolates | ||
| 294 (WT) | 0.16 ± 0.02 (6) | 0.68 ± 0.13 (6) |
| 615.8 (ACVr) | 0.25 ± 0.05 (6) | 5.6 ± 1.0 (6) |
| 615.9 (ACVr) | 0.15 ± 0.02 (6) | 29.8 ± 3.6 (6) |
Values represent the means ± the SEM from six or eight independent determinations, as indicated by the numbers in parentheses.
WT, wild type.
Comparative in vivo activities of BILS 45 BS and ACV against HSV-1 dlsptk.
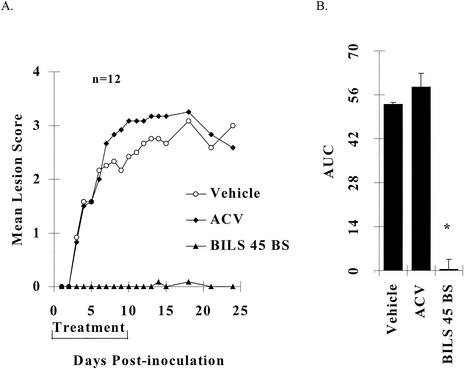
Cutaneous inoculation of athymic nude mice with 107 PFU of HSV-1 strain dlsptk per inoculation site induced reproducible and persistent topical lesions. Infected animals that did not receive any treatment reached a mean maximum lesion score of 3.0 ± 0.1 on day 12 postinoculation, with a mean AUC over 24 days of 50 ± 3 (n = 12). Treatment with the vehicle did not significantly affect the maximum lesion score (2.8 ± 0.3) or AUC (53 ± 5; P > 0.05; Fig. 2). Oral treatment with ACV at 125 mg/kg/day for 10 days was completely ineffective (Fig. 2). However, BILS 45 BS at the same oral dosage almost totally abolished HSV-1 dlsptk-mediated topical lesions (Fig. 2). Only 1 of the 12 inoculated sites developed very few small vesicles that were observed only during days 14 to 18 postinoculation and spontaneously resolved later.
FIG. 2.
Comparative effects of orally administered ACV and BILS 45 BS against HSV-1 dlsptk-induced cutaneous lesions in nude mice. Animals were cutaneously inoculated with 107 PFU per site as described in Materials and Methods. ACV and BILS 45 BS (125 mg/kg/day, three times a day for 10 days) were administered orally as described in the text. (A) Mean lesion scores (12 mice) were significantly (P < 0.05) reduced (after day 3) by BILS 45 BS but not by ACV. (B) AUCs of lesion scores represented as the mean + the SEM of 12 mice per group. The asterisk indicates a P value of <0.05 as determined by ANOVA, followed by Student-Newman-Keuls multiple comparisons.
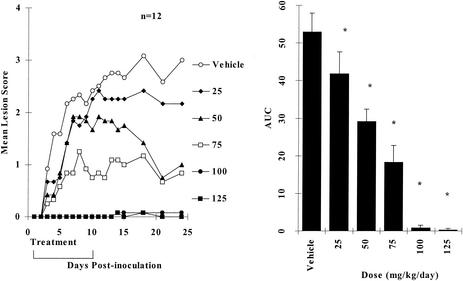
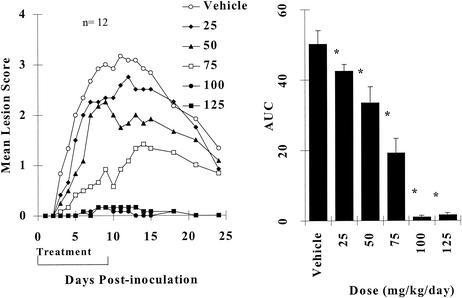
The dose-dependent antiviral effects of orally administered BILS 45 BS are summarized in Fig. 3. It is clear that maximum efficacy was achieved at an oral dose of around 100 mg/kg/day. The lowest dose of BILS 45 BS tested (25 mg/kg/day) significantly reduced cutaneous lesions, and the ED50 was 56.7 mg/kg/day (Fig. 3).
FIG. 3.
Dose-dependent effects of orally administered BILS 45 BS against HSV-1 dlsptk-induced cutaneous lesions in nude mice. Animals were cutaneously inoculated with 107 PFU per site as described in Materials and Methods. BILS 45 BS (three times per day for 10 days) was administered orally at the doses indicated. (A) Mean lesion scores (12 mice per group). (B) AUCs of lesion scores represented as the mean + the SEM of 12 mice per group. The asterisk indicates a P value of <0.05 as determined by ANOVA, followed by Student-Newman-Keuls multiple comparisons.
Comparative activities of BILS 45 BS and ACV against HSV-1 PAAr5.
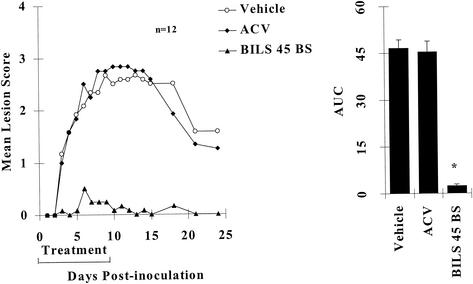
HSV-1 PAAr5-induced cutaneous lesions reached a maximum within about 10 days, and partial regression started at about 2 weeks postinoculation. The vehicle or ACV at an oral dose of 100 mg/kg/day did not affect any of the experimental parameters (Fig. 4). In contrast, BILS 45 BS at the same dosage reduced the AUC of topical-lesion scores by more than 98% (Fig. 4). A dose-response study of BILS 45 BS at 0 to 125 mg/kg/day for 10 days showed antiviral activity similar to that observed against HSV-1 dlsptk (Fig. 5). Maximum efficacy was achieved at about 100 mg/kg/day. The lowest dose tested reduced topical lesions significantly, and the oral ED50 was 61 mg/kg/day. Therefore, BILS 45 BS demonstrated similar efficacies and potencies against both the HSV-1 dlsptk and PAAr5 mutants.
FIG. 4.
Comparative effects of orally administered ACV and BILS 45 BS against HSV-1 PAAr5-induced cutaneous lesions in nude mice. Animals were cutaneously inoculated with 107 PFU per site as described in Materials and Methods. ACV and BILS 45 BS (100 mg/kg/day, three times a day for 10 days) were administered orally as described in the text. (A) Mean lesion scores were significantly reduced after day 3 by BILS 45 BS but not by ACV (12 mice per group; P < 0.05). (B) AUCs of lesion scores represented as the mean + the SEM of 12 mice per group. The asterisk indicates a P value of <0.05 as determined by ANOVA, followed by Student-Newman-Keuls multiple comparisons.
FIG. 5.
Dose-dependent effects of orally administered BILS 45 BS against HSV-1 PAAr5-induced cutaneous lesions in nude mice. Animals were cutaneously inoculated with 107 PFU per site as described in Materials and Methods. BILS 45 BS (three times per day for 10 days) were administered orally at the doses indicated. (A) Mean lesion scores (12 mice per group). (B) AUCs of lesion scores represented as the mean + the SEM of 12 mice per group. The asterisk indicates a P value of <0.05 as determined by ANOVA, followed by Student-Newman-Keuls multiple comparisons.
Bioavailability of orally administered BILS 45 BS.
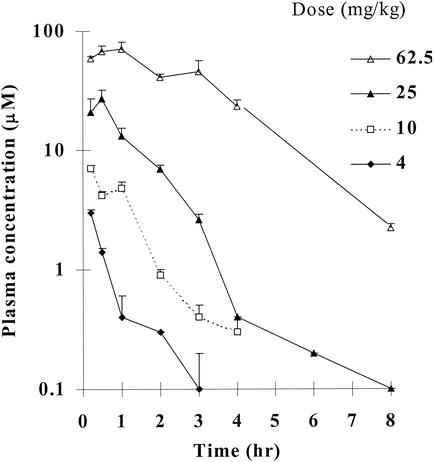
As Fig. 6 shows, BILS 45 BS was absorbed rapidly, reaching the Cmax within 0.5 h at doses of up to 25 mg/kg. The Tmax increased at higher doses. The AUC of BILS 45 BS in plasma increased disproportionally as the dose was increased (Fig. 6). This nonlinear profile was also identified with i.v. administration of BILS 45 BS over a dose range of 1.6 to 25 mg/kg, indicating saturable elimination at higher doses. On the basis of this observation, the bioavailability of BILS 45 BS after oral administration was estimated from equal doses of oral and i.v. drug administration at 25 mg/kg as summarized in Table 2 (27, 32). It may be important to point out that this dose used for calculation of bioavailability after oral administration is equivalent to its 60 to 70% effective dose. Under these experimental conditions, the estimated bioavailability of BILS 45 BS after oral administration is 49%.
FIG. 6.
PK profile of BILS 45 BS following administration of a single oral dose to hairless mice. Doses were administered as indicated by oral gavage. Values represent the mean + the SEM for three, three, eight, and three mice given doses of 4, 10, 25, and 62.5 mg/kg, respectively.
TABLE 2.
PK parameters of BILS 45 BS following administration of an oral or i.v. dose of 25 mg/kga
| Parameterb | Oral (n = 8) | I.v. (n = 4) |
|---|---|---|
| Cmax (μM) | 31.5 ± 3.0 | |
| Tmax (h) | 0.4 ± 0.1 | |
| t1/2 (h) | 0.98 ± 0.03 | |
| AUC (μM · h) | 45.4 ± 3.7 | 92.9 ± 3.8 |
| MRT (h) | 1.34 ± 0.07 | 1.29 ± 0.03 |
| Cltot (ml/min/kg) | 10.2 ± 0.4 | |
| Vss (liters/kg) | 0.79 ± 0.05 | |
| Bioavailability (%) | 49 |
Values indicate the mean ± the SEM for four or eight individual experiments, as indicated.
t1/2, elimination half-life; MRT, mean residence time; CLtot, total clearance; Vss, volume of distribution at steady state.
DISCUSSION
Since its launch in 1981, ACV has been established as the “gold standard” for the treatment of HSV infections (1, 11). This acyclic nucleoside analog has provided efficacy and a good safety profile (11). Its close analog penciclovir and the prodrugs valaciclovir and famciclovir were subsequently developed and shown to exhibit increased bioavailability and efficacy (1). However, two challenges remain for antiherpetic chemotherapy with ACV or its analogs, namely, HSV latency (17) and ACVr infections (4, 5, 22, 29). In 1991, Safrin et al. reported the antiviral effects of foscarnet against ACVr HSV infections in AIDS patients; however, in that study, foscarnet was administered i.v. and there was a high frequency of relapse (31). The recent discovery of acyclic nucleoside phosphonates led to the development of HPMPC (cidofovir; 12, 26, 34, 36), a polymerase inhibitor with potency against a broad spectrum of viruses, including several strains of wild-type and ACVr herpesviruses (28, 36). Unfortunately, this compound is associated with severe renal toxicity (24, 28). Combination with oral probenecid reduced the potential for renal toxicity; however, probenecid itself is also associated with toxicity. Therefore, the clinical indication for HPMPC is limited to the treatment of cytomegalovirus-mediated retinitis in AIDS patients in combination with probenecid (24).
The results presented here and elsewhere (8, 10, 35) demonstrate that inhibition of helicase-primase represents an alternative mechanism by which to prevent and treat HSV infections. HSV helicase-primase plays an essential role in HSV DNA replication and thus constitutes an excellent target for antiviral therapy (8, 10, 13, 20, 21). As demonstrated from the present study, BILS 45 BS, a selective aminothiazolyl-phenyl-based HSV helicase-primase inhibitor, is very effective against ACVr cutaneous HSV-1 infections in the nude mouse model. It reduced topical lesions in the treated groups that were inoculated with both the dlsptk and PAAr5 mutants much more effectively than did ribonucleotide reductase inhibitors (14-16), indicating that helicase-primase inhibition may represent a more effective target for antiviral therapy. The in vivo dose-dependent antiviral effects of orally administered BILS 45 BS against both mutants revealed similar potency profiles, consistent with its comparable potency in vitro. BILS 45 BS, like its close analog BILS 179 BS, is also effective against wild-type HSV-1 and HSV-2 infections in vivo (data not shown). These results therefore demonstrate the potential clinical utility of helicase-primase inhibitors and suggest that these inhibitors may be effective at treating humans infected with HSV strains that are resistant to nucleoside-based antiherpetic agents. Thus, helicase-primase inhibitors may represent novel therapeutic agents for the treatment of HSV infections in general. Nevertheless, since treatment in the present study was initiated 3 h postinoculation, further experiments are required to address the question of therapeutic potential when treatment is initiated after a longer delay.
Poor bioavailability after oral administration has been a limitation of many potent antiviral agents, including ACV and HPMPC (1, 28). The present study shows that BILS 45 BS is rapidly absorbed after oral administration. The disproportionality (or nonlinear PK profile) observed between the plasma AUC and oral doses becomes more pronounced at higher doses, probably because of a saturable mechanism of compound elimination. Under these circumstances, accurate estimation of a compound's bioavailability after oral administration is quite challenging and no ideal solution has been established (27, 32). Since the rate of compound elimination from plasma changes nonlinearly with drug exposure, accurate estimation of bioavailability after oral administration relies on the same level of drug exposure by both the oral and i.v. routes, which is very difficult to achieve experimentally. One of the well-used conservative approaches is to calculate bioavailability after oral administration on the basis of AUCs obtained with the same oral and i.v. doses (27, 32). Under these conditions, drug exposure in plasma is, for most drugs, higher following administration of an i.v. dose than that following administration of the same dose orally, thus resulting in a certain level of underestimation (27, 32). With this method, a bioavailability after oral administration of 49% was obtained for BILS 45 BS. Although it may be an underestimate, this bioavailability of BILS 45 BS after oral administration is superior to those of ACV and HPMPC in mice and could be expected to be even higher in humans considering the faster metabolism and elimination rate of smaller animals compared to those of humans (33). Liver microsome metabolism studies (data not shown) have indicated that further modifications of BILS 45 BS may lead to improved PK profiles by stabilizing the inhibitors against degradation.
In conclusion, the present report describes the properties of BILS 45 BS, a potent and selective HSV helicase-primase inhibitor with good bioavailability after oral administration and in vivo efficacy. This inhibitor had similar in vitro potencies against all of the HSV strains tested, including ACVr HSV strains. It also had similar in vivo efficacy and potency against ACVr HSV-1 infections after oral administration in the nude mouse model, in which ACV is inactive. Therefore, selective inhibitors of HSV helicase-primase may be useful orally active therapeutic agents for the treatment of HSV infections in humans mediated by wild-type, as well as nucleoside-resistant, HSV.
Acknowledgments
We acknowledge valuable discussions with P. Anderson. We thank G. Di Lella for technical assistance. We also acknowledge D. Coen for providing ACVr mutants and S. L. Sacks for providing clinical HSV-1 isolates.
REFERENCES
- 1.Boon, R. 1997. Antiviral treatment: from concept to reality. Antivir. Chem. Chemother. 8(Suppl. 1):5-10. [Google Scholar]
- 2.Brandt, C. R., R. L. Kintner, A. M. Pumfery, R. J. Visalli, and D. R. Grau. 1991. The herpes simplex virus ribonucleotide reductase is required for ocular virulence. J. Gen. Virol. 72:2043-2049. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael, E. P., and S. K. Weller. 1989. Herpes simplex virus type 1 DNA synthesis requires the product of the UL8 gene: isolation and characterization of an ICP6::lacZ insertion mutation. J. Virol. 63:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatis, P. A., and C. S. Crumpacker. 1992. Resistance of herpesviruses to antiviral drugs. Antimicrob. Agents Chemother. 36:1589-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coen, D. M. 1991. The implications of resistance to antiviral agents for herpesvirus drug targets and drug therapy. Antivir. Res. 15:287-300. [DOI] [PubMed] [Google Scholar]
- 6.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crute, J. J., and I. R. Lehman. 1991. Herpes simplex virus-1 helicase-primase, physical and catalytic properties. J. Biol. Chem. 266:4484-4488. [PubMed] [Google Scholar]
- 8.Crute, J. J., I. R. Lehman, J. Gambino, T.-F. Yang, P. Medveczky, M. Medveczky, N. N. Khan, C. Mulder, J. Monroe, and G. E. Wright. 1995. Inhibition of herpes simplex virus type 1 helicase-primase by (dichloroanilino)purines and -pyrimidines. J. Med. Chem. 38:1820-1825. [DOI] [PubMed] [Google Scholar]
- 9.Crute, J. J., T. Tsurumi, L. A. Zhu, S. K. Weller, P. D. Olivo, M. D. Challberg, E. S. Mocarski, and I. R. Lehman. 1989. Herpes simplex virus 1 helicase-primase: a complex of three herpes-encoded gene products. Proc. Natl. Acad. Sci. USA 86:2186-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crute, J. J., C. A. Grygon, K. D. Hargrave, B. Simoneau, A.-M. Faucher, G. Bolger, P. Kibler, M. Liuzzi, and M. G. Cordingley. 2001. Herpes simplex virus helicase-primase inhibitors are active in animal models of human disease. Nat. Med. 8:386-391. [DOI] [PubMed] [Google Scholar]
- 11.Darby, G. 1994. A history of antiherpes research. Antivir. Chem. Chemother. 5(Suppl. 1):3-9. [Google Scholar]
- 12.De Clercq, E., and A. Holy. 1991. Efficacy of (s)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine in various models of herpes simplex virus infection in mice. Antimicrob. Agents Chemother. 35:701-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dracheva, S., E. V. Koonin, and J. J. Crute. 1995. Identification of the primase active site of the herpes simplex virus type 1 helicase-primase. J. Biol. Chem. 270:14148-14153. [DOI] [PubMed] [Google Scholar]
- 14.Duan, J., M. Liuzzi, W. Paris, M. Lambert, C. Lawetz, N. Moss, J. Jaramillo, J. Gauthier, R. Déziel, and M. G. Cordingley. 1998. Antiviral activity of a selective ribonucleotide reductase inhibitor against acyclovir-resistant herpes simplex virus type 1 infection in vivo. Antimicrob. Agents Chemother. 42:1629-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis, M. N., D. C. Lobe, and T. Spector. 1989. Synergistic therapy by acyclovir and A1110U for mice orofacially infected with herpes simplex viruses. Antimicrob. Agents Chemother. 33:1691-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis, M. N., R. Waters, E. L. Hill, D. C. Lobe, D. W. Selleseth, and D. W. Barry. 1989. Orofacial infection of athymic mice with defined mixtures of acyclovir-susceptible and acyclovir-resistant herpes simplex virus type 1. Antimicrob. Agents Chemother. 33:304-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field, H. J., and A. M. Thackray. 1997. Can herpes simplex virus latency be prevented using conventional nucleoside analogue chemotherapy? Antivir. Chem. Chemother. 8(Suppl. 1):59-66. [Google Scholar]
- 18.Furman, P. A., D. M. Coen, M. H. St. Clair, and P. A. Schaffer. 1981. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J. Virol. 40:936-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein, D. J., and S. K. Weller. 1988. An ICP6::lacZ insertional mutation is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J. Virol. 62:2970-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graves-Woodward, K. L., J. Gottlieb, M. D. Challberg, and S. K. Weller. 1997. Biochemical analysis of mutations in the HSV-1 helicase-primase that alter ATP hydrolysis, DNA unwinding, and coupling between hydrolysis and unwinding. J. Biol. Chem. 272:4623-4630. [DOI] [PubMed] [Google Scholar]
- 21.Healy, S., X. You, and M. Dodson. 1997. Interactions of a subassembly of the herpes simplex virus type 1 helicase-primase with DNA. J. Biol. Chem. 272:3411-3415. [DOI] [PubMed] [Google Scholar]
- 22.Kimberlin, D. W., C. S. Crumpacker, S. E. Straus, K. K. Biron, W. L. Drew, F. G. Hayden, M. McKinlay, D. D. Richman, and R. J. Whitley. 1995. Antiviral resistance in clinical practice. Antivir. Res. 26:423-438. [DOI] [PubMed] [Google Scholar]
- 23.Klinedinst, D., and M. D. Challberg. 1994. Helicase-primase complex of herpes simplex virus type 1: a mutation in the UL52 subunit abolishes primase activity. J. Virol. 68:3693-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalezari, J. P., W. L. Drew, E. Glutzer, D. Miner, S. Safrin, W. F. Owen, Jr., J. M. Davidson, P. E. Fisher, and H. S. Jaffe. 1994. Treatment with intravenous (s)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]-cytosine of acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with AIDS. J. Infect. Dis. 170:570-572. [DOI] [PubMed] [Google Scholar]
- 25.Lawetz, C., and M. Liuzzi. 1998. The antiviral activity of the ribonucleotide reductase inhibitor BILD 1351 SE in combination with acyclovir against HSV type-1 in cell culture. Antivir. Res. 39:35-46. [DOI] [PubMed] [Google Scholar]
- 26.Marinez, C. M., and D. B. Luks-Golger. 1997. Cidofovir use in acyclovir-resistant herpes infection. Ann. Pharmacother. 31:1519-1521. [PubMed] [Google Scholar]
- 27.Martis, L., and R. H. Levy. 1973. Bioavailability calculations for drugs showing simultaneous first-order and capacity-limited elimination kinetics. J. Pharmacokinet. Biopharm. 1:283-294. [Google Scholar]
- 28.Naesens, L., R. Snoeck, G. Andrei, J. Balzarini, J. Neyts, and E. De Clercq. 1997. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleotide phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 8:1-23. [Google Scholar]
- 29.Pottage, J. C., Jr., and H. A. Kessler. 1995. Herpes simplex virus resistance to acyclovir: clinical relevance. Infect. Agents Dis. 4:115-124. [PubMed] [Google Scholar]
- 30.Sacks, S. L., R. J. Wanklin, D. E. Reece, K. A. Hicks, K. L. Tyler, and D. M. Coen. 1989. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity. Ann. Intern. Med. 111:893-899. [DOI] [PubMed] [Google Scholar]
- 31.Safrin, S., C. Crumpacker, P. Chatis, R. Davis, R. Hafner, J. Rush, H. A. Kessler, B. Landry, and J. Mills. 1991. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. N. Engl. J. Med. 325:551-555. [DOI] [PubMed] [Google Scholar]
- 32.Shepard, T. A., N. Lordi, and P. E. Sparrow. 1993. Influence of dose range on degree of nonlinearity detected in dose-proportionality studies for drugs with saturable elimination: single-dose and steady-state studies. Pharm. Res. 10:289-293. [DOI] [PubMed] [Google Scholar]
- 33.Singer, M. A. 2001. Of mice and men and elephants: metabolic rate sets glomerular filtration rate. Am. J. Kid. Dis. 37:164-178. [DOI] [PubMed] [Google Scholar]
- 34.Snoeck, R., G. Andrei, M. Gerard, A. Silverman, A. Hedderman, J. Balzarini, C. Sadzot-Delvaux, G. Tricot, N. Clumeck, and E. De Clercq. 1994. Successful treatment of progressive mucocutaneous infection due to acyclovir- and foscarnet-resistant herpes simplex virus with (s)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC). Clin. Infect. Dis. 18:570-578. [DOI] [PubMed] [Google Scholar]
- 35.Spector, F. C., H. Liang, H. Giordano, M. Sivaraja, and M. G. Paterson. 1998. Inhibition of herpes simplex virus replication by a 2-aminothiazole via interactions with the helicase component of the UL5-UL8-UL52 complex. J. Virol. 72:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zabawski, E. J., J. Do, and C. J. Cockerell. 1998. Topical and intralesional cidofovir: a review of pharmacology and therapeutic effects. J. Am. Acad. Dermatol. 39:741-745. [DOI] [PubMed] [Google Scholar]